2. Epidemiology
This infection is distributed worldwide, although there are endemic genera and species. It can affect any ethnic group, age, and sex, predominating in adult men between the third and fourth decade of life in tropical and subtropical climates [
6]. Some species have limited distribution, such as
Rhinocladiella mackenziei in the Middle East,
Veronaea botryosa and
F. monophora in southern China, or
Scytalidium hyalinum and
Neoscytalidium dimidiatum in Southeast Asia and Caribbean countries such as Trinidad and Tobago, Colombia, and Venezuela [
7].
Galezowski A. et al. described the clinical characteristics of patients with deep skin mycoses in a 20-year retrospective study (1998–2016) in France. They found 21 patients with phaeohyphomycosis out of 46 patients with deep fungal infections produced by other causative agents, with a median age of 56.5 years [
7,
8] and a male-to-female ratio of 2.54:1. A mean time of 28 months was observed between transplantation and the diagnosis of deep mycosis. The most frequent type of transplant in these patients was renal transplantation (67%), followed by heart transplantation (11%). Some post-transplant drugs were prednisone, calcineurin inhibitors, mycophenolate mofetil, cyclosporine, azathioprine, and everolimus [
4,
8].
He Y. et al. conducted an epidemiological description of 174 cases of phaeohyphomycosis reported in China from 1987 to 2021. Furthermore,
Phialophora americana has been reported so far in a 28-year-old man from China [
9].
Corynespora cassiicola has been underreported, with cases in Colombia, Africa, and Asia [
10]. The highest number of reported cases in China has been observed between latitudes 18° N and 53° N, covering Taiwan, Zhejiang, Jiangsu, and Guangdong province [
5].
Bonifaz A. et al. reported a series of 160 cases of mycosis and deep pseudomycosis of the foot studied for 20 years in Mexico, finding only three cases (1.8%) of phaeohyphomycosis. All cases involved male subjects with a history of trauma who presented cysts with hyphae and black yeasts as the parasitic forms. The causal agents were
Pseudochaetospheronema martinelli,
E. spinifera, and
E. oligosperma [
11].
Of all phaeohyphomycosis cases caused by
Cladophialophora spp.,
C. bantiana has been reported in India (39 cases, 32.5%), the United States (29 cases, 24.2%, of which five subjects were African, five Caucasian, and one East Asian), followed by Brazil (5 cases, 4.2%), Canada, France, Spain, South Africa, and the United Kingdom (4 cases, 3.3%), and Italy, Thailand, and Turkey (2.5%). Sporadic cases were reported in 15 other countries, such as Chile, Cuba, Venezuela (1.7%), Australia, Belgium, Colombia, China, Congo, Ireland, Japan, Nigeria, Singapore, Slovakia, Switzerland, and Vietnam (0.8%) [
12].
In India, cases of
Alternaria alstromeriae,
Epicoccum tritici, and
Phialemonium obovatum have been reported [
13].
3. Causative Agents
Currently, about 70 genera and 150 species of fungi have been reported to cause phaeohyphomycosis, among which
Exophiala (
Wangiella),
Fonsecaea,
Curvularia, and
Lomentospora produce most cases [
14]. These organisms belong to the orders of the Capnodiales, Dothideales, Pleosporales, Botryosphaeriales, Coniochaetales, Chaetothyriales, Microascales, Sordariales, Calosphaeriales, and Ophiostomatals [
7].
The causative agents are filamentous saprophytic fungi found in the environment, mainly in the soil and plants. They all produce melanic pigment, a vital virulence factor inhibiting phagocytosis and binding to hydrolytic enzymes, preventing its action on membranes, which may explain their pathogenic potential in immunocompetent patients [
4]. They also have protease enzymes, peptidases, hyaluronidases, and chitin synthetase that confer resistance mechanisms. Some are limited to the stratum corneum and subcutaneous cellular tissue, while others are neurotropic or disseminated [
7].
Numerous members of the genus
Exophiala have been associated with this deep mycosis, which are members of the order
Chaetothyriales, family
Herpotrichiellaceae, black yeast-like fungi. Specifically,
E. dermatitidis is the most isolated species worldwide. It colonizes the respiratory tract of 19% of patients with cystic fibrosis and presents most frequently as cerebral and disseminated clinical forms [
15,
16,
17].
E. jeanselmei (3.7%)
, E. oligosperma (18.6%), and
E. xenobiotica (19.7%) are the most frequent species reported in Japan. Other causative species are
E. lecaniicorni (6.9%) and
Exophiala spp. [
18,
19]. This genus is saprophytic, isolated from oligotrophic, humid, and hot environments [
15,
20].
Galezowski A, et al. reported 21 cases (46%) of phaeohyphomycosis in 46 patients with deep skin mycoses, of which 16 (35%) were alternariosis (
A alternata) [
8].
Alternaria is a saprophytic fungus of the soil, air, or plants, considered one of the frequent agents of phaeohyphomycosis. The species that mostly affect humans are
A. alternata, A. tennuissima, and
A. chartarum. A. infectoria is an emerging species of this disease [
21,
22].
C. bantiana is a highly neurotropic dematiaceous fungus in the order
Chaetothyriales and the most common cause of human cerebral phaeohyphomycosis [
12,
23].
N dimidiatum is a dematiaceous saprophyte fungus that can be isolated from soil and plants that secretes keratinase, which facilitates skin invasion [
24].
The genus
Phialemoniopsis (
Sordariomycetes) currently includes six species, four of which have been isolated from human fluids, skin, or nails.
Phialemoniopsis limonesiae sp. nov. has also been noted as a phaeohyphomycosis agent [
25].
There are other phaeohyphomycosis agents rarely reported in the literature. The study conducted by Yu Y. et al. in Taiwan reported three cases of deep cutaneous mycosis caused by
Pleosporales:
Nigrograna mackinnonii,
Medicopsis romeroi, and
Parathyridaria percutanea. These agents have been registered in Latin America, the Middle East, and Western Europe [
2].
Pallidocercospora crystallina is a rare non-sporulating causative agent of phaeohyphomycosis, with no more than five cases reported in the literature. It belongs to
Pseudocercospora (Mycosphaerellaceae in its teleomorphic state), a large group of phytopathogenic fungi that usually causes stippling in the leaves and fruits [
14]. Noolêto et al. reported a case of phaeohyphomycosis by
Peyronellae spp. in a 63-year-old male with type 2 diabetes mellitus [
26].
P. Martinelli was noted in a series of Mexican cases [
11].
C. cassiicola has only been reported in less than 10 cases [
10]. Sharma et al. identified two new agents isolated for the first time in the world (
A. alstromeriae,
E. tritici) and another one isolated for the first time in India (
P. obovatum) [
13].
P. verrucosa can be isolated from soil, wood, and vegetation and has been reported in cases of subcutaneous phaeohyphomycosis [
27].
In China, He Y. et al. reported several typical causative agents in a series of phaeohyphomycosis cases studied over 30 years. The most frequent agent was
Exophiala spp. According to the type of infection, 365 cases associated with the central nervous system were caused by
E. dermatitidis and 18% by
C. bantiana. In disseminated forms,
E. dermatitidis was observed in 27% of cases and
E spinifera in 18%. As for pulmonary forms, 2% were caused by
Chaetomium spp., and 25% by
Exophiala spp. Twenty-nine percent of subcutaneous lesions were caused by
Exophiala spp., 7% by
Cladosporium spp., 6% by
Phialophora spp., 6% by
Veronaea spp., 5% by
Alternaria spp. and
Arthrinium spp., and 4.5% by
Corynespora spp. In addition, they reported rare cases, primarily soft tissue infections, caused by
C. sphaerospermum,
Knufia epidermidis,
E. asiatica,
C. cassiicola, Ochroconis tshawytschae,
E. hongkongensis,
P. hongkongensis,
Hongkongmyces pedis,
Bipolaris oryzae,
P. crystallina,
Pleurostoma hongkongense [
5].
4. Risk Factors and Pathogenesis
The history of fungal implantation by trauma in exposed areas is relevant as it is reported in most phaeohyphomycosis cases, where a primary nodular lesion is formed, subsequently growing into a subcutaneous abscess [
18]. This inoculation process is observed mainly in farmers, agriculture, and livestock workers. Other at-risk populations are florists or gardeners, greengrocers, and coal miners, especially in cases of skin infection by
C. bantiana [
12]. Also, studies have found cases of
E. dermatiditis in subjects living in train stations and sleeping on train tracks [
28].
Climate is also a factor, with more cases reported in tropical or subtropical areas [
5].
About 78% of cases involve patients with some immunosuppression. Those reported include solid organ transplant recipients, type 2 diabetes mellitus, rheumatoid arthritis, cancer, chronic use of catheters, chronic sinusitis, chronic use of systemic corticosteroids and other immunosuppressive agents such as chemotherapy, neutropenia after treatment with cytostatics, liver cirrhosis, and human immunodeficiency syndrome (AIDS) [
2,
8,
18,
21]. The disease rarely occurs in healthy patients [
2]. Guo Y. et al. described the homozygous mutation in the protein of the caspase recruiting domain family (CARD9) as a possible risk factor, as it encodes signaling proteins that are positioned in the lower middle part of many C-type lectin receptors. This gene plays a vital role in inducing the response to IL-17 in local or systemic fungal infections [
14,
27,
29] caused by agents such as
E. dermatitidis,
C. cassiicola,
P. americana,
P. verrucosa [
9,
10,
19]. The latter is because this protein shows a selective alteration of proinflammatory cytokines and production of chemokines in the activation of FN-Bκ and the TH22 and TH17 responses. On the other hand, phagocytosis and reactive oxygen species are not altered [
30].
TH17 cell deficiency has also been associated with a predisposition to this mycosis, as Wan X et al. demonstrated in the four reported cases of
P. verrucosa [
27]. Another risk factor observed mainly in fungal cyst formation is handling materials contaminated with these agents, like the cases involving
Neoscytallidium [
4].
Drummond R.A. et al. evaluated a patient with progressive, refractory
C. cassiicola phaeohyphomycosis. They found that he carried biallelic deleterious mutations in CLEC7A encoding the CARD9-coupled, β-glucan-binding receptor, Dectin-1, which failed to produce TNF-α and IL-1β in response to fungi. Thus, they developed a mouse model of this infection to confirm the cellular and molecular requirements for immunity against
C. cassiicola and proved that deficiency of either Dectin-1 or CARD9 was associated with more severe fungal disease [
31].
During the COVID-19 pandemic, the number of phaeohyphomycosis cases increased worldwide because of the infection as such and the use of immunosuppressive agents like steroids [
5].
For cases caused by
E dermatitidis, studies have been conducted to identify the triggering factors of the disease. This fungus is assumed to originate in tropical forests, like the beds of wild fruits and berries. One possible route of infection is dispersal through the feces of frugivorous birds and bats. It has been isolated from dishwashers, steam baths, and sauna facilities, environments characterized by high temperatures, humidity, and pH variations, and railroad ties at a rate of 13%. Due to the high isolation rate in dishwasher wastewater samples, it is thought that the dishwasher is a possible transmission route between the environment and the human host, primarily through aerosol inhalation [
15,
32].
5. Diagnosis
Infections are classified into [
4,
5,
7]:
Superficial (the most frequent): tinea nigra, mucous membranes, keratomycosis, and fungal melanonychia;
Subcutaneous: Fungal cyst, papules, warty plaques;
Deep: primary local infection: endophthalmitis, invasive rhinosinusitis, miscellaneous infections;
Systemic: Deep phaeohyphomycosis, central nervous system involvement (primary or secondary; in the form of abscesses, meningitis, encephalitis, myelitis, meningoencephalitis) and pulmonary (primary or secondary, presenting as pneumonia, asymptomatic solitary pulmonary nodule, endobronchial lesions);
Disseminated.
Phaeohyphomycosis goes unnoticed in the early stages of infection. It occurs more frequently in extremities, with one or more lesions in the same affected site (
Figure 1, lesions close to the knee). The lesions are polymorphous, with nodules, papules, abscesses, cysts, ulcerated or necrotic lesions, blisters, lesions like ecthyma gangrenosum, warty papules or nodules similar to multiple pyogenic granulomas, plaques resembling dermatophytosis, erythema plaques, desquamation and eczema that ulcerates and erodes, and lesions similar to onychomycosis [
8,
33].
On the other hand, the fungal cyst appears weeks to months after trauma. The lesions present as an asymptomatic and solitary subcutaneous nodule. Less frequently, they appear as plaques or multiple erythematous nodules. The most frequent locations include feet, fingers and toes, knees, elbows, legs, and forearms [
7].
The disseminated nodular subcutaneous form is less common than the previous one. It is mainly seen on the extremities as papules, then single or multiple warty-looking confluent nodules [
7].
The presentation may be different depending on the causative species.
Infections caused by
Pleorosporales display lesions in forearms, consisting of erythematous plaques with pustules, hyperpigmentation, with or without nodules, and erythematous patches [
2].
As for the species of the genus
Exophiala, clinical lesions present as erythematous plaques with superficial pustules, cysts, and nodules on hands and forearms [
18].
The only case related to
P. crystallina, which occurred in a 35-year-old woman, reported the presence of a dark red plaque on the left cheek, with 16 years of evolution [
14].
Another unique case caused by
Peyronellae spp. involved a 64-year-old Brazilian man with type 2 diabetes mellitus, with nodules on the left ankle [
26].
In a case reported by Bonifaz A. et al.,
P. martinelli was observed as a single, painless cyst in a male with a history of trauma [
11].
Neoscytallidium infections may present as abscesses, endophthalmitis, sinusitis, empyema, osteomyelitis, central nervous system involvement, or disseminated infection in immunocompromised subjects. Also, they will cause superficial infection of the skin and nails, being the most common sites of invasion: the soles of the feet, the interdigital membranes, and the toenails [
24].
C. cassiicola infection presents as a warty ulcer with extensive necrosis and crusting that can affect the face, as reported by Arango-Franco C et al. [
10].
Alternaria spp. infection includes single or multiple infiltrated lesions, erythematous-violet plaques, or nodules, with or without central serohematic crusts that appear mainly in the lower extremities [
21]. Sporotrichoid patterns have been reported in some cases.
A. alstromeriae has been reported in the fourth and fifth interdigital space of the right foot, with erythema, skin maceration, and fissures [
13].
A single case reporting
P. americana describes a gray-colored warty plaque with an ulcerated surface and facial involvement [
9].
P. verrucosa presents as red plaques and facial nodules [
29].
E. tritici was reported as erythematous-squamous plaques in the lower extremities, while
P obovatum has been reported in the left foot, with scaly lesions, hyperkeratosis, and erythema [
13].
P. verrucosa affects the face with red, ulcerated plaques and nodules, scars, erosions, and crusting [
27].
P. limonesiae grows an indurated painful violet plaque crowned by papules and nodules, as well as ulcers with seropurulent secretion and pimples [
25].
In China, 174 cases of phaeohyphomycosis were reported over 30 years. Men were the most affected (61.5%), showing a male-to-female ratio of 1.6:1 with a median age of 48 (2–89 years). As for the clinical presentation, most were subcutaneous infections (85 cases), followed by keratomycosis and superficial infections (26 and 23 cases, respectively, observing 11 cases of central nervous system involvement and disseminated forms each; 10 cases of deep mycosis and only 10 cases of the pulmonary form). The outer diameter of most lesions measured less than 15 cm. Invasions were observed more frequently in severe infections in fat tissue, muscle, mucosa, and bone. Plaques, nodules, warts, and abscesses were the most frequent morphological types. The most affected sites for single lesions were the upper and lower extremities. The lower and upper extremities were more affected when more than two injuries were involved. Also, 23% of patients showed lesions in three body parts [
5].
Mycological Samples
The 20% KOH staining test of fresh material is used for diagnosis. It shows brown or dark, thick, long, and short septate hyphae, as well as pseudohyphae and blastoconidium, depending on the species [
7,
34] (
Figure 2).
The samples are obtained differently for each clinical presentation [
7]:
Superficial: a sample of corneal scraping is obtained for keratitis. For skin or nails, samples of superficial scraping, including stratum corneum scales, are used;
Subcutaneous: a specimen of the affected tissue, or the secretion of the lesion, is obtained;
Deep: the sample is obtained from the damaged tissue or the lesion exudate;
Systemic: A lung or bronchial tissue, sputum, or bronchoalveolar lavage sample is obtained. For central nervous system affection, the sample is obtained from cerebrospinal fluid;
Disseminated: The causative agent is isolated from blood samples or other tissue in two contiguous sites.
6. Histopathology
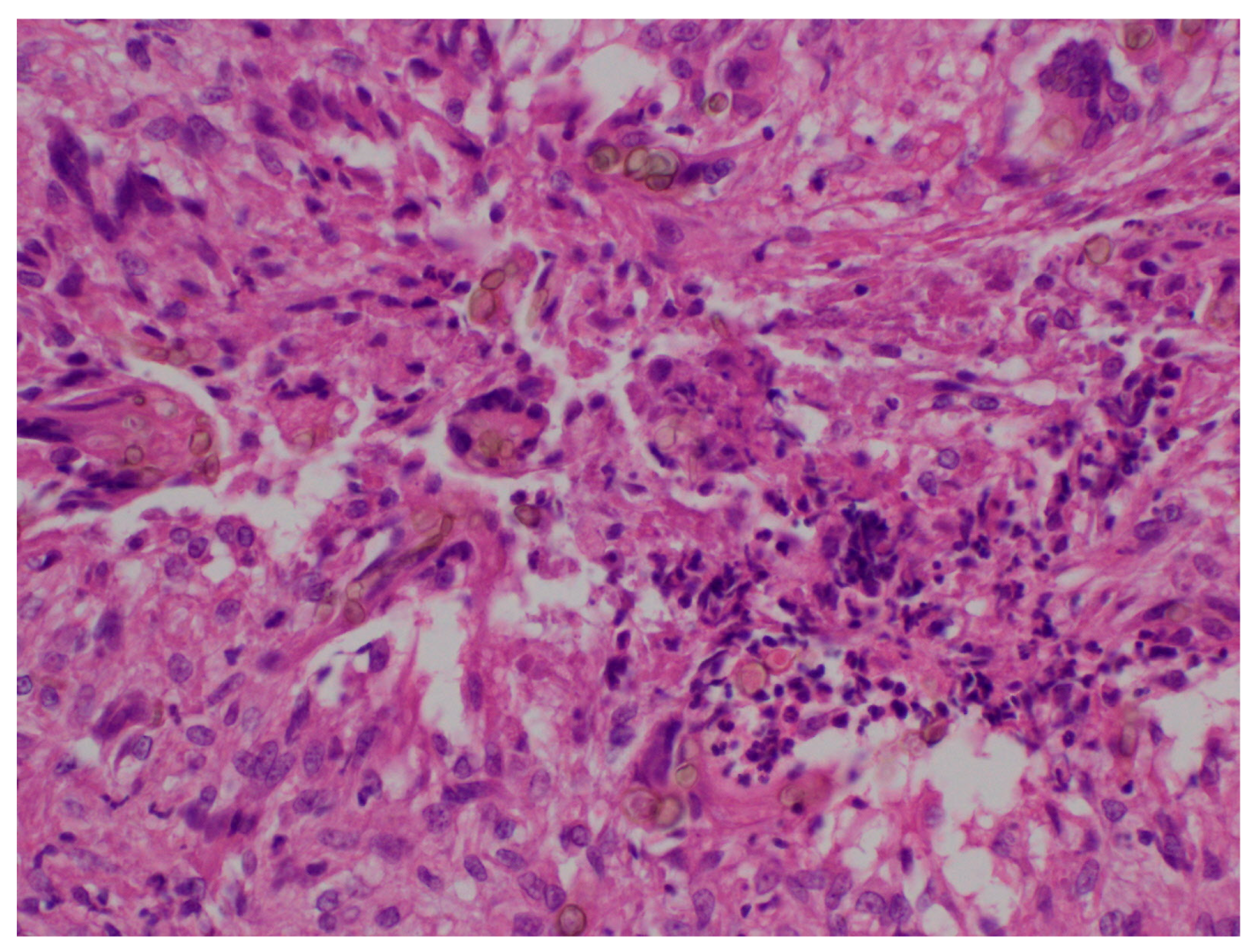
Granulomas with multinucleated giant cells, suppurative granulomas, and dermal fibrosis may be observed [
8]. Hyphae and pigmented spores may also be observed, especially in the center of the lesion [
18] (
Figure 3).
In cases of rare black fungi, such as
P. crystallina and
C. cassiicola, pseudoepitheliomatous hyperplasia with hyperkeratosis and microabscesses in the epidermis can be observed. A mixed infiltrate with neutrophils, macrophages, lymphocytes, and plasma cells can be seen in the dermis. Also, pigmented septate hyphae and ovoid yeasts can be observed from the stratum corneum to the deep dermis, surrounded by necrotic tissue and multinucleated giant cells [
14].
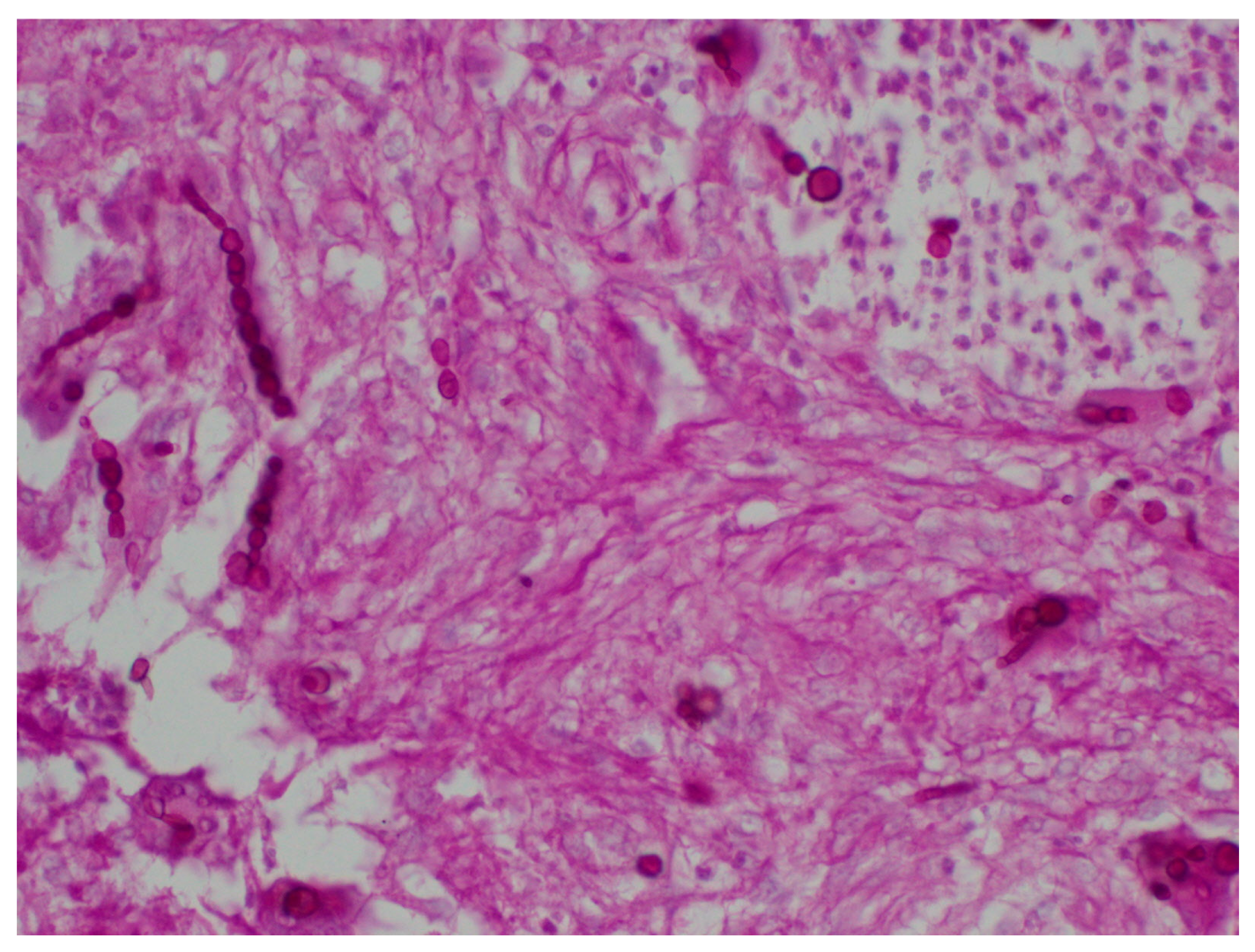
The special stains used to identify fungal structures are periodic acid-Schiff (PAS), Grocott Methenamine Silver stain (the polysaccharide of the fungal cell wall is detected by the reduction of Grocott’s alkaline hexamine-silver solution. It forms precipitates of silver ions turning black the cell wall of the fungi, which is known as the argentaffin reaction) and Fontana-Masson (used to identify dematiaceous fungi from hyaline hyphomycetes as it identifies argentaffin granules and melanin pigment) to observed filaments and yeasts [
8] (
Figure 4 and
Figure 5).
Systemic involvement should be suspected when angioinvasion is observed through these special staining techniques, especially Methenamine Silver [
5].
7. Cultures
Commonly used culture media are employed, such as Sabouraud’s dextrose agar without cycloheximide at 25–28 °C. The cultures can fully grow in 20–30 days. Generally, phaeohyphomycosis agents liquefy gelatin, coagulate milk, and digest starch [
7].
In cases caused by Pleosporales, the culture presents dark blackish and gray molds [
2]. Species of the genus
Exophiala, such as
E. jeanselmei,
E. spinifera, E. dermatitidis, and
E. oligosperma, present velvety gray colonies with a black olive-colored reverse. They first form yeast-like colonies and later filamentous ones [
18].
E. dermatitidis grows with a restricted, smooth, waxy, dark appearance, often with dark pigments exuded in the culture medium [
15].
P. crystallina displays as a black colony resembling a “volcanic island” [
14].
Peyronellaea spp. grows as a filamentous fungus that forms hyaline colonies at first, with subsequent black coloration [
26].
A. alternata grows black, gray, or dark green colonies with woolly surfaces, which can cover the entire culture medium in 5 to 10 days [
4,
21].
N. dimidiatum shows a dark grey villous mold [
24].
C. bantiana develops in seven days folded, velvety, dark or gray-greenish colonies, and a black reverse [
4,
35].
C. cassiicola shows numerous velvety gray colonies with short aerial mycelia [
10].
The colonies of
P. americana are brown to olive green, with abundant short, gray aerial hyphae [
9], while those of
P. verrucosa are black and villous [
27]. The colonies of
A. alstromeriae are greyish black, cottony, and fluffy with a scarce inverse black aerial mycelium [
13].
E. tritici shows white, brown to pink, pigmented, dense, circular, velvety colonies with complete edges [
13].
The colonies of
P. obovatum are white at first and then become pale ochraceous, moisty but not viscous and slightly floccose [
13].
Phialemoniopsis, especially
P. limonesiae, can grow on Oatmeal Agar (OA), 2% Potato Dextrose Agar (PDA), and 2% Malt Extract Agar (MEA). In PDA, it reaches 55 mm in diameter, is flat, floccose, olive-gray in the center, white on the periphery, and colorless on the reverse. In OA, it reaches 50 mm in diameter, is flat, dusty, with a hazel-colored center, white periphery, and colorless reverse. In MEA, it measures 52 mm in diameter, is flat, radially folded, floccose, dirty white, and saffron colored in the reverse [
25].
8. Microscopic Examination
It is used to see the forms of reproduction of the fungi that grew in the culture media mentioned above under the light microscope, using stains such as Parker blue ink, methylene blue, and lactophenol blue [
18].
In general, the genus
Exophiala form phialides collarettes, sympodial conidiophores, or conidial chains in various proportions.
E. jeanselmei has brown septate hyphae filaments and branched conidiophores, and
E. oligosperm displays conidiogenic cells in the form of rockets and mature coenocytic thallus [
18].
E. jeanselmei,
E. spinifera,
E dermatitidis, and
E. oligosperma reproduce by yeasts, phialides and annellides, and their conidia can form chains.
E. spinifera produces very elongated conidiophores.
E. jeanselmei forms septate and branched hyphae with terminal or lateral conidiophores; cylindrical or elongated conidiogenic cells with thin annellides that generate subglobose and hyaline aneloconidia.
E. spinifera has septate, brown, branched hyphae, the conidiophores are lateral and darker, the conidiogenic cells are annellides, and unicellular, hyaline or subhyaline, subglobose or ellipsoidal annelloconidia.
E.
dermatitidis presents annellides and phialides, and the conidia are unicellular, globose, or subglobose, hyaline, or brown [
4].
Microscopically,
N. dimidiatum has dark brown septate hyphae and ellipsoidal or oval-shaped chains of unicellular or bicellular arthroconidia [
24].
C. bantiana has a long cladosporium-like reproduction with elongated brown and septate conidiophores, and single-celled conidia in long chains; the spores are pigmented, spherical or elliptical, and measure 2 μm to 2.5 μm by 4 μm to 7 μm [
4,
35].
P. crystallina is observed as septate hyphae without sporulation [
14].
A. alternata presents simple or branched conidiophores, which show a small pore at its distal end, ellipsoidal conidia with more than eight transverse or oblique septa, which may be spread or forming chains [
21].
A. alstromeriae has simple, geniculate conidiophores measuring 15–35 × 3.0–4.0 μm. Their conidia are brown when mature, solitary or in short 2–4 chains, broad, ovoid to ellipsoidal measuring 20–30 × 8–12 μm with 3–6 transverse septa and a longiseptum in 1–2 of the wider transverse segments [
13].
P. americana presents hyphae with membranes and clusters of spherical conidia aggregated on top of the hyphae [
9].
P. verrucosa presents septate hyphae with phialides in clusters [
27].
E. tritici has septated hyaline hyphae, dispersed or aggregate black sporodochia, hemispherical to spherical stroma, composed of brown pseudoparenchymatous cells and pale brown ampulliform conidiophores, measuring 6.4–14.4 × 3.0–6.5 μm, arranged as a palisade layer. Conidia are initially yellowish-brown, then become dark brown, irregularly multiseptated, globose to subglobose, vase-shaped with a truncated base, densely warty, measuring 19.2–25.6 × 17.6–23.7 μm [
13].
The hyphae of
P. obovatum are short peduncles, 0.6–2 μm wide, with phialide-like pins measuring 1–9 × 0.5–1 μm. The conidia are hyaline, obovate with an apiculate and meticulously truncated base, with smooth walls of 3.5–6 × 1.2–1.7 μm and oval-cylindrical chlamydospores of 6.0–8.0 × 2.0–4.0 μm [
13].
Phialemoniopsis has phialides and adelophialides with collarettes and develops conidiomata similar to sporodochium or pycnidium.
P. limonesiae has a mycelium consisting of branched, septate, hyaline, smooth-walled, thin-walled hyphae, mostly 1–2 [
4,
6] μm wide having 1–2 levels with 2–3 phialides per node, up to 55 μm long, 1.5–2.5 μm wide at the hyaline, smooth-walled base, with cell walls generally thicker than those of vegetative hyphae. Conidiogenic monophialidic, adelophialidic or polyphialidic cells, terminal, lateral, straight to slightly flexuous, cylindrical, 12–33 μm long, 1.5–2.5 μm wide at the base, with evident collarette and characteristic periclinal thickening at conidiogenic site, hyaline, thick and smooth-walled adelophialides of 4.2–20.2 × 1.1–2.2 μm, polyphialides with up to three commonly present conidiogenic loci. Conidia formed in small globose pins at the apex of the phialides, ellipsoidal to cylindrical with rounded, aseptate, hyaline edges [
25].
9. Molecular Biology
Molecular biology has enhanced the identification of the causative agents of this mycosis through DNA extraction and subsequent sequencing [
2]. In most cases, this is achieved by amplifying the ITS and D regions of the 28S rRNA large subunit of the genomic DNA from the tissue [
5,
7,
15].
Yu Y. et al. determined the
Pleorosporales species of their three cases of deep mycoses with DNA extraction from the mycelia, using Smart Lab Assist
TM (TANBead, Taoyuan, Taiwan) automatic DNA extraction system. The internal transcribed spacer regions of rDNA (ITS) and the D1/D2 region of the rRNA 28S gene were amplified with the following pairs of primers for the three cases: ITS1, 50-TCCGTAGGTGAACCTGCGG-30; ITS4, 50-TCCTCCGCTTATTGATATGC-30; 5NL1, 50-GCATAT-CAATAAGCGGAGGAAAAG-30; NL4, 50-GGTCCGTGTTTCAAGACGG-30. These sequences were uploaded to the National Center for Biotechnology and Information and a BLAST analysis was performed comparing the data deposited in GenBank [
2].
Fungal DNA from
P. crystallina was extracted using an Ezup Fungi DNA Extraction kit (Sangon Biotech, Shanghai, China). DNA sequences of actin regions (GenBank access number MF135482), elongation factor (EF-1a, MF135483), 18S rRNA (MF135484), and ITS1-5.8S-ITS2 (internal transcribed spacer, ITS, MF135485) were amplified by PCR. The total genomic DNA of
P. crystallina was extracted using the Rapid Fungi Genomic DNA Isolation Kit following the manufacturer’s recommendations (Sangon Biotech, Shanghai, China). Complete genome sequencing was performed with an Illumina HiSeq (Illumina Inc., San Diego, CA, USA) [
14].
Peyronellaea spp. can be identified using the ITS1-5.8 rDNA-ITS2 primers [
26].
C. cassiicola employs the primers of the D1 and D2 domain; as well as the 1 ITS region of the 28S ribosomal DNA subunit [
10].
11. Treatment
For fungal cyst-type cutaneous cases, the most recommended treatment of choice is surgical excision [
7].
The use of systemic antifungals has shown good results in all clinical forms, except for the fungal cyst, mainly in the deep, systemic, and disseminated forms. It is divided into targeted treatment, which is administered when the causative agent has already been identified; and empirical treatment, when only clinical suspicion is available, without identification of the species and its antifungal susceptibility [
7,
18].
The empirical treatment of first choice is itraconazole, due to its broad antifungal spectrum, followed by voriconazole (especially for central nervous system infections) and posaconazole (which is used as rescue therapy when other antifungals fail) [
7,
18]. In cases where patients are on systemic steroid therapy, it is suggested to provide antifungal treatment together with the steroid, reducing doses until discontinuation of treatment and use steroid-sparing agents like azathioprine. The above is recommended to avoid the exacerbation of the underlying disease. In cases where the immunosuppressive treatment cannot be discontinued, it is recommended to bring down the therapy to the minimum effective dose [
7,
21].
A series of cases studied over the course of 30 years in China reported the use of antifungals for each type of clinical presentation. The most used drug overall was itraconazole (39%), followed by voriconazole (9%). In central nervous system infections, voriconazole was the most employed (9%); while in disseminated lesions, itraconazole and voriconazole were utilized in the same proportion (27%). In the pulmonary forms, itraconazole was the most used (50%), as well as in the deep forms (30%), subcutaneous (57%), keratitis (27%), and superficial forms (13%). In addition, antifungal susceptibility was determined, finding a high resistance (95%) to fluconazole. There was good susceptibility to terbinafine (89% of cases), voriconazole (86%), and itraconazole (77%). Of these treatments, the one with the greatest clinical efficacy was itraconazole, with a cure rate of 81% [
5].
The dose and length vary for both empirical and directed treatments, for example: for Pleorosporales cases (
N. mackinnonii,
M. romeroi, P. percutanea), 200 mg daily of oral itraconazole for 2–10 months has shown complete recovery of the disease [
2].
Some reported cases of infections caused by the genus
Exophiala were treated empirically with fosravuconazole and terbinafine for the species
E.
jeanselmei and
E. oligosperma, respectively. However, based on the reviewed studies, itraconazole is the first choice at doses ranging from 50–400 mg over a period of 5–60 weeks, with partial or total responses. Terbinafine is another treatment that has shown efficacy with a dose of 125 mg per day for 24 weeks. It has been studied that this genus has a minimum inhibitory concentration of 0.512–1 μg/mL, specifically with
E. jeanselmei. Cases treated with surgery and antifungals have been reported, with variable results after total resection. The determination of serum levels of β-D-glucan has been suggested to monitor response to treatment [
18,
36]. Voriconazole, itraconazole, and posaconazole have been shown to be active against
E. dermatitidis [
15].
N. dimidiatum may not respond to treatment with voriconazole or itraconazole. For these patients, amphotericin B has shown good response [
24].
In the case caused by
P. crystallina, reported by Guo Y. et al., treatment was given with itraconazole 200 mg every 12 h together with topical oxiconazole twice daily for 5 months; followed by terbinafine plus itraconazole for 4 months. However, the disease persisted with skin lesions, thus requiring a surgical excision and another cycle of itraconazole and terbinafine for 2 more months. After 7 months of treatment, there was no recurrence of the disease [
14].
Peyronellaea spp. has been treated with itraconazole 200 mg daily for 4 months with regression of the disease [
26].
C. cassiicola has been treated for systemic cases and skin and soft tissue affection with liposomal amphotericin B (5 mg/kg/daily) and oral posaconazole (20 mg/kg/daily) for one month, observing clinical improvement and subsequently offering maintenance therapy with oral posaconazole plus terbinafine (125 mg/daily) and surgical debridement of the necrotic area [
10].
Alternaria spp. has a good response to itraconazole. In some species such as
A. infectoria, treatment with this antifungal has been given at doses of 200 mg/daily for 6 months. In the cases reported by Kieselová K, et al., some nodules were treated with cryosurgery, with good response to treatment [
21].
The treatments reported for cases caused by
C. bantiana, mainly in cerebrospinal infections, are amphotericin B (0.7 to 1.0 mg/kg/daily), ketaconazole always in combination with flucytosine (150 mg/kg/daily), fluconazole (200 to 400 mg/daily), itraconazole (200 to 600 mg/daily); voriconazole (dose of 4 mg/kg/daily and subsequently two loading doses of 6 mg/kg/daily and subsequently 4 mg/kg/daily), posaconazole, and caspofungin (0.7 mg/kg/daily) have been administered in several relatively recent cases [
12,
35].
Phialemoniopsis, especially
P. limonesiae sp. nov., has the following antifungal susceptibility (MIC): flucytosine > 64; posaconazole 0.06; voriconazole 0.12; itraconazole 0.06; fluconazole 16; amphotericin B 2 [
25].
Specifically, in patients with CARD9 deficiency and phaeohyphomycosis, the response to antifungals is poor, resulting in recalcitrant disease. Therefore, the duration and choice of treatment should be adjusted depending on the clinical presentation and the patient’s response throughout their lives. Treatment with posaconazole oral suspension (800 mg/day: 20 mL/day) shows a dramatic improvement in the lesions after one month of treatment. However, some cases described that the lesions did not entirely subside and could potentially relapse upon drug withdrawal. A very useful tool for following treatment response in these patients is the decrease of serum (1–3)-β-D-glucan antigen (BG) assays, but there is still no reference point to determine a good response [
37].
13. Conclusions
This work aims to review phaeohyphomycosis, including newly identified agents and their clinical and morphological features. Moreover, we highlight the role of the CARD 9 mutation in the pathophysiology of the disease and response to treatment. As for refractory cases, the use of posaconazole as a therapeutic option is emphasized, as well as the use of (1–3)-β-D-glucan for assessing the severity and monitoring patients. It is important to remember that polymorphic lesions in the lower extremities in the context of a tropical region should make us suspect the disease. In the first instance, direct examination of the lesions and histopathological study are important to initiate antifungal treatment, while waiting for the culture result to offer targeted therapy. Cryosurgery or complete surgical excision is ideal in single lesions, with good results and low recurrence. In endemic areas, it is important to wear footwear to avoid skin trauma.
It is worth reviewing this disease, especially in tropical countries, because this infection increases the cost of health resources and causes increased morbidity.