Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability
Abstract
1. Introduction
2. Taxonomy and Characteristics
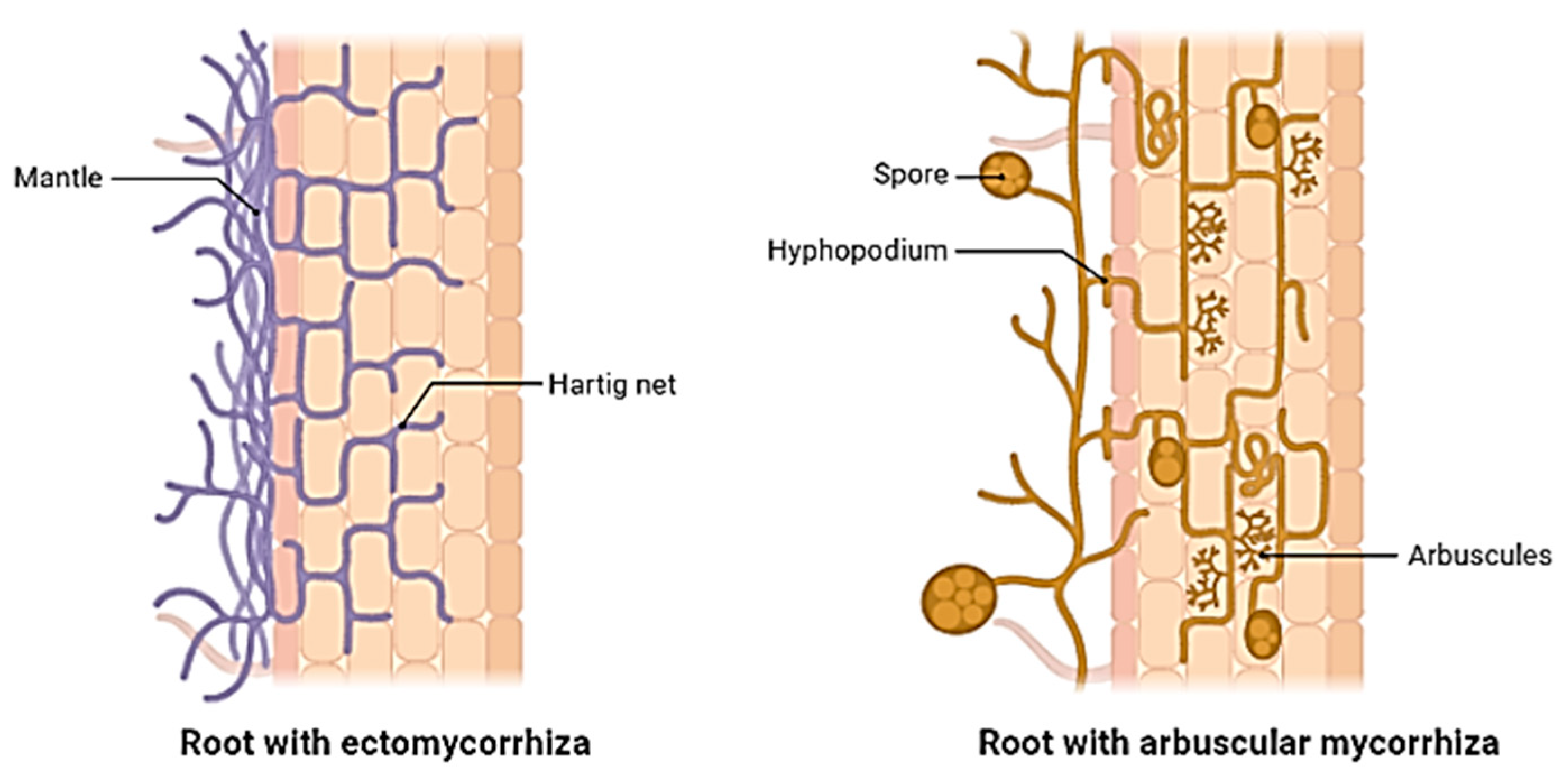
3. Mycorrhizal Symbiosis
4. Role of R. intraradices in Promoting Plant Growth
5. Nutrient Cycling and Soil Health
6. Environmental Restoration and Ecosystem Resilience
7. Sustainable Agriculture and Organic Farming
8. Agro-Ecological Relevance of Glomeromycota and R. intraradices
- (a)
- Plant growth promotion based on nutrient solubilization and phytohormones
- (b) Mycorrhiza–plant interaction: yielding plant disease biocontrol
- (c) Mycorrhiza–microorganisms interaction
- (d) Mycorrhiza–soil interaction
- (e) Biogeochemical cycles and mycorrhiza
9. Genomic Research in Glomeromycota
10. Negative Effects of AMF
11. Challenges and Future Perspectives
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schenck, N.C.; Smith, G.S. Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 1982, 74, 77–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Liu, K.; Chen, Y.; Yuan, J.; Chang, Q. Rhizoglomus intraradices improves plant growth, root morphology and Phytohormone balance of Robinia pseudoacacia in arsenic-contaminated soils. Front. Microbiol. 2020, 11, 1428. [Google Scholar] [CrossRef]
- Schüssler, A.; Walker, C. The Glomeromycota: A Species List with New Families and New Gener; Schüßler and Walker: Gloucester, UK, 2010; pp. 1–56. [Google Scholar]
- Butler, E. The occurrences and systematic position of the vesicular-arbuscular type of mycorrhizal fungi. Trans. Br. Mycol. Soc. 1939, 22, 274–301, IN7. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Trappe, J.M. The Endogonaceae in the Pacific Northwest; New York Botanical Society and The Mycological Society of America: New York, NY, USA, 1974; Volume 5, pp. 1–76. [Google Scholar]
- Sieverding, E.; da Silva, G.A.; Berndt, R.; Oehl, F. Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon 2015, 129, 373–386. [Google Scholar] [CrossRef]
- Walker, C.; Trappe, J.M.; Schüßler, A.; Hawksworth, D.L.; Cazares, E.; Elliott, T.F.; Redecker, D.; McNeill, J.; Redhead, S.A.; Wiersema, J.H. (2491) Proposal to conserve the name Rhizophagus with a conserved type (Fungi: Glomeromycota: Glomeraceae). Taxon 2017, 66, 199–200. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.H.; Li, D.Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar]
- Krüger, M.; Krüger, C.; Walker, C.; Stockinger, H.; Schüßler, A. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 2011, 193, 970–984. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.R.; Bello-Bello, E.; Rellán-Álvarez, R.; Sawers, R.J.H.; Olalde-Portugal, V. Inoculation with the mycorrhizal fungus Rhizophagus irregularis modulates the relationship between root growth and nutrient content in maize (Zea mays ssp. mays L.). Plant Direct 2019, 3, e00192. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.-J.; Hao, Z.-P.; Li, H.; Chen, B.-D. Aquaporin genes GintAQPF1 and GintAQPF2 from Glomus intraradices contribute to plant drought tolerance. Plant Signal. Behav. 2013, 8, e24030. [Google Scholar] [CrossRef]
- Croll, D.; Sanders, I.R. Recombination in Glomus intraradices, a supposed ancient asexual arbuscular mycorrhizal fungus. BMC Evol. Biol. 2009, 9, 13. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.; Merckx, V.; Tedersoo, L. FungalRoot: Global online database of plant mycorrhizal associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef]
- Maherali, H. Is there an association between root architecture and mycorrhizal growth response? New Phytol. 2014, 204, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.B.; Connolly, E.L. Plant-Soil Interactions: Nutrient Uptake. Nat. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Chialva, M.; di Fossalunga, A.S.; Daghino, S.; Ghignone, S.; Bagnaresi, P.; Chiapello, M.; Novero, M.; Spadaro, D.; Perotto, S.; Bonfante, P. Native soils with their microbiotas elicit a state of alert in tomato plants. New Phytol. 2018, 220, 1296–1308. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Pons, S.; Fournier, S.; Chervin, C.; Bécard, G.; Rochange, S.; Frey, N.F.D.; Pagès, V.P. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef] [PubMed]
- Roussis, I.; Beslemes, D.; Kosma, C.; Triantafyllidis, V.; Zotos, A.; Tigka, E.; Mavroeidis, A.; Karydogianni, S.; Kouneli, V.; Travlos, I.; et al. The influence of arbuscular mycorrhizal fungus Rhizophagus irregularis on the growth and quality of processing tomato (Lycopersicon esculentum Mill.) seedlings. Sustainability 2022, 14, 9001. [Google Scholar] [CrossRef]
- Fracasso, A.; Telò, L.; Lanfranco, L.; Bonfante, P.; Amaducci, S. The physiological beneficial effect of Rhizophagus intraradices inoculation on tomato plant yield under water deficit conditions. Agronomy 2020, 10, 71. [Google Scholar] [CrossRef]
- Ibiang, S.R.; Sakamoto, K.; Kuwahara, N. Performance of tomato and lettuce to arbuscular mycorrhizal fungi and Penicillium pinophilum EU0013 inoculation varies with soil, culture media of inoculum, and fungal consortium composition. Rhizosphere 2020, 16, 100246. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, K.; Feng, Z.; Qin, Y.; Zhou, Y.; Feng, G.; Zhu, H.; Yao, Q. Tomato plant growth promotion and drought tolerance conferred by three arbuscular mycorrhizal fungi is mediated by lipid metabolism. Plant Physiol. Biochem. 2024, 208, 108478. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, X.; Elzenga, J.T.M.; van Elsas, J.D. Effect of soil bacteriomes on mycorrhizal colonization by Rhizophagus irregularis—Interactive effects on maize (Zea mays L.) growth under salt stress. Biol. Fertil. Soils 2022, 58, 515–525. [Google Scholar] [CrossRef]
- Xie, L.; Lehvävirta, S.; Timonen, S.; Kasurinen, J.; Niemikapee, J.; Valkonen, J.P.T. Species-specific synergistic effects of two plant growth-Promoting microbes on green roof plant biomass and photosynthetic efficiency. PLoS ONE 2018, 13, e0209432. [Google Scholar] [CrossRef]
- Saia, S.; Tamayo, E.; Schillaci, C.; De Vita, P. Arbuscular Mycorrhizal Fungi and Nutrient Cycling in Cropping Systems. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R., Pathan, S., Ceccherini, M., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, Q.; Geng, X.; Lin, K.; Li, Z.; Li, Y.; Chen, J.; Li, X. Arbuscular Mycorrhizal Fungi Alter Rhizosphere Bacterial Diversity, Network Stability and Function of Lettuce in Barren Soil. Sci. Hortic. 2024, 323, 112533. [Google Scholar] [CrossRef]
- Saia, S.; Jansa, J. Editorial: Arbuscular Mycorrhizal Fungi: The Bridge Between Plants, Soils, and Humans. Front. Plant Sci. 2022, 13, 875958. [Google Scholar] [CrossRef]
- Luginbuehl, L.H.; Oldroyd, G.E. Understanding the Arbuscule at the Heart of Endomycorrhizal Symbioses in Plants. Curr. Biol. 2017, 27, R952–R963. [Google Scholar] [CrossRef] [PubMed]
- Huey, C.J.; Gopinath, S.C.B.; Uda, M.N.A.; Zulhaimi, H.I.; Jaafar, M.N.; Kasim, F.H.; Yaakub, A.R.W. Mycorrhiza: A Natural Resource Assists Plant Growth under Varied Soil Conditions. 3 Biotech 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate-Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L. Arbuscular Mycorrhizal Fungi Reduce Potassium, Cadmium and Ammonium Losses but Increases Nitrate Loss under High Intensity Leaching Events. BMC Plant Biol. 2022, 22, 365. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Guo, M.; Qu, L.; Biere, A. Effects of arbuscular mycorrhizal fungi on plant growth and herbivore infestation depend on availability of soil water and nutrients. Front. Plant Sci. 2023, 14, 1101932. [Google Scholar] [CrossRef]
- De Andrade, S.A.L.; Domingues, A.P., Jr.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Herold, A.; Walker, D.A. Transport across Chloroplast Envelopes the Role of Phosphate. In Transport across Single Biological Membranes, 1st ed.; Tosteson, D.C., Ed.; Springer: Berlin/Heidelberg, Germany, 1979; Volume 2, pp. 411–439. [Google Scholar]
- Andrino, A.; Guggenberger, G.; Kernchen, S.; Mikutta, R.; Sauheitl, L.; Boy, J. Production of organic acids by arbuscular mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxides. Front. Plant Sci. 2021, 12, 661842. [Google Scholar] [CrossRef]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef]
- Liu, R.-C.; Meng, L.-L.; Zou, Y.-N.; He, X.-H.; Wu, Q.-S. Introduction of earthworms into mycorrhizosphere of white clover facilitates N storage in glomalin-related soil protein and contribution to soil total N. Appl. Soil Ecol. 2022, 179, 104597. [Google Scholar] [CrossRef]
- Meng, L.-L.; Srivastava, A.; Kuča, K.; Wu, Q.-S. Earthworm (Pheretima guillelmi)-mycorrhizal fungi (Funneliformis mosseae) association mediates rhizosphere responses in white clover. Appl. Soil Ecol. 2022, 172, 104371. [Google Scholar] [CrossRef]
- He, J.-D.; Chi, G.-G.; Zou, Y.-N.; Shu, B.; Wu, Q.-S.; Srivastava, A.; Kuča, K. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil Ecol. 2020, 154, 103592. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Xie, M.-M.; Li, Y.; Liu, B.-Y.; Liu, C.-Y.; Wu, Q.-S.; Kuča, K. Effects of field inoculation with arbuscular mycorrhizal fungi and endophytic fungi on fruit quality and soil properties of Newhall navel orange. Appl. Soil Ecol. 2022, 170, 104308. [Google Scholar] [CrossRef]
- Hovland, M.; Mata-González, R.; Schreiner, R.P.; Rodhouse, T.J. Fungal facilitation in rangelands: Do arbuscular mycorrhizal fungi mediate resilience and resistance in sagebrush steppe? Rangel. Ecol. Manag. 2019, 72, 678–691. [Google Scholar] [CrossRef]
- Sugiura, Y.; Akiyama, R.; Tanaka, S.; Yano, K.; Kameoka, H.; Kawaguchi, M.; Akiyama, K.; Saito, K. Myristate is a carbon and energy source for the asymbiotic growth of the arbuscular Mycorrhizal fungus Rhizophagus intraradices. BioRxiv 2019, 731489. [Google Scholar] [CrossRef]
- Gou, X.; Hu, Y.; Ni, H.; Wang, X.; Qiu, L.; Chang, X.; Shao, M.; Wei, G.; Wei, X. Arbuscular mycorrhizal fungi alleviate erosional soil nitrogen loss by regulating nitrogen cycling genes and enzymes in experimental agro-ecosystems. Sci. Total Environ. 2024, 906, 167425. [Google Scholar] [CrossRef]
- Bukovská, P.; Rozmoš, M.; Kotianová, M.; Gančarčíková, K.; Dudáš, M.; Hršelová, H.; Jansa, J. Arbuscular Mycorrhiza Mediates Efficient Recycling from Soil to Plants of Nitrogen Bound in Chitin. Front. Microbiol. 2021, 12, 574060. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Liu, Y.; Yi, Q.; Hall, M.; Saha, N.; Wang, J.; Huang, Y.; Huang, L. Arbuscular mycorrhizal fungi regulate plant mineral nutrient uptake and partitioning in iron ore tailings undergoing eco-engineered pedogenesis. Pedosphere 2024, 34, 385–398. [Google Scholar] [CrossRef]
- Kalamulla, R.; Karunarathna, S.C.; Tibpromma, S.; Galappaththi, M.C.A.; Suwannarach, N.; Stephenson, S.L.; Asad, S.; Salem, Z.S.; Yapa, N. Arbuscular Mycorrhizal Fungi in Sustainable Agriculture. Sustainability 2022, 14, 12250. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Anderson, I.C.; Antonovics, J.; Ballhausen, M.; Bergmann, J.; Bielcik, M.; Chaudhary, V.B.; Deveautour, C.; Grünfeld, L.; et al. Myristate and the ecology of AM fungi: Significance, opportunities, applications and challenges. New Phytol. 2020, 227, 1610–1614. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Riley, R.C.; Cavagnaro, T.R.; Brien, C.; Smith, F.A.; Smith, S.E.; Berger, B.; Garnett, T.; Stonor, R.; Schilling, R.K.; Chen, Z.; et al. Resource allocation to growth or luxury consumption drives mycorrhizal responses. Ecol. Lett. 2019, 22, 1757–1766. [Google Scholar] [CrossRef]
- Asmelash, F.; Bekele, T.; Birhane, E. The Potential Role of Arbuscular Mycorrhizal Fungi in the Restoration of Degraded Lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar] [CrossRef]
- Kuila, D.; Ghosh, S. Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 2022, 3, 100107. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Bender, S.F.; van der Heijden MG, A. Soil biota enhance agricultural sustainability by improving crop yield, and nutrient uptake and reducing nitrogen leaching losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Walker, C.; Schüßler, A.; Vincent, B.; Cranenbrouck, S.; Declerck, S. Anchoring the species Rhizophagus intraradices (formerly Glomus intraradices). Fungal Syst. Evol. 2021, 8, 179–201. [Google Scholar] [CrossRef]
- Bhardwaj, I.; Garg, N. Phytohormones and arbuscular mycorrhizal Rhizoglomus intraradices together modulate defense mechanisms in mungbean to reduce Ni toxicity. Rhizosphere 2023, 27, 100723. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Liu, C.; Guo, C.; Zhang, Y.; Ma, C.; Duan, X. Individual and combined effects of arbuscular mycorrhizal fungi and phytohormones on the growth and physiobiochemical characteristics of tea cutting seedlings. Front. Plant Sci. 2023, 14, 1140267. [Google Scholar] [CrossRef]
- Hossain, M.B. Glomalin and contribution of glomalin to carbon sequestration in soil: A review. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 191–196. [Google Scholar] [CrossRef]
- Basyal, B. Plant-Arbuscular Mycorrhizal Fungi Association Under Drought Stress. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Parihar, M., Rakshit, A., Adholeya, A., Chen, Y., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Ibrahim, D.S.; Riad, S.N.; Abo-Elyousr, K.A.; Nashwa, S.M.; Khalil Bagy, H.M.; Abdelrazek, S.; Abdellatif, A.A. Unraveling the Mysteries of Mycorrhiza-Plant Interactions: Mechanisms of Protection and Ecological Factors Influencing Symbioses. In Mycorrhizal Symbiosis and Agroecosystem Restoration; Ansari, R.A., Rizvi, R., Mahmood, I., Eds.; Springer: Singapore, 2024; pp. 197–226. [Google Scholar] [CrossRef]
- Weng, W.; Yan, J.; Zhou, M.; Yao, X.; Gao, A.; Ma, C.; Cheng, J.; Ruan, J. Roles of Arbuscular mycorrhizal fungi as a biocontrol agent in the control of plant diseases. Microorganisms 2022, 10, 1266. [Google Scholar] [CrossRef]
- Dey, M.; Ghosh, S. Arbuscular mycorrhizae in plant immunity and crop pathogen control. Rhizosphere 2022, 22, 100524. [Google Scholar] [CrossRef]
- Dutta, S.S.; Ghosh, S. Mycorrhizae: Potential Biocontrol for Crop Plants. Fungal Genom Biol. 2024, 14, 241. [Google Scholar]
- Vishwakarma, S.K.; Ilyas, T.; Malviya, D.; Shafi, Z.; Shahid, M.; Yadav, B.; Singh, U.B.; Rai, J.P.; Singh, H.B.; Singh, H.V. Arbuscular mycorrhizal fungi (AMF) as potential biocontrol agents. In Rhizosphere Microbes: Biotic Stress Management; Springer: Singapore, 2022; pp. 197–222. [Google Scholar]
- Hussain, T.; Usmaan, M.; Numan, M.; Khan, A.A.; Abbas, F.; Gul, A. Mycorrhiza: Plant growth-promoting and biocontrol agent ability under the abiotic stress conditions. In Soil Microbiomes for Sustainable Agriculture: Functional Annotation; Springer: Cham, Switzerland, 2021; pp. 503–527. [Google Scholar]
- Jin, Z.; Jiang, F.; Wang, L.; Declerck, S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi and Streptomyces: Brothers in arms to shape the structure and function of the hyphosphere microbiome in the early stage of interaction. Microbiome 2024, 12, 83. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sumathi, C.S.; Rajeshkannan, V.; Bagyaraj, D.J. Mycorrhizosphere Revisited: Multitrophic Interactions. In Re-Visiting the Rhizosphere Eco-System for Agricultural Sustainability; Rhizosphere Biology; Singh, U.B., Rai, J.P., Sharma, A.K., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Sangwan, S.; Prasanna, R. Mycorrhizae helper bacteria: Unlocking their potential as bioenhancers of plant–arbuscular mycorrhizal fungal associations. Microb. Ecol. 2021, 84, 1–10. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, H.; Zhang, S.; Jiang, J.; Zhu, H.; Yang, H.; Li, L. Isolation and identification of mycorrhizal helper bacteria of Vaccinium uliginosum and their interaction with mycorrhizal fungi. Front. Microbiol. 2023, 14, 1180319. [Google Scholar] [CrossRef]
- Berrios, L.; Yeam, J.; Holm, L.; Robinson, W.; Pellitier, P.T.; Chin, M.L.; Henkel, T.W.; Peay, K.G. Positive interactions between mycorrhizal fungi and bacteria are widespread and benefit plant growth. Curr. Biol. 2023, 33, 2878–2887.e4. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Tao, W.; Liu, G.; Liu, W.; Wang, L.; Ma, L. Interactions between arbuscular mycorrhizal fungi and soil properties jointly influence plant C, N, and P stoichiometry in West Lake, Hangzhou. RSC Adv. 2020, 10, 39943–39953. [Google Scholar] [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup, H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [Google Scholar] [CrossRef]
- de Novais, C.B.; Avio, L.; Giovannetti, M.; de Faria, S.M.; Siqueira, J.O.; Sbrana, C. Interconnectedness, length and viability of arbuscular mycorrhizal mycelium as affected by selected herbicides and fungicides. Appl. Soil Ecol. 2019, 143, 144–152. [Google Scholar] [CrossRef]
- Singh, A.K.; Zhu, X.; Chen, C.; Wu, J.; Yang, B.; Zakari, S.; Jiang, X.J.; Singh, N.; Liu, W. The role of glomalin in mitigation of multiple soil degradation problems. Crit. Rev. Environ. Sci. Technol. 2020, 52, 1604–1638. [Google Scholar] [CrossRef]
- Hu, D.; Baskin, J.M.; Baskin, C.C.; Wang, Z.; Zhang, S.; Yang, X.; Huang, Z. Arbuscular mycorrhizal symbiosis and achene mucilage have independent functions in seedling growth of a desert shrub. J. Plant Physiol. 2018, 232, 1–11. [Google Scholar] [CrossRef]
- Mubekaphi, C. Soil Organic Carbon, Glomalin Related Soil Protein and Related Physical Properties after 15 Years of Different Management Practices in a Subtropical Region of South Africa. Doctoral Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2019. [Google Scholar]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Tiwari, G.; Mishra, P.K.; Pattanayak, A.; Bisht, J.K.; et al. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2020, 202, 1581–1596. [Google Scholar] [CrossRef]
- Pellegrino, E.; Gamper, H.A.; Ciccolini, V.; Ercoli, L. Forage rotations conserve diversity of arbuscular mycorrhizal fungi and soil fertility. Front. Microbiol. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Boyno, G.; Yerli, C.; Çakmakci, T.; Sahin, U.; Demir, S. The effect of arbuscular mycorrhizal fungi on carbon dioxide (CO2) emission from turfgrass soil under different irrigation intervals. J. Water Clim. Chang. 2024, 15, 541–553. [Google Scholar] [CrossRef]
- Bisht, A.; Sharma, V.; Garg, N. Deciphering the Role of Arbuscular Mycorrhizal Fungi in Mitigating the Negative Effects of Abiotic Stresses in Legume Crops. In Arbuscular Mycorrhizal Fungi in Sustainable Agriculture: Nutrient and Crop Management; Parihar, M., Rakshit, A., Adholeya, A., Chen, Y., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Zhang, T.; Feng, G. Arbuscular mycorrhizal fungi alleviate the negative effects of increases in phosphorus (P) resource diversity on plant community structure by improving P resource utilization. Plant Soil 2021, 461, 295–307. [Google Scholar] [CrossRef]
- Rosling, A.; Sahraei, S.E.; Khan, F.K.; Desirò, A.; Bryson, A.E.; Mondo, S.J.; Grigoriev, I.V.; Bonito, G.; Sánchez-García, M. Evolutionary history of arbuscular mycorrhizal fungi and genomic signatures of obligate symbiosis. BMC Genom. 2024, 25, 529. [Google Scholar] [CrossRef]
- Wang, X.-X.; Wang, X.; Sun, Y.; Cheng, Y.; Liu, S.; Chen, X.; Feng, G.; Kuyper, T.W. Arbuscular mycorrhizal fungi negatively affect nitrogen acquisition and grain yield of maize in a n deficient soil. Front. Microbiol. 2018, 9, 418. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Rodriguez-Morelos, V.H.; Calonne-Salmon, M.; Bremhorst, V.; Garcés-Ruiz, M.; Declerck, S. Fungicides with Contrasting Modes of Action Differentially Affect Hyphal Healing Mechanism in Gigaspora sp. and Rhizophagus irregularis. Front. Plant Sci. 2021, 12, 642094. [Google Scholar] [CrossRef]
- Kuyper, T.W.; Jansa, J. Arbuscular mycorrhiza: Advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses. Plant Soil 2023, 489, 41–88. [Google Scholar] [CrossRef]

| Taxonomy | Classification |
|---|---|
| Domain | Eukaryota |
| Kingdom | Fungi |
| Division | Glomeromycota |
| Class | Glomeromycetes |
| Order | Glomerales |
| Family | Glomeraceae |
| Genus | Rhizophagus |
| Species | R. intraradices |
| Features | Description |
|---|---|
| Spores | Color: Pale yellow, greyish yellow. |
| Shape: Elliptical with irregularities. | |
| Size: Generally, between 40–140 μm. Formation: Predominantly forms spores intraradically. | |
| Hyphae | Shape: Cylindrical or slightly flared. |
| Size: Width: 11–18 μm. | |
| Distribution | Found in almost all soils, especially those populated with common host plants, and in forests and grasslands. |
| Colonization | Colonization peaks earlier than many other fungi in Rhizophagus, with extensive hyphal networking and intense intraradical spores associated with the older roots of host plants. |
| Reproduction | Colonizes new plants using spores, hyphae, or fragments of roots colonized by the fungus. |
| Features | Description |
|---|---|
| Metabolism | Capable of osmotic adjustment, antioxidation, and expression of aquaporin Plasma Membrane Intrinsic Proteins, PIP genes under drought stress [11]. |
| Meiosis and recombination | Possesses homologs of 51 meiotic genes, indicating the capability of undergoing conventional meiosis and genetic recombination [12]. |
| Mycorrhizal association | Forms arbuscular mycorrhizal symbiosis with plant roots [2]. |
| Growth temperature range | Mesophilic, optimum growth temperature around 25–30 °C |
| Growth substrate | Grows in soil, forming mycorrhizal networks with plant roots [10]. |
| Nutrient utilization and uptake | Utilizes organic carbon compounds for growth. Can use both organic and inorganic nitrogen sources. Efficiently absorbs and transports phosphorus to the host [10]. |
| Benefits | Description |
|---|---|
| Mycorrhizal Symbiosis | Arbuscular mycorrhizae, such as Rhizophagus intraradices, substantially affect the absorption of nutrients by plants and the growth of the root system. Mycorrhizal application improves the consistency of crops, reduces transplant losses, and increases the yield of numerous horticultural crops [13,14]. |
| Plant Growth Promotion | Inoculation with Rhizophagus intraradices improves seedling growth, root development, and biomass. Rhizophagus intraradices stimulates root growth, nutrient uptake, and growth parameters under different environmental conditions. Combined inoculation with Rhizophagus intraradices and other microbes can increase shoot weight and photosynthetic efficiency [2,10,22]. |
| Nutrient Cycling | Mycorrhizal fungi such as Rhizophagus intraradices affect photosynthesis by improving nutrient absorption by plants, leading to changes in chlorophyll levels and the availability of phosphorus. Arbuscular mycorrhizal fungi help in obtaining nitrogen from organic material, affecting nitrogen cycling and ecosystem functioning [38,40]. |
| Environmental Restoration and Ecosystem Resilience | Mycorrhizal fungi such as Rhizophagus intraradices play a crucial role in soil health, plant physiology, and ecological interactions, improving the function of plants and ecosystem resilience. Arbuscular mycorrhizal fungi enhance soil organic matter content and water retention, thereby preventing the scarcity of water and improving the preservation of the soil ecosystem [46,51]. |
| Sustainable Agriculture and Organic Farming | Arbuscular mycorrhizal fungi are important in sustainable agriculture for improving plant nutrition, growth, and stress tolerance. Mycorrhizal fungi can function as bio-fertilizers, enhancing soil quality, fertility, and resistance to pathogens, thereby improving organic farming practices [50,55,56]. |
| Negative Effects of AMF | Description |
|---|---|
| Plant Growth Suppression | The introduction of arbuscular mycorrhizal fungi (AMF) suppresses plant height, particularly under conditions of low water availability, as observed by Wang et al. [35]. AMF presence also leads to a reduction in plant biomass, specifically noticeable under circumstances of low water and nutrient levels [35]. |
| Root Morphology Alteration | AMF inoculation enhances specific root length and decreases average root diameter, especially at low water and nutrient levels, according to research by Wang et al. [35]. |
| Nutrient Alteration | Wang et al. [35] found that AMF application decreases leaf phosphorus concentrations, especially under conditions of high nutrient availability. |
| Herbivore Population Control | AMF presence decreases the population of the foliar herbivore Chrysolina aeruginosa on plants cultivated in low-nutrient soil, as observed by Wang et al. [35], possibly linked to diminished leaf phosphorus content. This contrasts with the increased abundance observed in fertilized plants with high water levels [35]. |
| Impaired Nitrogen Acquisition | Arbuscular mycorrhizal fungi (AMF) impede nitrogen (N) acquisition, resulting in diminished maize grain yield in N-deficient soils, as demonstrated in field conditions by Wang et al. [88]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyeaka, H.N.; Akinsemolu, A.A.; Siyanbola, K.F.; Adetunji, V.A. Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability. Microbiol. Res. 2024, 15, 1028-1049. https://doi.org/10.3390/microbiolres15020068
Onyeaka HN, Akinsemolu AA, Siyanbola KF, Adetunji VA. Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability. Microbiology Research. 2024; 15(2):1028-1049. https://doi.org/10.3390/microbiolres15020068
Chicago/Turabian StyleOnyeaka, Helen N., Adenike A. Akinsemolu, Kehinde Favour Siyanbola, and Victoria Ademide Adetunji. 2024. "Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability" Microbiology Research 15, no. 2: 1028-1049. https://doi.org/10.3390/microbiolres15020068
APA StyleOnyeaka, H. N., Akinsemolu, A. A., Siyanbola, K. F., & Adetunji, V. A. (2024). Green Microbe Profile: Rhizophagus intraradices—A Review of Benevolent Fungi Promoting Plant Health and Sustainability. Microbiology Research, 15(2), 1028-1049. https://doi.org/10.3390/microbiolres15020068







