Abstract
Co-infection with Pseudomonas (Pa) and Aspergillus (Af) commonly occurs in the airways of immune-compromised patients or in cystic fibrosis and frequently results in more severe outcomes than mono-infection. We affixed both pathogens to agar beads, separately (Af beads, Pa beads) or on the same bead (AfPa beads) and infected immunocompetent mice, an in vivo Af-Pa interaction model. Endotracheal administration was superior to intranasal, allowing larger beads to be administered resulting in longer lung residence. The CFU of the Af beads, diameter 150–250 µm, were detectable for ≤21 days. Af-bead-infected mice cleared the Af infection more than mice infected with AfPa beads, but Af clearance was the same with a combination of beads (Af beads + Pa beads). Pa-infected mice had more Pa clearance in the presence of Af than with Pa beads alone. In vitro studies supported our conclusion that the close proximity of Af and Pa (on AfPa beads) was disadvantageous for Af, whereas a larger distance (Af + Pa beads) was not. We demonstrated that the interaction between Pseudomonas and Aspergillus during co-infection can be studied in immunocompetent mice. The mutual inhibition of Af and Pa in vivo appears to be dependent on their proximity. We review the literature relating to animal models of infection with Af, Pa, or both.
1. Introduction
P. aeruginosa (Pa) and A. fumigatus (Af) are the most prominent bacterium and fungus, respectively, in the airways of persons with cystic fibrosis (CF), as well as immune-compromised patients [1,2,3,4,5,6,7]. Both pathogens, especially in cooperation, have been associated with deterioration of lung function [1,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
Interactions between P. aeruginosa and A. fumigatus have been studied in vitro for many years [24], with the majority of studies pointing toward an anti-fungal role of P. aeruginosa, interfering with fungal metabolism or growth via molecules such as phenazines, i.e., pyocyanin (PCN, 5-N-methyl-1-hydroxyphenazine) [25,26], 1-hydroxyphenazine (1HP) [25,26], phenazine-1-carboxamide (PCB) [26], phenazine-1-carboxylic acid (PCA) [26], and di-rhamnolipids [27]. The most prominent P. aeruginosa virulence factor under limiting iron conditions is its major siderophore, pyoverdine [28,29,30]. A. fumigatus siderophores counteract this action efficiently [31]. Under non-limiting iron conditions, pyoverdine is not present, and we identified phenazines, especially pyocyanin, as contributors to P. aeruginosa’s anti-fungal activity [32]. We also showed that the Pa product 3,4-dihydroxy-2-heptylquinoline (PQS) uniquely affects Af metabolism in two ways, depending on the concentrations of iron present: damaging the fungus under low-iron conditions while promoting fungal metabolism under high-iron conditions [33].
Any in vitro system lacks the influence of the immune system and of host physiologic properties (e.g., airway mucus, mucociliary clearance, the inflammatory response). Animal models have great utility in addressing biological questions (Table 1), as detailed in several review articles on aspergillosis [34,35,36,37,38,39,40].

Table 1.
Potential uses of murine models of Aspergillus and/or Pseudomonas in studies.
Rodents, guinea pigs, rabbits, birds, flies, and moth larvae have been used for this purpose. Of these, murine models have important advantages, as mice have a low purchase cost, are the least expensive mammals to board, are best characterized genetically and immunologically, and inbred strains are readily available. Many mouse models for pulmonary Pseudomonas infection rely on immune suppression or genetic abnormalities for establishing infection [41,42,43,44,45,46,47], although, as reviewed in [48], normal mice have also been used [47,49,50,51,52,53,54,55,56]. Agar-bead-incorporated Pseudomonas or Aspergillus has successfully been used to prolong lung infections in mice [47,49,50,52,53,54,55,57,58,59].
Here, we present a model in which mono-infection was verified and co-infection explored using immunocompetent mice, and this model may be useful for future studies of intermicrobial interactions. Such a model could provide a better understanding of Aspergillus and Pseudomonas interactions in patients and allow for the development of medications that consider microbial interactions. Our choice of outbred mice reduces expenses in establishing such a model. We also review the literature relevant to Aspergillus and Pseudomonas pulmonary models.
2. Materials and Methods
Materials: Sabouraud Dextrose Agar (SDA), Potato Dextrose Agar (PDA), Trypticase Soy Agar (TSA), and Trypticase Soy Broth (TSB) were purchased from Becton Dickinson and Co. (Sparks, MD, USA). Bacto agar was obtained from Carolina Biological Supply Co., Burlington, NC, USA. Mineral oil; phosphate-buffered saline (PBS), pH 7.4; and cetrimide agar (CET) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Isolates: All isolates used in this study are provided in Table 2. The use of all microbes in our laboratory was approved by the CIMR Biological Use Committee (approval no. 001-03 Yr.17).

Table 2.
Isolates used in this study.
Bacterial culture: Bacteria from overnight cultures of PA14 on TSA plates were added to 3 mL of TSB and allowed to grow at 37 °C 100 rpm for 24 h. One mL of that culture was diluted with 5 mL of fresh TSB and allowed to grow at 37 °C and 100 rpm for another 24 h. OD was determined using a spectrophotometer (Genesys 20, Thermo Fisher Scientific Inc., Waltham, MA, USA) and adjusted to OD610 = 1.5. This culture was used for bead preparations immediately.
Fungal culture: 10AF conidia were distributed on PDA plates and incubated at 37 °C for 3 days. Conidia were harvested using 10 mL of PBS with Tween 0.05%. Conidia were counted using an Improved Neubauer Chamber (Hausser Scientific, Horsham, PA, USA) and used for bead production at concentrations of 108 to 109 conidia/mL. A culture of Blastomyces yeasts as a comparator was studied as previously described [64], and the airway inoculation technique was the same as described below.
Bead preparation: Five mL of agar in water per bead preparation (double-concentrated in comparison with the manufacturer’s instructions) was autoclaved and adjusted to 50 °C in a water bath. We compared Bacto agar, TSA, and PDA but did not find differences in the outcomes of experiments when using beads based on the respective agars. Fifty mL of mineral oil was autoclaved per bead preparation and adjusted to 50 °C in a water bath. For Aspergillus beads (Af beads), 2.5 mL of prewarmed conidia suspension and 2.5 mL of prewarmed TSB were added to agar. For Pseudomonas beads (Pa beads), 2.5 mL of prewarmed bacterial suspension in TSB and 2.5 mL of saline were added to agar. For Aspergillus + Pseudomonas beads (AfPa beads), 2.5 mL of prewarmed conidia and 2.5 mL of prewarmed bacterial suspension in TSB were pre-mixed and added to agar. Additionally, agar mixtures were prepared that contained no organisms and only 2.5 mL of saline and 2.5 mL of TSB (empty beads). Mixtures were added to mineral oil under constant stirring using 10 mL syringes and pressing through a 23-gauge needle. At that moment, beads of various sizes formed in each preparation. Bead suspensions in mineral oil were allowed to cool to room temperature for 7 min, followed by chilling in an ice bath for 5 min while stirring constantly. Bead suspensions were then transferred to sterile 50 mL plastic tubes and centrifuged at 4 °C and 4000× g for 30 min to pellet the beads. The mineral oil was discarded, and each bead preparation was washed four times in 25 mL of sterile phosphate-buffered saline (PBS) (400 g, room temperature, 15 min) until no oil residues were visible anymore. Each bead preparation was suspended in 15 mL of sterile PBS and passed through a sterile rough metal mesh (about 1 mm mesh size) to remove very large beads. The filtrate was passed through a 250 µm tissue strainer (PierceTM, Thermo Scientific, Rockford, IL, USA) to remove beads larger than 250 µm. The filtrate contained beads < 250 µm and was passed through a 150 µm cell strainer (Sysmex Partec GmbH, Goerlitz, Germany). This cell strainer retained beads of 150 to 250 µm diameter, and these were collected with 1 mL of sterile PBS. The filtrate contained beads < 150 µm and was passed through a 100 µm cell strainer (Sysmex Partec GmbH). This cell strainer retained beads of 100 to 150 µm diameter and was washed with 1 mL of sterile PBS. The filtrate contained beads < 100 µm and was passed through a 50 µm cell strainer (Sysmex Partec GmbH). This cell strainer retained beads of 50 to 100 µm diameter, and these were collected with 1 mL of sterile PBS. The filtrate contained beads < 50 µm and was discarded. We finally obtained three preparations of beads that had diameters of 50–100, 100–150, and 150–250 µm. We prepared beads with Af only, Pa only, Af + Pa. or no microbes. To determine bead numbers in preparations, part of each organism-containing bead preparation was diluted in sterile PBS and plated on SDA with chloramphenicol (to suppress Pseudomonas CFU and enable the counting of Aspergillus CFU) and CET plates (selective agar, allowing for the counting of Pseudomonas CFU) and incubated for 40 h at 37 °C. For uninfected beads, 25 µL of undiluted bead preparation was plated on SDA and CET plates and examined for CFU at the same time the organism-containing bead plates were evaluated. One CFU on plates represented one bead, as a bead containing multiple organisms would grow in one spot. Figure 1 shows an example of Af, AfPa, and Af + Pa beads (size 150–250 µm) at a 100-fold magnification (taken via digital microscopy using the M83EZ-C02 microscope; OMAX Microscopes, China, in combination with OMAX Toup View Software). The number of uninfected beads was determined by counting under the microscope at 40× magnification.
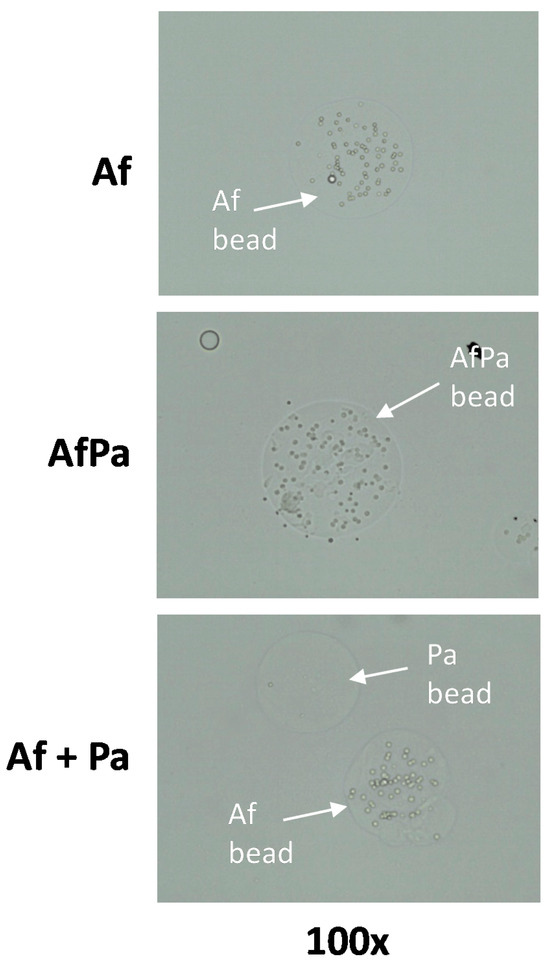
Figure 1.
Bead preparation: Agar beads carrying P. aeruginosa PA14 and/or A. fumigatus 10AF. Example pictures of beads carrying 10AF (Af beads), PA14 (Pa beads), or 10AF and PA14 together (AfPa beads) were taken via digital microscopy at a magnification of 100×. The bead size is approximately 200 µm. This was taken 6 h after microbes were added to the beads.
Preparation of inoculum: Beads were diluted in sterile PBS to their final concentrations, which are indicated in the Results, Figures, and Figure Legends. When Af or Pa beads were compared with their combination, the total bead number was equalized by adding uninfected beads.
Challenge in vivo: Female CD-1 mice, 5 weeks of age, were obtained from Charles River Laboratories, Gilroy, CA, and housed in groups of 5 in micro-isolator cages. The use of animals was approved by the California Institute for Medical Research Institutional Animal Care and Use Committee (approval no. 21-01:01). Sterilized food and acidified water were provided ad libitum. Mice were acclimatized for one week before any procedure, at which time, the average weight/mouse was about 25 g. Study groups were a minimum of 4 per group in each experiment. Experiments were repeated at least twice. For intranasal inoculation, mice were sedated briefly with isoflurane. For endotracheal challenge, mice were injected with dexmedetomidine hydrochloride (Dexdomitor, Zoetis, Kirkland, QC, Canada) according to the manufacturer’s instructions for calming before brief exposure to isoflurane. Adding a Dexdomitor application to the isoflurane exposure reduced stress for the animals during inoculation. Inoculation was performed through the open mouth into the trachea, requiring the positioning of mice on their backs and keeping the mouth open with a slightly protracted tongue via the gentle use of forceps. Endotracheal application was performed using Hamilton syringes and 22 s needles (Hamilton Company, Inc., Reno, NV, USA). The entrance to the trachea can be located using the tracheal rings, which the operator feels while holding the syringe and passing the Hamilton syringe needle gently along the posterior part of the airway. This was more time-consuming than intranasal application. Dexdomitor/isoflurane treatment induced unconsciousness for approximately one minute, so mice did not need to be restrained during the procedure. Immediately after successful inoculation, mice were injected intra-peritoneally with atipamezole (Antisedan, Zoetis, Canada) according to the manufacturer’s instructions and were mobile within a minute.
Determination of CFU in lung tissue: Examination of the beads after application of Af showed conversion of conidia into the hyphal form in vitro (Figure 2).
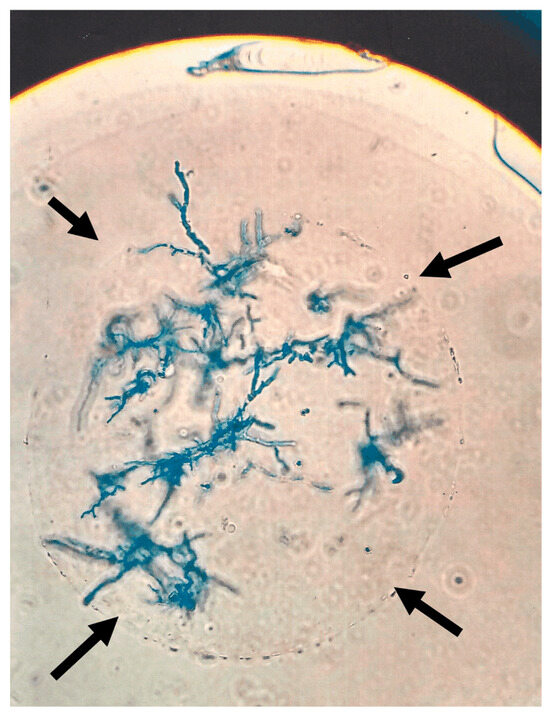
Figure 2.
Aspergillus hyphae on a bead after incubation. One 10AF bead (outline indicated by black arrows) carrying hyphae (shown after Giemsa staining) after overnight incubation in RPMI 1640 at 37 °C. The picture was taken under light microscopy.
In an initial experiment, 6 mice were each infected with 2500 Af beads (determined via the enumeration of 2500 CFU), 150–250 µm diameter. Seven days after infection, mice were sacrificed. Lung impressions were taken on sterile glass slides and lung smears examined via light microscopy.
In subsequent experiments, at times indicated in the Results and Figure Legends, mice were sacrificed via CO2 asphyxiation. For determination of CFU in lung tissue, organ weight was determined, and lung homogenates in 5 mL of sterile saline were prepared and distributed on SDA with chloramphenicol (Aspergillus growth) or CET plates (Pseudomonas growth). Plates were incubated at 37 °C for 48 h. CFU were counted and are presented as CFUlog10 for each lung. Although it is possible some small fraction of an inoculum introduced to the respiratory tree could find its way into the upper gastrointestinal tree, similar prior reports of studies with beads and respiratory challenges [47,49,50,52,53,54,55,57,58,65] have not reported gastrointestinal pathology or dissemination as a feature of infection, and in our prior study of pulmonary challenge with conidia in immunocompromised mice, these features were not noted at necropsy [66]. At necropsy in our study, no disseminated disease was noted.
Interaction of beads in vitro: For in vitro experiments, 1000 beads of each kind (Af, Pa, and AfPa, all 150–250 µm) were combined in 1 mL of RPMI medium and incubated at 37 °C for 24 h. To determine CFU/mL after incubation, aliquots were distributed on SDA-chloramphenicol plates (determination of Aspergillus CFU) or on CET plates (determination of Pseudomonas CFU), and CFU/mL were calculated.
Light microscopic evaluation of fungal growth from beads was performed as follows: one sterile 10 mm coverslip (Roundcover10; BIPEE, Shenzhen, China) was placed in each well of a 24-well plate. Beads (corresponding to 103 cells/mL/well) were seeded in a volume of 2 mL RPMI in combinations indicated in the Results and Figure Legends. Beads were incubated at 37 °C for 24 h. To take pictures, coverslips were removed from their wells and placed face down on glass microscope slides (12-544-7; Fisher Scientific, Waltham, MA, USA). Pictures were taken using a digital microscope (M83EZ-C02, OMAX Microscopes, in combination with OMAX Toup View Software) at a magnification of 400×. Representative pictures are shown. For size references of structures in micrographs, bead sizes are indicated in the text, the diameter of an A. fumigatus conidium is 2.75 ± 0.25 µm, the width of A. fumigatus hyphae is 5.3 ± 2.7 µm, the length of a Pseudomonas bacillus is 1.75 ± 0.25 µm.
Statistical analysis: Results were analyzed using Student’s t-test if two groups were compared and 1-way ANOVA combined with a Tukey’s post-test for multiple comparisons. All data in this study are expressed as a mean ± SD. Data are reported as log10CFU. Each study was performed at least twice with a minimum of four mice per group.
3. Results
3.1. Inoculum Diameter and Infection Route Determine Infection Efficiency and Duration
With the goal of creating an optimized model for Aspergillus–Pseudomonas interactions in vivo, we first compared the infection efficiency of inocula of different diameters using intranasal infection. Figure 3A shows that infection efficiency, determined 1 h after infection with CFU that reached the lung, decreased significantly with a larger-diameter inoculum (Aspergillus conidia: 2–3 µm > Blastomyces cells: 10–15 µm > Af beads: 100–150 µm). Likewise, Af beads showed better infection efficiency when the bead diameter was smaller (50–100 µm > 100–150 µm > 150–250 µm) (Figure 3B). We also found that, after intranasal infection, Aspergillus CFU declined rapidly as soon as 7 days post-infection (Figure 3C). A higher concentration of beads for infection did not increase the initial pathogen load significantly and could not compensate for the CFU loss over time (Figure 3C).
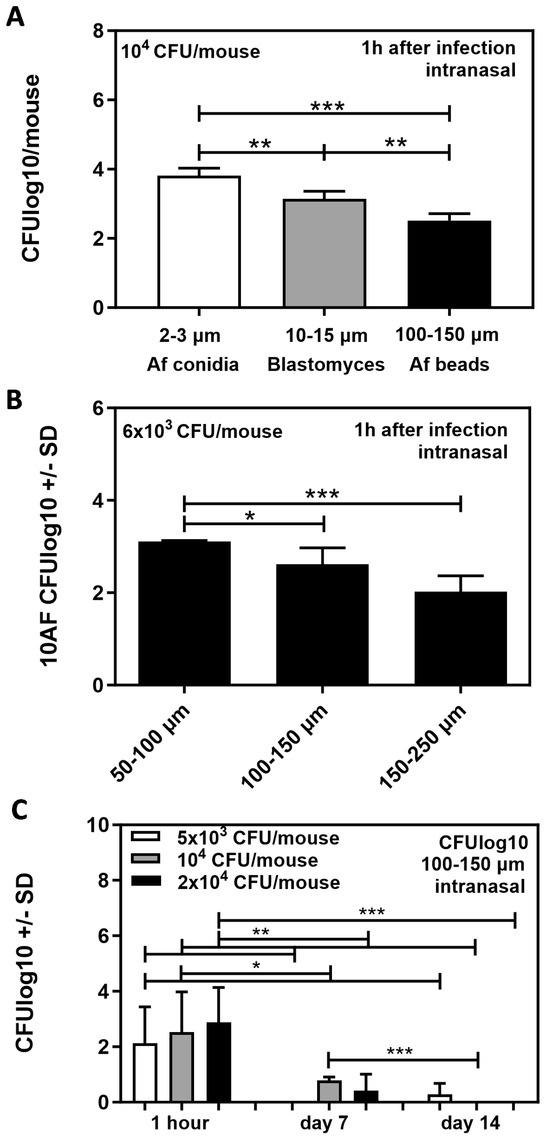
Figure 3.
Infection efficiency for beads of different sizes, following intranasal infection. (A) CD1 mice were infected intranasally with 104 agar beads containing organisms of different sizes (Aspergillus conidia: 2–3 µm, Blastomyces cells: 10–15 µm, Af beads: 100–150 µm). CFU in lungs of mice were determined 1 h after infection. (B) CD1 mice were infected intranasally with 6 × 103 agar beads of three different sizes: 50–100 µm, 100–150 µm, or 150–250 µm. CFU in lungs of mice were determined 1 h after infection. (C) CD1 mice were infected intranasally with 100–150 µm agar beads of three different concentrations: 5 × 103, 104, or 2 × 104. CFU in lungs of mice were determined 1 h, 7 days, or 14 days after infection. (A–C) Comparisons as indicated by the ends of the brackets. Statistical analysis: t-test. One, two, or three asterisks = p ≤ 0.005, p ≤ 0.01, or p ≤ 0.001, respectively. Only significant differences are shown.
We also observed that larger beads (150–250 µm) that theoretically had a higher chance of not being cleared rapidly after infection frequently blocked mouse airways when administered intranasally, resulting in a loss of mice.
After disappointing results for longer-term infection via the intranasal route, as illustrated in Figure 3C, we switched to endotracheal application, which not only allowed for the use of mid-sized beads (100–150 µm; Figure 4A) but also larger beads (150–250 µm; Figure 4B). Both bead sizes produced about equal longevity of infection, determined by enumerating the CFU in the lungs of mice 7, 14, or 21 days after infection (compare Figure 4A with Figure 4B).
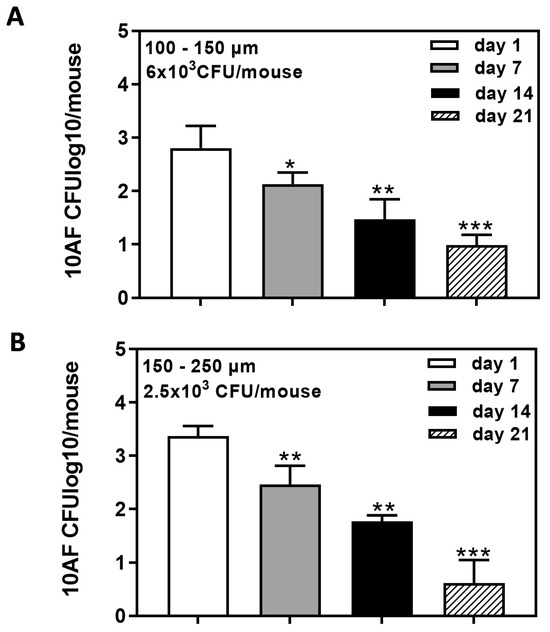
Figure 4.
Long-term detection of 10AF beads following endotracheal infection. CD1 mice were infected via tracheal infection with agar beads of 100–150 µm (A; 6 × 103 beads/mouse) or 150–250 µm (B; 2.5 × 103 beads/mouse). CFU in lungs of mice were determined 1, 7, 14, and 21 days after infection. Comparisons: CFU detected on day 1 vs. all other days. Statistical analysis: t-test. One, two, or three asterisks = p ≤ 0.005, p ≤ 0.01, or p ≤ 0.001, respectively. Only significant differences are shown.
Figure 5 illustrates the transformation of conidia into hyphae in the lungs after our inoculation of the airway with Af beads. Hyphal elements of Af can be seen in the lung, developing from conidia inoculated on beads, similar to those shown in Figure 2 after incubation in vitro.
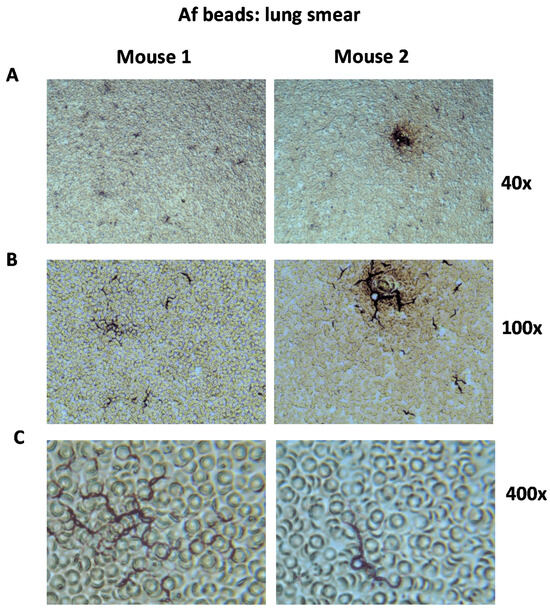
Figure 5.
Lung smears after 7 days of infection with Af beads carrying A. fumigatus 10AF. Mice were infected with beads of diameters of 150 to 250 µm carrying A. fumigatus 10AF (Af beads). Seven days after infection, mice were sacrificed, lung impressions were taken on sterile glass slides, and the smears were examined via light microscopy. The sample photomicrographs shown here were taken via digital microscopy at (A) 40×, (B) 100×, or (C) 400× magnification. The dark hyphae of Af are seen in the smears among murine erythrocytes. Examples from two mice are shown.
Hyphal Af forms associated with beads have been previously demonstrated in the literature [59,65] (note Figure 2 in each of these publications), using the techniques we adapted from these prior studies. As further experiments also showed that large beads had a slight advantage in the duration of residence in the lungs of infected mice, we decided on the use of 150–250 µm beads for all subsequent combination studies.
3.2. Pseudomonas Affects Aspergillus Growth in Mice When Located in Close Proximity to the Fungus
Based on our favorable observations of large 10AF beads (150–250 µm) over a period of 14 days (Figure 4B), we also prepared PA14 beads, as well as beads carrying 10AF and PA14 simultaneously in beads of this size. Seven days (Figure 6A), or fourteen days (Figure 6B) after infecting CD1 mice via the trachea with 2.5 × 103 Af beads alone or in combination with Pa beads (Af + Pa), we did not detect differences in Aspergillus CFU in the lungs. In contrast, when mice were infected with 2.5 × 103 beads that contained Aspergillus and Pseudomonas on the same bead (AfPa), we detected less Aspergillus CFU in mice infected with AfPa beads 7 (Figure 6A) and 14 days after infection (Figure 6B). Mice infected with Pa beads alone, as expected, did not show Aspergillus CFU.
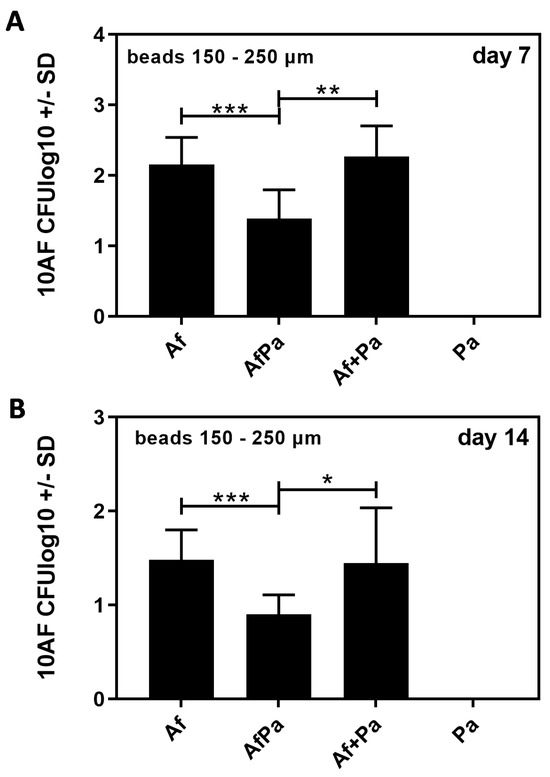
Figure 6.
Aspergillus detection following endotracheal infection with 10AF beads, PA14 beads, or beads carrying both pathogens. CD1 mice were infected endotracheally with 2.5 × 103 agar beads of 150–250 µm carrying 10AF (Af beads), PA14 (Pa beads), an equal mixture of both beads, or beads carrying both pathogens simultaneously (AfPa beads). The inoculum of Af on beads was the same for mono-infected beads as when Pa was present with Af in the challenge. CFU in lungs of mice were determined 7 (A) and 14 days (B) after infection. Comparisons as indicated by the ends of the brackets. Statistical analysis: t-test. One, two, or three asterisks = p ≤ 0.005, p ≤ 0.01, or p ≤ 0.001, respectively. Only significant differences are shown.
Our results indicate negative effects of Pseudomonas on Aspergillus if in close proximity. (“Proximity” is based on beads 150–250 µm size, and both pathogens were present in this environment in great numbers. The distance necessary for interaction, therefore, was <250 µm, but it was likely smaller).
3.3. Aspergillus Increases Clearance of Pseudomonas In Vivo
Concurrently with evaluations summarized in Figure 6, we determined CFU for Pseudomonas. Seven (Figure 7A) as well as fourteen days after infection (Figure 7B), we found significantly more CFU in the lungs of mice infected with Pa beads alone than we found in the lungs of mice infected with the same number of Pa beads but in the presence of Af beads.
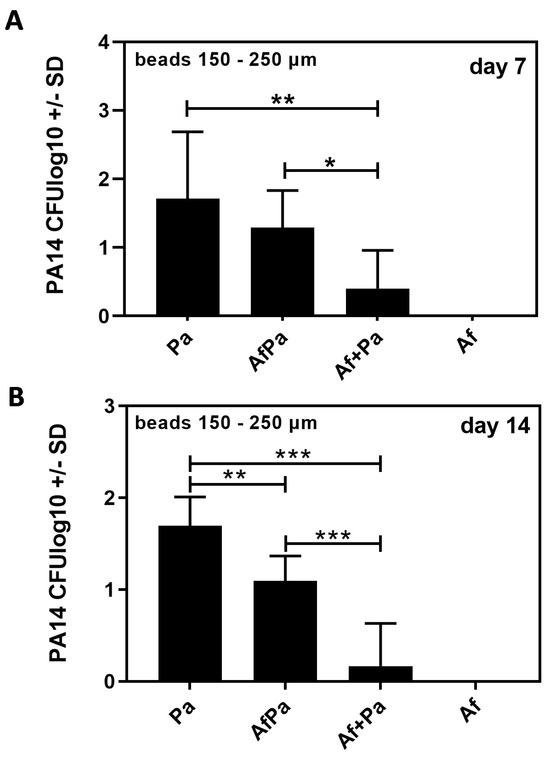
Figure 7.
Pseudomonas detection following endotracheal infection with 10AF beads, PA14 beads, or beads carrying both pathogens. CD1 mice were infected endotracheally with 2.5 × 103 agar beads of 150–250 µm carrying 10AF (Af beads), PA14 (Pa beads), an equal mixture of both beads, or beads carrying both pathogens simultaneously (AfPa beads). The inoculum of Pa on beads was the same for mono-infected beads as when Pa was present with Af in the challenge. CFU in lungs of mice were determined 7 (A) and 14 days (B) after infection. Comparisons as indicated by the ends of the brackets. Statistical analysis: t-test. One, two, or three asterisks = p ≤ 0.005, p ≤ 0.01, or p ≤ 0.001, respectively. Only significant differences are shown.
The presence of Aspergillus, therefore, seemed to support the sterilization or clearance of Pseudomonas. This effect of Af on Pa occurred to a lesser extent when Aspergillus was in close proximity to Pseudomonas (AfPa beads), a situation where Pseudomonas might have weakened the fungus in close proximity, resulting in less fungal antibacterial activity; the recovery of Pa after the Pa bead challenge was only significantly greater than after the AfPa beads on day 14 (Figure 7). Mice infected with Af beads alone, as expected, had no Pseudomonas CFU when the beads were cultured after residence in vivo.
3.4. Interaction of Bead-Bound Aspergillus and Pseudomonas In Vitro
Similar to our in vivo observations (Figure 6), we found that A. fumigatus growth in vitro was affected by P. aeruginosa only when both pathogens were located in close proximity (AfPa beads) but not when located on separate beads (Af beads + Pa beads) (Figure 8A).
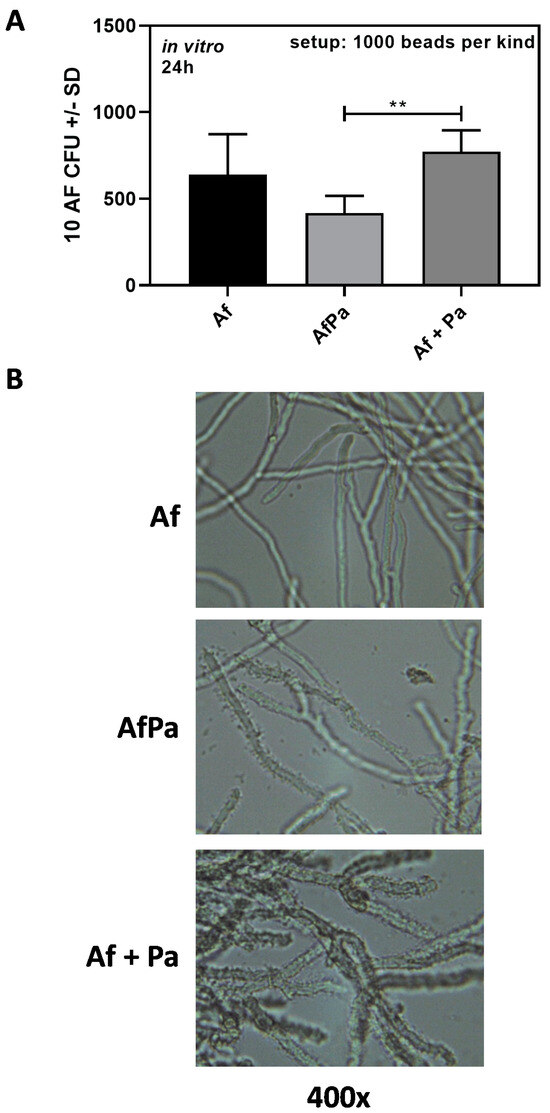
Figure 8.
Interaction between bead-bound Aspergillus and Pseudomonas in vitro. (A) 1000 10AF (Af beads) alone or in a mixture with 1000 PA14 (Pa beads) or 1000 beads carrying both pathogens simultaneously (AfPa beads) were incubated in a volume of 1 mL in 4 mL tubes in RPMI 1640 medium. Aspergillus CFU in each tube were determined via plating on SDA plates after incubation at 37 °C for 24 h. Comparisons as indicated by the ends of the brackets. Statistical analysis: t-test: Two asterisks = p ≤ 0.01. Only significant differences are shown. (B) 10AF (Af beads) alone or in mixture with PA14 (Pa beads) or beads carrying both pathogens simultaneously (AfPa beads) were incubated in a volume of 1 mL in RPMI 1640 in wells of a 24-well plate at 37 °C for 24 h. Pictures were taken via digital microscopy at a magnification of 400×.
Pseudomonas proliferated in vitro from initially 1000 CFU (based on the production of one CFU/bead) to high CFU numbers within 24 h of incubation, indicating that, in the absence of an immune system, proliferating bacteria left their beads. The proximity of Af on the same bead appeared to slow Pa proliferation (Table 3, example from one experiment).

Table 3.
Example for Pseudomonas proliferation with (Af beads + Pa beads; AfPa beads) or without the presence of Aspergillus (Pa beads). For each kind of bead, 1000 CFU were used for setup. Pseudomonas (Pa) CFU were counted on CET agar after incubation at 37 °C for 24 h.
We also determined Aspergillus growth patterns via digital microscopy. At 24 h, Aspergillus hyphae derived from AfPa beads were similar to hyphae derived from Af beads (compare the upper picture with the middle picture in Figure 8B). Hyphae derived from Af + Pa beads appeared more compact than hyphae derived from Af beads (compare the upper picture with the lower picture in Figure 8B). At higher magnification, it was obvious that Pseudomonas in Af + Pa bead studies more densely covered Aspergillus hyphae than it did in AfPa studies (compare the middle picture with the lower picture in Figure 8B). It is possible that some of the Pa released from Pa beads adhered readily to Af on Af beads, whereas, on AfPa beads, there was an inhibition of Pa attachment or an inhibition of Pa proliferation caused by Af, suggested by and consistent with the quantitative finding in Table 3 and the findings in vivo. Hyphae in the Af + Pa setup appeared sturdier and had shorter branches than hyphae in the Af or AfPa setups (compare the lower picture with the upper and middle pictures in Figure 8B). Despite its distinctive appearance, the Af + Pa arrangement did not produce less Aspergillus CFU in vitro compared with Af beads alone (Figure 8A) or in vivo, perhaps because of the late arrival of shed Pa onto hyphae.
4. Discussion
Microbial interactions have the potential to affect host morbidity and responses to therapy [67,68,69]. In the case of Pseudomonas + Aspergillus interaction in lungs, e.g., of persons with CF, co-infection seems to be associated with more rapid deterioration of lung function [1,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. In vitro, a number of interactions between Pseudomonas and Aspergillus have been described that either weaken the fungus [25,26,27,28,29,30,70,71] or the bacterium [31]. Others have described beneficial effects of Pseudomonas on Aspergillus in vitro [72]. A clinical study indicated Pseudomonas protected against the potential negative effect of Aspergillus on lung function [73].
Disseminated aspergillosis in immunocompetent mice, involving the intravenous injection of the inoculum, is a common model for study [35,74]. In addition to the models of invasive pulmonary aspergillosis, in reviews cited earlier, models of pulmonary aspergillosis have also been applied to airway reactivity in aspergillosis [75], allergic bronchopulmonary aspergillosis [76,77], and aspergillosis in transplantation [78]. Animal models and in vitro studies of Aspergillus have been of interest in order to examine the effect of the fungus and the inflammatory response it engenders in clinical CF [79,80,81,82,83,84]. Pulmonary models for aspergillosis, if based on the inoculation of conidia, require the use of immune-suppressed or genetically altered mice to produce ongoing infection. Otherwise, infection is eliminated swiftly. When using bead-bound Aspergillus, elimination is prolonged. Chronic pulmonary aspergillosis in immunocompetent mice has also been studied with intratracheal hyphal balls [85]. Intrapulmonary challenge with Aspergillus-infected agarose beads plus steroids has been shown to result in progressive murine pulmonary aspergillosis [58]. It is well established in the literature that introducing beads with Aspergillus conidia into murine airways produces a local neutrophil response [65], whereas sterile beads produce a minimal inflammatory response, as we have corroborated (unpublished observations). This utility of beads to establish a prolonged pulmonary infection is also true for Pseudomonas, where the immune system rapidly removes free Pseudomonas in immunocompetent mice. It is also well established that Pseudomonas infection on beads results in a prompt neutrophil response in murine airways [47,49,50,52,53,54,55].
Beads that have been used in the literature include agar, agarose, and alginate. Agar beads can be generated by electrostatic or coaxial bead generators, but the method we used, after some exploratory tweaking as described, is both simple and cheap. When comparing application routes, bead size, and the residence of beads in the lungs, we found that smaller beads reach the lung more easily than larger beads, via intranasal or by endotracheal application. The application of beads through the endotracheal route proved advantageous, as it allowed for the use of larger beads without the risk of choking for the animals. Larger beads were found to reside in lungs longer than small beads, allowing for the establishment of a longer-term model. We used fully immunocompetent mice that would ordinarily quickly remove pathogens from their lungs. Our experiments showed that, in vitro, 103 Pa beads multiplied to almost 108 CFU within 24 h of incubation, whereas, in our immunocompetent animal model, only a fraction of the originally attributed beads were found in the form of CFU in mouse lungs 7 or 14 days after infection. This finding confirms that free Pseudomonas is eliminated rapidly, but Pseudomonas associated with beads is partially protected from the immune system and viable in the lung for at least 14 days.
It has been documented that Aspergillus conidia on beads germinate into hyphae [86], as we corroborated here. Regarding Aspergillus, we found no multiplication of bead CFU in vitro or in vivo, indicating that all Aspergillus stays connected to the beads and, in vivo, is cleared from the normal lung. In vitro, Aspergillus stemming from one bead can spread via hyphal growth. Although many pictures have been presented in the literature [65,86] of Aspergillus on beads that has germinated from conidia (Figure 2 and Figure 3), it is not yet completely clear how far from a bead Aspergillus spreads in vivo, but hyphal growth is very likely limited by the immune response and clearance from the respiratory tree caused by the mucociliary escalator. The CFU values after 7 or 14 days were much reduced in our experiments compared with the initial inoculum, indicating that Aspergillus does not develop conidia as a means of multiplication on beads, and rarely are conidia-producing fruiting bodies of Af noted in tissue in invasive aspergillosis in experimental animals or humans.
In a previous, similar study [65] using intratracheal agar beads in immunocompetent female C57/BL6 mice and Aspergillus strain Af293, the neutrophilic focal airway infiltration started on day 1 after challenge and was maximal at day 7, at which time, peri-bronchial inflammation was documented. In that model, the persistence of infection up to 28 days after challenge was reported, which is longer than the course of Aspergillus infection we describe, but their finding was based on the detection of galactomannan, which is not equivalent to assays for live fungi in whole lungs, as we performed, and/or the histologic visualization of fungi in beads, where the viability of the fungal particles is not assured. Another model using beads reported Aspergillus colonization in immunocompetent mice [86] and a duration of 28 days.
Considerable effort in many laboratories has been placed on developing murine models of chronic Pseudomonas pulmonary infection. A stimulus for this has been persons with CF, where Pseudomonas residence in airways is a prominent cause of morbidity and mortality. The development of pulmonary models in mice with genetic abnormalities mimicking CF has been reviewed [41,42,43,44,45,46,87], as well as with other genetic abnormalities [47,49]. Problems employing such CF models include the expense of generation and maintaining such colonies, the development of disease in organs other than the lung that can lead to early death, and the incomplete anatomical identity of defects in mice and humans. To complement such efforts and for simpler solutions for studying Pseudomonas-pulmonary interactions, pulmonary models have been developed that use normal mice (as reviewed in [47,48,49,50,51,52,53,54,55,56,59]). Studies of rats have produced similar results. Chronic pulmonary Pseudomonas infection has also been established in monkeys [88,89].
Both CF and non-CF models employ beads to introduce infection, although persistent Pseudomonas infections have been produced without beads in some instances [56,90,91]. Few models have studied the duration of Pseudomonas pulmonary infections, with some studies sacrificing animals at an early time point. In such models, the persistence of infection has varied from 3 to >30 days [50,54,59], with persistence appearing to correlate with inoculum size and large doses even producing mortality [50]. Infection with a CF-derived Pseudomonas strain was studied for at least 28 days in mice. This model was established in the nasopharynx and relies on the migration of Pseudomonas to the lung [90,91]. Persistence appears to also be affected by murine host inbred strain differences and could have been a factor in clearance in our studies, which employed the robust outbred strain CD-1.
There have been few studies of co-infection with Aspergillus and Pseudomonas. Infection with either Aspergillus or Pseudomonas in immunocompromised mice is usually lethal, and one study of immunocompromised (leukopenic) mice examined co-infection and found negligible intermicrobial effects [92]. A previous study of agar beads in mouse lungs provided rather inconclusive results with respect to Aspergillus and Pseudomonas in combination [59].
The proximity of Aspergillus and Pseudomonas in vivo appears to have played a role in their interaction in our studies. When mice were infected with Aspergillus and Pseudomonas located on different beads, we found no Pseudomonas effects on the fungus but detrimental effects from the fungus on the bacteria. When Pseudomonas and Aspergillus were located on the same bead (AfPa beads), hence, in close proximity, it appeared that Aspergillus was adversely affected by Pseudomonas, resulting in less Aspergillus CFU compared with beads carrying the fungus alone. This finding indicates that Aspergillus affects Pseudomonas over a distance, maybe through a higher iron affinity or longer half-life of its siderophores compared with Pseudomonas siderophores, denying the essential nutrient iron to the bacteria or via fungal toxins [31,93]. When in close proximity, Pseudomonas siderophores might outpace Aspergillus siderophores in the race to bind iron, starving the fungus. Pseudomonas produces anti-fungal molecules other than siderophores that damage the fungus, e.g., rhamnolipids or phenazines, which need to be concentrated to affect the fungus, which is facilitated by close proximity. Pseudomonas proliferation in vivo was also adversely affected by the fungus.
5. Conclusions
In conclusion, in this study, we examined infections by Pseudomonas and Aspergillus that were recent and occurred concurrently. The effects on each microbe by the other might depend on the niche, the stage of infection, the relative numbers of each microbe, the presence of inflammation, anatomical variations in the airways (e.g., bronchiectasis), and the content of the airways (e.g., the thickness of secretions). In future studies with the present model, using complex and difficult lung lavage techniques, it is also of interest to investigate whether the neutrophil response is quantitatively different, comparing mono-infection vs. dual infection. The use of fluorescent microbes might increase our understanding of host–pathogen interactions in vivo in models. We did not investigate scenarios where a lung is infected with one pathogen and secondarily superinfected by the other. Superinfection would be an interesting research topic for the future.
Author Contributions
Conceptualization, D.A.S.; methodology, G.S.; software, G.S.; validation, D.A.S. and G.S.; formal analysis, D.A.S. and G.S.; investigation, G.S.; resources, D.A.S.; data curation, D.A.S. and G.S.; writing—original draft preparation, D.A.S.; writing—review and editing, D.A.S. and G.S.; visualization, G.S.; supervision, D.A.S.; project administration, D.A.S.; funding acquisition, D.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
These studies were partially supported by a grant from the Flatley Foundation, CIMR no. 3770. The funder had no role in study design, data collection, and interpretation or the decision to submit the work for publication.
Institutional Review Board Statement
This animal study protocol was approved by the Institutional Animal Care and Use Committee of the California Institute for Medical Research (approval no. 21-01:01, approved in June 2021 and prolonged in June 2022 under the number 21:01:02).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results can be obtained from the corresponding authors.
Acknowledgments
The authors thank Rajesh Anand, Karl V. Clemons, Kevin Cohen, Jose A.G. Ferreira, Marife Martinez, Jack Penner (California Institute for Medical Research, San Jose, CA, USA), and Raymond Sobel (Stanford University School of Medicine, Stanford, CA, USA), for their efforts in the early stages of this work. We thank the laboratory of Donald Sheppard, McGill University, Montreal, for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Williams, H.D.; Davies, J.C. Basic science for the chest physician: Pseudomonas aeruginosa and the cystic fibrosis airway. Thorax 2012, 67, 465–467. [Google Scholar] [CrossRef]
- Smyth, A.R.; Hurley, M.N. Targeting the Pseudomonas aeruginosa biofilm to combat infections in patients with cystic fibrosis. Drugs Fut. 2010, 35, 1007–1014. [Google Scholar] [CrossRef]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: An evolutionary perspective. Nat. Rev. Microbiol. 2012, 10, 841–851. [Google Scholar] [CrossRef]
- Sabino, R.; Ferreira, J.A.; Moss, R.B.; Valente, J.; Veríssimo, C.; Carolino, E.; Clemons, K.V.; Everson, C.; Banaei, N.; Penner, J.; et al. Molecular epidemiology of Aspergillus collected from cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 474–481. [Google Scholar] [CrossRef]
- de Bentzmann, S.; Plésiat, P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol. 2011, 13, 1655–1665. [Google Scholar] [CrossRef]
- Walsh, T.J.; Stevens, D.A. Aspergillosis, Cecil Textbook of Medicine, 24th ed.; Goldman, L., Schafer, A., Eds.; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Valenza, G.; Tappe, D.; Turnwald, D. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 123–127. [Google Scholar] [CrossRef]
- Fillaux, J.; Brémont, F.; Murris, M.; Cassaing, S.; Rittié, J.L.; Tétu, L.; Segonds, C.; Abbal, M.; Bieth, E.; Berry, A.; et al. Assessment of Aspergillus sensitization or persistent carriage as a factor in lung function impairment in cystic fibrosis patients. Scand. J. Infect. Dis. 2012, 44, 842–847. [Google Scholar] [CrossRef]
- Speirs, J.J.; van der Ent, C.K.; Beekman, J.M. Effects of Aspergillus fumigatus colonization on lung function in cystic fibrosis. Curr. Opin. Pulm. Med. 2012, 18, 632–638. [Google Scholar] [CrossRef]
- Ramsey, K.A.; Ranganathan, S.; Park, J.; Skoric, B.; Adams, A.M.; Simpson, S.J.; Robins-Browne, R.M.; Franklin, P.J.; de Klerk, N.H.; Sly, P.D.; et al. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2014, 190, 1111–1116. [Google Scholar] [CrossRef]
- de Boer, K.; Vandemheen, K.L.; Tullis, E.; Doucette, S.; Fergusson, D.; Freitag, A.; Paterson, N.; Jackson, M.; Lougheed, M.D.; Kumar, V.; et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011, 66, 680–685. [Google Scholar] [CrossRef]
- Nicolai, T.; Arleth, S.; Spaeth, A.; Bertele-Harms, R.M.; Harms, H.K. Correlation of IgE antibody titer to Aspergillus fumigatus with decreased lung function in cystic fibrosis. Pediatr. Pulmonol. 1990, 8, 12–15. [Google Scholar] [CrossRef]
- Forsyth, K.D.; Hohmann, A.W.; Martin, A.J.; Bradley, J. IgG antibodies to Aspergillus fumigatus in cystic fibrosis: A laboratory correlate of disease activity. Arch. Dis. Child. 1988, 63, 953–957. [Google Scholar] [CrossRef]
- Schønheyder, H.; Jensen, T.; Høiby, N.; Andersen, P.; Koch, C. Frequency of Aspergillus fumigatus isolates and antibodies to aspergillus antigens in cystic fibrosis. Acta Pathol. Microbiol. Immunol. Scand. B 1985, 93, 105–112. [Google Scholar] [CrossRef]
- Coughlan, C.A.; Chotirmall, S.H.; Renwick, J.; Hassan, T.; Low, T.B.; Bergsson, G.; Eshwika, A.; Bennett, K.; Dunne, K.; Greene, C.M.; et al. The effect of Aspergillus fumigatus infection on vitamin D receptor expression in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 999–1007. [Google Scholar] [CrossRef]
- Mirković, B.; Lavelle, G.M.; Azim, A.A.; Helma, K.; Gargoum, F.S.; Molloy, K.; Gernez, Y.; Dunne, K.; Renwick, J.; Murphy, P.; et al. The basophil surface marker CD203c identifies Aspergillus species sensitization in patients with cystic fibrosis. J. Allergy Clin. Immunol. 2016, 137, 436–443. [Google Scholar] [CrossRef]
- Baxter, C.G.; Moore, C.B.; Jones, A.M.; Webb, A.K.; Denning, D.W. IgE-mediated immune responses and airway detection of Aspergillus and Candida in adult cystic fibrosis. Chest 2013, 143, 1351–1357. [Google Scholar] [CrossRef]
- Shoseyov, D.; Brownlee, K.G.; Conway, S.P.; Kerem, E. Aspergillus bronchitis in cystic fibrosis. Chest 2006, 130, 222–226. [Google Scholar] [CrossRef]
- Amin, R.; Dupuis, A.; Aaron, S.D.; Ratjen, F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010, 137, 171–176. [Google Scholar] [CrossRef]
- Singh, A.; Ralhan, A.; Schwarz, C.; Hartl, D.; Hector, A. Fungal Pathogens in CF Airways: Leave or Treat? Mycopathologia 2018, 183, 119–137. [Google Scholar] [CrossRef]
- Yan, K.; Yin, H.; Wang, J.; Cai, Y. Subtle relationships between Pseudomonas aeruginosa and fungi in patients with cystic fibrosis. Acta Clin. Belg. 2022, 77, 425–435. [Google Scholar] [CrossRef]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: An Irish registry analysis. BMC Pulm. Med. 2017, 17, 70. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, W. Interaction between Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. Peer J. 2018, 6, e5931. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.-C.; Dietl, A.-M.; Visca, P.; Haas, H.; et al. Aspergillus-Pseudomonas interaction, relevant to competition in airways. Med. Mycol. 2019, 57, S228–S232. [Google Scholar] [CrossRef]
- Kerr, J.R.; Taylor, G.W.; Rutman, A.; Høiby, N.; Cole, P.J.; Wilson, R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999, 52, 385–387. [Google Scholar] [CrossRef]
- Briard, B.; Bomme, P.; Lechner, B.E.; Mislin, G.L.; Lair, V.; Prévost, M.C.; Latgé, J.P.; Haas, H.; Beauvais, A. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep. 2015, 5, 8220. [Google Scholar] [CrossRef]
- Briard, B.; Rasoldier, V.; Bomme, P.; ElAouad, N.; Guerreiro, C.; Chassagne, P.; Muszkieta, L.; Latgé, J.P.; Mulard, L.; Beauvais, A. Dirhamnolipids secreted from Pseudomonas aeruginosa modify anjpegungal susceptibility of Aspergillus fumigatus by inhibiting β1,3 glucan synthase activity. ISME J. 2017, 11, 1578–1591. [Google Scholar] [CrossRef]
- Doring, G.; Maier, M.; Muller, E.; Bibi, Z.; Tummler, B.; Kharazmi, A. Virulence factors of Pseudomonas aeruginosa. Antibiot. Chemother. 1987, 39, 136–148. [Google Scholar]
- Malhotra, S.; Hayes, D., Jr.; Wozniak, D.J. Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.C.; Dietl, A.M.; Visca, P.; Haas, H.; et al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J. Bacteriol. 2017, 200, e00345-17. [Google Scholar] [CrossRef][Green Version]
- Sass, G.; Ansari, S.R.; Dietl, A.M.; Déziel, E.; Haas, H.; Stevens, D.A. Intermicrobial interaction: Aspergillus fumigatus siderophores protect against competition by Pseudomonas aeruginosa. PLoS ONE 2019, 14, e0216085. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Chatterjee, P.; Stevens, D.A. Under nonlimiting iron conditions pyocyanin is a major antifungal molecule, and differences between prototypic Pseudomonas aeruginosa strains. Med. Mycol. 2021, 59, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Nazik, H.; Sass, G.; Ansari, S.R.; Ertekin, R.; Haas, H.; Déziel, E.; Stevens, D.A. Novel intermicrobial molecular interaction: Pseudomonas aeruginosa quinolone signal (PQS) modulates Aspergillus fumigatus response to iron. Microbiology 2020, 166, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Banfalvi, G. Improved and adopted murine models to combat pulmonary aspergillosis. Appl. Microbiol. Biotechnol. 2018, 102, 6865–6875. [Google Scholar] [CrossRef] [PubMed]
- Clemons, K.V.; Stevens, D.A. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med. Mycol. 2005, 43 (Suppl. S1), S101–S110. [Google Scholar] [CrossRef]
- Yamaguchi, H. Animal Models in Medical Mycology. In Opportunistic Fungal Infections; Miyaji, M., Ed.; CRC Press: Boca Raton, FL, USA, 1987; Charpt 4. [Google Scholar]
- Paulussen, C.; Boulet, G.A.; Cos, P.; Delputte, P.; Maes, L.J. Animal models of invasive aspergillosis for drug discovery. Drug Discov. Today 2014, 19, 1380–1386. [Google Scholar] [CrossRef]
- Clemons, K.V.; Stevens, D.A. Animal models of Aspergillus infection in preclinical trials, diagnostics and pharmacodynamics: What can we learn from them? Med. Mycol. 2006, 44 (Suppl. S1), S119–S126. [Google Scholar] [CrossRef][Green Version]
- Desoubeaux, G.; Cray, C. Animal Models of Aspergillosis. Comp. Med. 2018, 68, 109–123. [Google Scholar]
- Takazono, T.; Sheppard, D.C. Aspergillus in chronic lung disease: Modeling what goes on in the airways. Med. Mycol. 2017, 55, 39–47. [Google Scholar] [CrossRef]
- Lavelle, G.M.; White, M.M.; Browne, N.; McElvaney, N.G.; Reeves, E.P. Animal models of cystic fibrosis pathology: Phenotypic parallels and divergences. BioMed Res. Int. 2016, 2016, 5258727. [Google Scholar] [CrossRef]
- Semaniakou, A.; Croll, R.P.; Chappe, V. Animal models in the pathophysiology of cystic fibrosis. Front. Pharmacol. 2019, 9, 1475. [Google Scholar] [CrossRef]
- McCarron, A.; Donnelley, M.; Parsons, D. Airway disease phenotypes in animal models of cystic fibrosis. Respir. Res. 2018, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Becker, K.A.; Zhang, Y.; Gulbins, E. CFTR-dependent susceptibility of the cystic fibrosis-host to Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2010, 300, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.; Buijs-Offerman, R.M.; Aarbiou, J.; Colledge, W.H.; Sheppard, D.N.; Touqui, L.; Bot, A.; Jorna, H.; de Jonge, H.R.; Scholte, B.J. Mouse models of cystic fibrosis: Phenotypic analysis and research applications. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S152–S171. [Google Scholar] [CrossRef] [PubMed]
- Keiser, N.W.; Engelhardt, J.F. New animal models of cystic fibrosis: What are they teaching us? Curr. Opin. Pulm. Med. 2011, 17, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Bayes, H.K.; Ritchie, N.D.; Evans, T.J. Interleukin-17 is required for control of chronic lung infection caused by Pseudomonas aeruginosa. Infect. Immun. 2016, 84, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, C.H.; Vries, R.B.; Reijmer, J.; Holthaus, D.; Visser, D.; Heming, A.; Elzinga, J.; Kempkes, R.W.; Beumer, W.; Punt, C.; et al. Animal models for cystic fibrosis: A systematic search and mapping review of the literature. Part 2: Nongenetic models. Lab. Anim. 2021, 55, 307–316. [Google Scholar] [CrossRef]
- Bayes, H.K.; Ritchie, N.; Irvine, S.; Evans, T.J. A murine model of early Pseudomonas aeruginosa lung disease with transition to chronic infection. Sci. Rep. 2016, 6, 35838. [Google Scholar] [CrossRef]
- Bragonzi, A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. Int. J. Med. Microbiol. 2010, 300, 584–593. [Google Scholar] [CrossRef]
- van Heeckeren, A.M.; Schluchter, M.D. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 2002, 36, 291–312. [Google Scholar] [CrossRef]
- Heeckeren, A.; Walenga, R.; Konstan, M.W.; Bonfield, T.; Davis, P.B.; Ferkol, T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Investig. 1997, 100, 2810–2815. [Google Scholar] [CrossRef]
- Ding, F.-M.; Zhu, S.-L.; Shen, C.; Ji, X.-L.; Zhou, X. Regulatory T cell activity is partly inhibited in a mouse model of chronic Pseudomonas aeruginosa lung infection. Exp. Lung Res. 2015, 41, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, L.J.; Trøstrup, H.; Malling Damlund, D.S.; Bjarnsholt, T.; Thomsen, K.; Jensen, P.Ø.; Hougen, H.P.; Høiby, N.; Moser, C. Bead-size directed distribution of Pseudomonas aeruginosa results in distinct inflammatory response in a mouse model of chronic lung infection. Clin. Exp. Immunol. 2012, 170, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Jensen, P.O.; Kobayashi, O.; Hougen, H.P.; Song, Z.; Rygaard, J.; Kharazmi, A. Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin. Exp. Immunol. 2002, 127, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; Rasmussen, T.B.; Jensen, P.Ø.; Stub, C.; Hentzer, M.; Molin, S.; Ciofu, O.; Givskov, M.; Johansen, H.K.; Høiby, N. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect. Immun. 2005, 73, 2504–2514. [Google Scholar] [CrossRef]
- Nawada, R.; Amitani, R.; Tanaka, E.; Niimi, A.; Suzuki, K.; Murayama, T.; Kuze, F. Murine model of invasive pulmonary aspergillosis following an earlier stage, noninvasive Aspergillus infection. J. Clin. Microbiol. 1996, 34, 1433–1439. [Google Scholar] [CrossRef]
- Yonezawa, M.; Sugiyama, H.; Kizawa, K.; Hori, R.; Mitsuyama, J.; Araki, H.; Shimakura, M.; Minami, S.; Watanabe, Y.; Yamaguchi, K. A new model of pulmonary superinfection with Aspergillus fumigatus and Pseudomonas aeruginosa in mice. J. Infect. Chemother. 2000, 6, 155–161. [Google Scholar] [CrossRef]
- Facchini, M.; De Fino, I.; Riva, C.; Bragonzi, A. Long term chronic Pseudomonas aeruginosa airway infection in mice. J. Vis. Exp. 2014, 85, 51019. [Google Scholar] [CrossRef]
- Shankar, J.; Cerqueira, G.C.; Wortman, J.R.; Clemons, K.V.; Stevens, D.A. RNA-Seq profile reveals Th-1 and Th-17 type immune responses in mice infected systemically with Aspergillus fumigatus. Mycopathologia 2018, 183, 645–658. [Google Scholar] [CrossRef]
- Denning, D.W.; Stevens, D.A. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 1991, 35, 1329–1333. [Google Scholar] [CrossRef]
- Mikkelsen, H.; McMullan, R.; Filloux, A. The Pseudomonas reference strain PA14 displays increased virulence due to a mutation in ladS. PLoS ONE 2011, 6, e29113. [Google Scholar] [CrossRef]
- Lee, D.G.; Urbach, J.M.; Wu, G.; Liberati, N.T.; Feinbaum, R.L.; Miyata, S.; Diggins, L.T.; He, J.; Saucier, M.; Déziel, E.; et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006, 7, R90. [Google Scholar] [CrossRef] [PubMed]
- Clemons, K.V.; Stevens, D.A. Efficacy of Nikkomycin Z against experimental pulmonary blastomycosis. Antimicrob. Agents Chemother. 1997, 41, 2026–2028. [Google Scholar] [CrossRef] [PubMed]
- Urb, M.; Snarr, B.D.; Wojewodka, G.; Lehoux, M.; Lee, M.J.; Ralph, B.; Divangahi, M.; King, I.L.; McGovern, T.K.; Martin, J.G.; et al. Evolution of the immune response to chronic airway colonization with Aspergillus fumigatus hyphae. Infect. Immun. 2015, 83, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Chen, V.; Tong, A.-J.; Hamilton, K.; Clemons, K.V.; Stevens, D.A. Experimental evidence that granulocyte transfusions are efficacious in treatment of neutropenic hosts with pulmonary aspergillosis. Antimicrob. Agents Chemother. 2013, 57, 1882–1887. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Coenye, T.; Touqui, L.; Bomberger, J.M. Interplay between host-microbe and microbe-microbe interactions in cystic fibrosis. J. Cyst. Fibros. 2020, 19 (Suppl. S1), S47–S53. [Google Scholar] [CrossRef]
- Martín-Gómez, M.T. Taking a look on fungi in cystic fibrosis: More questions than answers. Rev. Iberoam. Micol. 2020, 37, 17–23. [Google Scholar] [CrossRef]
- Hamada, T.; Nowak, J.A.; Milner, D.A., Jr.; Song, M.; Ogino, S. Integration of microbiology, molecular pathology, and epidemiology: A new paradigm to explore the pathogenesis of microbiome-driven neoplasms. J. Pathol. 2019, 247, 615–628. [Google Scholar] [CrossRef]
- Penner, J.C.; Ferreira, J.A.G.; Secor, P.R.; Sweere, J.; Birukova, M.; Joubert, L.-M.; Haagensen, J.A.J.; Garcia, O.; Malkovskiy, A.V.; Kaber, G.; et al. Pf4 bacteriophage produced by Pseudomonas aeruginosa inhibits Aspergillus fumigatus metabolism via iron sequestration. Microbiology 2016, 162, 1583–1594. [Google Scholar] [CrossRef]
- Nazik, H.; Sass, G.; Deziel, E.; Stevens, D.A. Aspergillus is inhibited by Pseudomonas aeruginosa volatiles. J. Fungi 2020, 6, 118. [Google Scholar] [CrossRef]
- Scott, J.; Sueiro-Olivares, M.; Ahmed, W.; Heddergott, C.; Zhao, C.; Thomas, R.; Bromley, M.; Latgé, J.P.; Krappmann, S.; Fowler, S.; et al. Pseudomonas aeruginosa-derived volatile sulfur compounds promote distal Aspergillus fumigatus growth and a synergistic pathogen-pathogen interaction that increases pathogenicity in co-infection. Front. Microbiol. 2019, 10, 2311. [Google Scholar] [CrossRef]
- Al Shakirchi, M. Fungal Colonization and Infection in Cystic Fibrosis-Prevalence, Consequences and Intervention; Karolinska Institute: Stockholm, Sweden, 2022; ISBN 978-91-8016-615-7. [Google Scholar]
- Mirkov, I.; Stosic-Grujicic, S.; Kataranovski, M. Host immune defense against Aspergillus fumigatus: Insight from experimental systemic (disseminated) infection. Immunol. Res. 2012, 52, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Templeton, S.P.; Buskirk, A.D.; Green, B.J.; Beezhold, D.H.; Schmechel, D. Murine models of airway fungal exposure and allergic sensitization. Med. Mycol. 2010, 48, 217–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grunig, G.; Corry, D.B.; Coffman, R.L.; Rennick, D.M.; Kurup, V.P. Animal models of allergic bronchopulmonary aspergillosis. Immunol. Allergy Clin. N. Am. 1998, 18, 661–679. [Google Scholar] [CrossRef]
- Kurup, V.P.; Grunig, G. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia 2002, 153, 165–177. [Google Scholar] [CrossRef]
- Hsu, J.L.; Khan, M.A.; Sobel, R.A.; Jiang, X.; Clemons, K.V.; Nguyen, T.T.; Stevens, D.A.; Martinez, M.; Nicolls, M.R. Aspergillus fumigatus invasion increases with progressive airway ischemia. PLoS ONE 2013, 8, e77136. [Google Scholar] [CrossRef]
- Warris, A.; Bercusson, A.; Armstrong-James, D. Aspergillus colonization and antifungal immunity in cystic fibrosis patients. Med. Mycol. 2019, 57 (Suppl. S2), S118–S126. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Carvalho, A.; Cunha, C.; De Luca, A.; Giovannini, G.; Casagrande, A.; Zelante, T.; Vacca, C.; Fallarino, F.; Puccetti, P.; et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am. J. Respir. Crit. Care Med. 2013, 187, 609–620. [Google Scholar] [CrossRef]
- Reihill, J.A.; Moore, J.E.; Elborn, J.S.; Ennis, M. Effect of Aspergillus fumigatus and Candida albicans on pro-inflammatory response in cystic fibrosis epithelium. J. Cyst. Fibros. 2011, 10, 401–406. [Google Scholar] [CrossRef]
- Brunel, S.F.; Willment, J.A.; Brown, G.D.; Devereux, G.; Warris, A. Aspergillus-induced superoxide production by cystic fibrosis phagocytes is associated with disease severity. ERJ Open Res. 2018, 4, 00068–2017. [Google Scholar] [CrossRef]
- Currie, A.J.; Main, E.T.; Wilson, H.M.; Armstrong-James, D.; Warris, A. CFTR modulators dampen Aspergillus-induced reactive oxygen species production by cystic fibrosis phagocytes. Front. Cell Infect. Microbiol. 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, C.; Jiang, Y.; Kou, C.; Kong, Q.; Long, N.; Lu, L.; Sang, H. Innate and adaptive immune response to chronic pulmonary infection of hyphae of Aspergillus fumigatus in a new murine model. J. Med. Microbiol. 2017, 66, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Ralph, B.; Sheppard, D.C. A murine model for chronic A. fumigatus airway infections. Methods Mol. Biol. 2021, 2260, 215–224. [Google Scholar] [CrossRef]
- Stotland, P.K.; Radzioch, D.; Stevenson, M.M. Mouse models of chronic lung infection with Pseudomonas aeruginosa: Models for the study of cystic fibrosis. Pediatr. Pulmonol. 2000, 30, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.T.; Moss, R.B.; Kurland, G.; Leong, A.B.; Novick, W.J., Jr. Chronic Pseudomonas aeruginosa endobronchitis in rhesus monkeys: II. A histopathologic analysis. J. Med. Primatol. 1993, 22, 257–262. [Google Scholar] [CrossRef]
- Cheung, A.T.; Moss, R.B.; Leong, A.B.; Novick, W.J., Jr. Chronic Pseudomonas aeruginosa endobronchitis in rhesus monkeys: I. Effects of pentoxifylline on neutrophil influx. J. Med. Primatol. 1992, 21, 357–362. [Google Scholar] [CrossRef]
- Fothergill, J.L.; Neill, D.R.; Loman, N.; Winstanley, C.; Kadioglu, A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat. Commun. 2014, 5, 4780. [Google Scholar] [CrossRef]
- Waters, E.M.; Neill, D.R.; Kaman, B.; Sahota, J.S.; Clokie, M.R.J.; Winstanley, C.; Kadioglu, A. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax 2017, 72, 666–667. [Google Scholar] [CrossRef]
- Mitsuyama, J.; Kizawa, K.; Minami, S.; Watanabe, Y.; Yamaguchi, K. Evaluation of antimicrobial agents using an experimental pulmonary superinfection model with Aspergillus fumigatus and Pseudomonas aeruginosa in leukopenic mice. J. Infect. Chemother. 2003, 9, 144–150. [Google Scholar] [CrossRef]
- Reece, E.; Doyle, S.; Greally, P.; Renwick, J.; McClean, S. Aspergillus fumigatus inhibits Pseudomonas aeruginosa in co-culture: Implications of a mutually antagonistic relationship on virulence and inflammation in the CF Airway. Front. Microbiol. 2018, 9, 1205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).