Abstract
Xylella fastidiosa (Xf) is classified as a quarantine pest due to its consequences on economically significant crops. Its main form of transmission in Europe is through the insect Philaenus spumarius. Due to climate change, the populations of insect vectors have become more extensive, resulting in the dissemination of the bacteria over longer periods, but the destruction of these insects raises issues due to their role in nature. Upon infection, Xf causes the occlusion of xylem vessels via bacterial aggregates and tylosis production by the plant as a response to infection. Although symptomatic manifestations of Xf are often linked to water stress, a variety of plant species have been found to carry the pathogen without symptoms, making it all too easy to evade detection when relying on visual inspections. Beyond water stress, other conditions (individual plant resistance/tolerance, bacterial concentrations, transpiration rates, and interactions between subspecies) may be implicated in symptom development. A thorough understanding of how this disease develops, especially its capacity to spread from the initial focus and establish a systemic infection, is imperative. This review focuses on the Xf infection process, the development of symptoms, its spread within Portugal, and the actions that have been taken to counter it.
1. Introduction
Xylella fastidiosa (Xf) is a bacillary (rod-shaped) bacterium, about 0.25 to 0.35 μm wide and 0.9 to 3.5 μm long, devoid of flagella [1]. It is an aerobic [2] Gram-negative [3] bacterium that multiplies in the xylem of various host plant species [4]. Since 1981, it has been considered a quarantine pest, on the EPPO A list, for affecting economically important agricultural crops as well as ornamental plants [5]. The goal of this strategy is to prevent the introduction of new subspecies in countries where this bacterium already exists and prevent its introduction in countries where it has not yet been established [6]. Xf belongs to the class Gammaproteobacteria, order Lysobacterales, and family Lysobacteraceae. The genus Xylella contains two species: X. fastidiosa and X. taiwanensis [7]. According to serological and phylogenetic studies, its strains are divided into six subspecies: X. fastidiosa subsp. fastidiosa [8], X. fastidiosa subsp. multiplex [8], X. fastidiosa subsp. pauca [8], X. fastidiosa subsp. sandyi [9], and X. fastidiosa subsp. Morus [10], of which only the first two have been validly published in the List of Prokaryote Names with Standing in Nomenclature. A further subspecies, X. fastidiosa subsp. tashke, has also been reported once [11], but to date, its genotype has not been detected again [12].
Due to their ability to infect a diverse range of hosts, including economically significant crops, like grapevine, peach, almond, citrus, plum, and coffee, these organisms pose a threat to plant health and agricultural productivity [2]. Based on spatially explicit economic models, Xf presence could, in the next 50 years, have a direct impact on the profitability of olive cultivation, potentially resulting in a loss of revenue of 1.5 to 5.9 thousand million EUR. Additionally, there may be severe indirect effects on cultural heritage and landscape due to this disease [5].
It is imperative to understand the incidence of this bacteria and the corresponding risk factors, which are, to date, poorly understood. It is known that plant bacteria are spread via anthropogenic sources, agricultural tools, insect vectors, and wastewaters, but the way their dissemination reaches agricultural crops is not properly understood. Thus, this paper aims to provide an updated review of the available literature concerning this pathogen and clarify the environmental flow of this bacteria.
2. Distribution in Europe
Before 2013, there were only sporadic reports of the detection of X. fastidiosa in Europe, which were not investigated and did not raise major concerns [13]. The first outbreak of this plant pathogen in the European Union was found in 2013, in olive trees near the town of Gallipoli, in the province of Lecce, in the Puglia region, Italy [14,15]. Analysis of the genetics of the subspecies found in this region indicates a close relationship to the subspecies of Xf present in Costa Rica. This finding suggests that the introduction of the bacteria in the Mediterranean area was likely due to the import of ornamental plants from that region [16]. Subsequently, the bacteria were detected in France (the first outbreak was in Corsica in 2015, Provence in 2019, and Occitania in 2020) on Lavandula spp., on Myrtus communis L., on Salvia rosmarinus Spenn., and on Spartium junceum L. [17]. In 2013, different European countries reported the presence of infected coffee plants from Latin America (Mexico, Ecuador, Costa Rica, and Honduras) [7], suggesting that the global distribution of this agent continued to increase due to the movement of commodities and plant materials.
In Spain, the first outbreak occurred in the Balearic Islands in 2016 [18,19]. Three subspecies of the bacteria (multiplex, fastidiosa, and pauca) infected more than 20 plant species, including vine (Vitis spp.), almond (Prunus spp.), olive (Olea europaea L.), and fig (Ficus spp.) [20].
In December 2020, over 600 olive trees in the Balearic archipelago were found to be infected. By 2021, the total affected area had expanded to 2292 hectares, resulting in the destruction of more than 100,000 almond trees. In 2016, a single olive tree in Madrid tested positive for the multiplex subspecies of Xf. In the same year, an ornamental plant nursery in Almeria (Andalusia) detected three specimens of Polygala myrtifolia L., although the bacterium has since been considered eradicated in this region [16].
3. Current Distribution in Portugal
The introduction of X. fastidiosa subsp. multiplex in Portugal occurred in 2019 in asymptomatic plants of Lavandula dentata L. [21]. Currently, in Portugal, there are 18 Xf demarcated zones (DZs), as shown in Table 1, and 1 suppressed DZ in Tavira, in the Region of Algarve, where the disease was eradicated [22].

Table 1.
Demarcated zones (DZs) in Portugal (adapted from [22]).
These data show that Portugal is highly vulnerable to the emergence of the Xf, owing to its Mediterranean climate, which is marked by mild temperatures, frequent rainfall, and high humidity during winter, coupled with hot, dry summers. These conditions are ideal for the growth of the bacterium. According to Godefroid et al., many regions of Western Europe and the Mediterranean basin are highly suitable for this vector due to their climate but might experience a substantial decrease in climatic suitability for P. spumarius by the period 2040–2060 [23]. The presence of insect vectors and preferred host plants, such as olive, grape, citrus, almond, and oak trees, as well as ornamental plants, which are economically significant crops for Portuguese agriculture, increase the risk of infection [3].
4. Transmission
The transmission of Xf occurs primarily through xylem-sucking insects [24]. The bacteria can be transmitted by any insect feeding on the xylem, making all such insects potential vectors. Furthermore, the extensive planting of monocultures creates an environment that can facilitate the spread of the disease [16]. However, according to Siccard et al., in monoculture agricultural systems, the presence of bacteria leads to the emergence of more aggressive pathogen genotypes. It is suggested that this aggressiveness reaches its maximum potential at a specific threshold point, where the development of plant disease symptoms becomes incompatible with the preferences of vector hosts and the acquisition capabilities of the pathogen. As a result, this threshold point leads to a decrease in the spread rate of X. fastidiosa [13].
Over long distances, the spread of the bacteria is mediated by the transport of infected plants or by infectious insect vectors [2]. The list of these vectors is vast and includes 120 species from 4 families, which belong to the order Hemiptera [25] and mainly to the families Cicadellidae and Aphrophoridae [26].
In Europe, the insect Philaenus spumarius (Figure 1) is the most efficient vector for Xf [21]. So far, only P. spumarius has been proven to transmit the bacterium in natural conditions in the EU. The probability of P. spumarius transmitting the bacterium was estimated with a median of 0.13 [27]. Other species in other Auchenorrhynchan families or Cicadellidae subfamilies have been tested for Xf transmission, always with negative results [28]. Some studies have found that some phloem-feeding insects can also acquire the bacteria, as they occasionally feed on xylem to replenish osmotic potential [29,30]. However, according to Cavalieri et al., despite being able to acquire the bacteria, they cannot transmit it to plants [31].

Figure 1.
An important vector of X. fastidiosa in Europe—Philaenus spumarius.
According to the latest studies, the list of potential insect vectors is continuously growing. More recently, other insect species were also identified, in Europe, as competent vectors (such as Philaenus italosignus and Neophilaenus campestres) but have only been shown to possess the capacity to acquire the bacterium in natural conditions, while their ability to transmit the bacterium to a new host plant still needs to be confirmed [2,27].
The potential presence and spread of Xf through seeds have not been extensively studied. However, PCR analysis has detected the bacteria in various parts of the seeds of oranges affected by citrus variegated chlorosis (CVC) [32]. The researchers suggest that the seed coat may act as a reservoir for the bacteria and potentially contribute to the spread of the disease to new areas. However, Dalla et al. failed to detect Xf in plants obtained from seeds of CVC-affected fruits [33]. Cordeiro et al. also failed to detect Xf in orange seedlings propagated from seeds extracted from fruits with CVC symptoms. In seedlings of six lemon varieties, they also did not detect the bacteria or observe any of the CVC symptoms. Thus, it has been concluded that Xf is unlikely to be transmitted or spread by seeds from the fruit of any citrus varieties grown in areas where CVC is endemic [34]. It is worth noting, however, that the studies conducted on the transmission of Xf through seeds were limited to small citrus samples. Therefore, further research is necessary to confirm the potential for transmission via seeds and to determine the actual risk of transmission in the field.
5. Hosts
Xf has a wide range of host plant species. Despite the large number of host plants reported, X. fastidiosa is a bacterial species with distinct phylogenetic clades, each of which has a more restricted host range. In terms of pathogenicity toward plants, the various phylogenetic clades of X. fastidiosa demonstrate a limited host range. X. fastidiosa may be associated with a wide array of plant species as a commensalist, while only a few clades and specific bacterial genotypes are responsible for causing diseases in a small number of plants [13].
The list of known plant hosts of European and non-European isolates is listed in Annex I to Implementing Regulation (EU) 2020/1201, and the plant genera and species identified as susceptible to subspecies of the bacteria anywhere in the world are listed in Annex II to Commission Implementing Regulation (EU) 2020/1201 [35]. According to data from EFSA’s 2023 report, there are currently 690 plant species that have been identified as susceptible to the bacteria, corresponding to 306 genera and 88 families [36]. In comparison with the previous database, published in December 2022, 12 new species (and 2 genera) have been identified as Xf hosts [16]. In the EU, nine new plant host species were identified in Portugal as naturally infected by subsp. multiplex or unknown (i.e., not reported). Three plant species were successfully artificially infected by subsp. fastidiosa [36].
In Portugal, the species Vitis vinifera (grapevine), Olea europaea L. (olive tree), Nerium L. (barley or oleander), Prunus persica (peach), Prunus dulcis (almond), Citrus sinensis (orange), Quercus sp., Vinca sp. L., Malva sp. L., Sorghum sp. L., Catharanthus sp., Portulaca sp. L., Polygala myrtifolia, Westringia fruticosa, Acacia saligna, Spartium junceum, Rosmarinus sp., Myrtus comunis, and Rhamnus alaternos are particularly noteworthy [37].
6. Inoculation of the Bacteria by the Insect Vector
Xf is naturally transmitted from one plant to another by insect vectors belonging to the order Hemiptera, mainly cycads, aphrophids, and cercopids [22]. Most of the main European insect vectors belong to the Aphrophoridae family, which includes Philaenus spumarius, Philaenus italosignus, and Neophilaenus campestris [4,31].
An adult Philaenus spumarius (Figure 1) is a small insect, measuring between 5.3 and 6.9 mm in length, and displays a wide range of dorsal coloration patterns. Both nymphs and adults of this species feed on crude sap, which is low in sugar but high in water, amino acids, and mineral salts found in the xylem vessels. They use their modified mouthparts, the stylet, to access the sap, and it is here where the bacteria Xf can attach [38]. While feeding in the nymph stage, the insect secretes a mass of foam that serves as protection from predators and desiccation. This foam production begins within minutes of feeding and is produced from a fluid originating from the abdomen, along with a surfactant secreted by the epidermal glands of the seventh and eighth abdominal segments [39].
P. spumarius is known for its highly polyphagous nature, with a preference for plants belonging to the Fabaceae and Asteraceae families. Additionally, they can survive on various host plants from distinct plant families [6]. Based on their biological cycle (Figure 2), these insects spend the winter as eggs. After a diapause period of approximately 100 days, the eggs hatch in early spring. The nymphs then progress through five developmental stages over a period of 5–8 weeks, during which they remain covered by a protective mucilaginous foam [40]. Adults typically begin to emerge in April or May and start mating during early summer, after which they tend to remain in the surrounding vegetation [41]. During spring, the nymphs can be found in the weeds, while adults are typically found in the canopy from May to summer. In autumn, the adults return to the weeds within the plot and surrounding areas or to other plants in the vicinity [24].
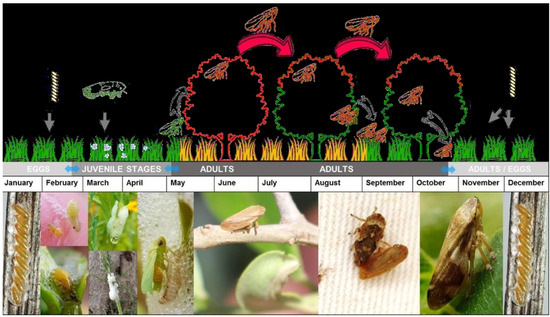
Figure 2.
Biological cycle of Philaenus spumarius in Italy [24].
The insects acquire the bacteria by feeding on infected plants and will subsequently host the bacterial cells themselves. They then proceed to release the pathogen into the transport system of host plants by inserting their stylet into the leaf petiole [3]; from there, the bacteria will spread to the xylem of the branches and stem [4]. Transmission of Xf does not require an incubation period in the vector. The bacterium is persistently transmitted [21] by both nymphs and adults.
Once they have fed on the xylem of an infected plant, insects are able to immediately transmit the pathogen to healthy plants [7]. However, it has been discovered that nymphs lose their infectivity after ecdysis, or molting. During this process, there is an exchange of the buccal armor where the bacteria are attached, and they are subsequently eliminated [21,40]. To reacquire the bacteria, the new adults will need to feed on an infected plant.
When a vector feeds on an infected plant, the process of bacteria adhesion to the insect occurs. This process is directly regulated by the expression of the rpfF gene, which induces the synthesis of a diffusible signaling factor (DSF) [42], which, when detected by other bacterial cells, induces the expression of adhesins [43]. These adhesins are indispensable for the adhesion of the pathogen to the insect and for the formation of a biofilm inside its body. Newman et al. found that bacteria with mutations in the rpfF gene are unable to produce DSF, which prevents biofilm formation on the insect vector, decreasing the bacteria’s ability to colonize it. As a result, the transmissibility of the bacteria is reduced [44]. Killiny et al. found that, once established in a biofilm inside the insect, the bacteria are able to remain viable [43]. Additionally, both nymphs and adults, can retain the pathogen for several months after acquisition, allowing Xf to spread to plants far from the original infection, mostly by anthropogenic influences [42]. To accomplish this, Xf secretes a chitinase that is capable of digesting the inner surface of the insect vector’s anterior gut [43,45]. Once an infected vector feeds, the bacteria detach from the foregut surface to enter the xylem of the plant. According to Killiny et al., the turbulence caused by ingestion is adequate to partially disaggregate the bacterial biofilm so that free cells can be injected into the plant [45].
Redak et al. also reported that the transmission process is highly efficient, as less than 200 viable bacterial cells in the gut of the vector are sufficient for producing infection [46]. Rapicavoli et al. also suggest that Xf can prevent initial recognition by the plant, thereby delaying the triggering of the plant’s immune response [47]. This may explain the effective way in which the bacteria establish themselves in their plant hosts.
7. Bacterial Action in the Xylem
The development of the disease in plants will now primarily depend on the bacteria’s ability to move from the point of inoculation and establish a systemic population in the infected plants [3]. After inoculation, the bacterial cells multiply, forming a biofilm [3] composed of bacterial cells, and secrete nucleic acids, proteins, and exopolysaccharides (EPSs) that can completely plug xylem vessels, blocking the transport of water and mineral salts [48].
Upon increasing the bacterial concentration, the synthesis of diffusible signaling factor (DSF) also increases, inducing the expression of adhesins. These adhesins promote the subsequent formation of the first colonies on the inner walls of the xylem. During the formation of the first colonies, cell aggregation is controlled by a two-component regulatory system known as the phoP/phoQ system. This system can respond to the relatively harsh environment of the xylem, particularly to acidic pH, by inducing adaptive changes and protective phenotypes in the pathogen. These changes include the formation of cell aggregates that are better enabled to cope with environmental stress [49].
The synthesis and secretion of exopolysaccharides and biofilm are further under the control of Gum genes [50]. According to Killiny et al., bacteria bearing mutations in this gene have impaired movement within the host plant [51]. On the contrary, mutants with the negative genetic regulator of Gum, PD1671, display a hypervirulent phenotype [52].
Biofilms are recognized for their ability to enhance the resistance of bacteria to stress and treatment. In the case of Xf, the formation of cell aggregates in biofilms, along with the plant’s production of tyloses to isolate the pathogen, can contribute to its persistence and spread [53], causing occlusions in the vessels and a decrease in water transport to the leaves, thereby compromising photosynthesis and transpiration rates [54].
8. Movement and Distribution of the Bacteria in the Plant
As the xylem is responsible for transporting water from the roots to the leaves, Xf is capable of spreading along the vasculature, even against the flow of sap. This allows the bacteria to effectively move throughout the plant and cause systemic infections [4]. In this process, Xf needs to cross the xylem cells through the existing xylem pores (PMs). PMs, ranging from 5 to 20 nm in diameter, are composed of hemicellulose, cellulose microfibers, and pectins and connect adjacent plant cells, forming a porous structure. They function to limit the passage of bacteria and air bubbles, protecting plants from embolisms [2]. The body size of Xf cells, on the other hand, is in the range of 250–2400 nm [1]. It has been proposed that the induction of various cell wall degradation enzymes [54] allows for an increase in the pores between adjacent xylem vessels, thereby enabling bacteria to traffic from one vessel to the next [44]. Sun et al. also reported degrading activity in the cell wall of xylem membranes in infected vines and an increased risk of embolisms [54]. Montillon et al. revealed that the average proportion of occlusions in the varieties Leccino and Cellina di Nardo indicates that the bacteria exploit PMs to spread systemically within the susceptible varieties. In these varieties, a clear degradation of the middle lamellae was observed, which allowed the bacteria to pass through. In contrast, this phenomenon was not observed in the resistant variety Leccino, which had intact lamellae [2]. During infections, decomposition enzymes produced by bacteria can degrade the components of the PMs, leading to an increase in their porosity.
This allows the bacteria to displace and diffuse along the xylem vasculature, which is a characteristic of their pathogenic virulence [55]. This fact may explain why some subspecies show higher virulence. As described by Chatterjee et al., virulence in Xf is associated with characteristics that allow it to move within and between xylem vessels [56]. Ionescu et al. linked the virulence of the bacteria to an environment with low pectin content and low DSF. This inhibits the adhesion of Xf to xylem walls, allowing it to spread rapidly without biofilm formation [57]. Benedictis et al. and Cardinale et al. reported notable differences in the distribution of occlusions between different twigs due to the erratic mode of colonization of the bacteria. Tyloses were found as responsible for the occlusions in twigs from older plants [53,58], whereas Montilon et al. reported that the occlusions observed in younger plants were composed of structures that are characteristic of an early event in the mechanism of pathogenesis [2]. Also, Lima et al. found that, in coffee bushes, the bacteria was distributed throughout the plant, confirming its downward translocation. Leite et al. observed that, in plum plants, high concentrations occurred in the aerial part. Almeida et al. and He et al. also verified that the bacteria showed ascending and descending translocation, being found in the roots of citrus plants inoculated in the aerial part [59,60]. Cardinale et al., meanwhile, reported a low concentration of bacterial cells in vascular occlusions of Ogliarola and Salentina stems and a higher presence of bacterial aggregates in leaf petioles [61]. These variations may be related to the size of the xylem pores, which enlarge in diameter from the top of the branch downward. As a result, the majority of the hydraulic resistance is concentrated in the lower portion of the branches, and pathogen invasion in the narrower vessels is less significant [61]. When examining the presence of Xf in embolized vessels, a smaller number of bacterial cells were found in the basal vessels compared to those found in vessels of the apical part of the plant. This suggests that the bacteria have a functional preference for aerobic respiration [62]. As air bubbles are filtered through the small membrane pores at the top, the basal area of the plant is less oxygen-rich. That causes an accumulation of bacteria in the apical zones [63]. The age of the plant could also impact the translocation of the bacteria, with older plants experiencing faster movement due to changes in transpiration and anatomical differences in the constitution of the xylem [64]. Therefore, further research is necessary to determine the spatial and quantitative distribution of the bacteria on host plants and the seasonal dynamics of this pathogen.
9. Symptoms
To date, it is generally accepted that following infection, water and nutrient transport is impaired due to the occlusion of xylem vessels by bacterial aggregates and the production of tylosis by the plant as a response to infection [2]. In infected grapevine and citrus, Goodwin et al. and Machado et al. found that impaired water and nutrient transport led to decreased photosynthesis rate, reduced transpiration rate, and high concentrations of abscisic acid, fructose, glucose, Ca2+, and Mg2+. They also observed low concentrations of Zn2+ and K2+. Leaf senescence was associated with chlorosis, high levels of proline and abscisic acid, and increased stomatal resistance [65,66].
These mechanisms induce the emergence of disease symptoms from the apical organs to the roots [3]. The symptoms are, in general, associated with manifestations similar to those observed during water stress, as visualized in Figure 3, which include chlorosis in the marginal zone of the leaves, followed by necrosis with a yellowish halo around them, wilting, burning (necrosis), and, in more serious cases, the death of the plant [21]. In some cases, it resembles mineral nutrient deficiency, such as marbling and chlorosis between veins. Depending on the plant species, irregular lignification of the bark, stunting, premature leaf fall, distortion, reduced size, and reduced fruit yield may also occur [7].

Figure 3.
Xyllela fastidiosa symptoms in an olive tree.
Several important diseases, as listed in Table 2, can also be associated with Xf infection, depending on the host and the observed symptomatology.

Table 2.
Description of symptoms produced by Xf according to host plant (adapted from [7]).
10. Absence of Symptoms
While in some hosts the infection induces visual changes, there are several species in which these bacteria colonize without causing symptoms [67]. Some authors suggest that in asymptomatic plants, these bacteria eventually die. Purcell suggests that infection or colonization by X. fastidiosa does not always result in disease development. In fact, there are processes in place that help maintain the populations of X. fastidiosa at low levels, which can also contribute to protecting plants from severe symptoms. X. fastidiosa is able to successfully colonize its hosts to ensure its survival and enable transmission through vectors while also minimizing harm to the host by regulating its gene expression in response to external signals [68].
According to Bragard et al., the asymptomatic period after infection can vary greatly, ranging from 1 month in ornamental plants to as long as 3–4 years in some hosts. This extended and variable asymptomatic period can hinder successful detection, especially when surveillance relies on visual inspection [69]. Some authors also mention that the most limiting factor in the manifestation of this disease is weather conditions. Harsh winters limit the spread of the disease since the bacterium is sensitive to low temperatures [70]. The growth and survival of Xf in cell cultures in vitro are differentially influenced by extremely low or high temperatures [71]. According to McElrone et al., in times of severe drought, the symptoms of infection will be aggravated by increased water stress [72]. Therefore, it is expected that phytopathologies caused by the bacteria will increase in response to global climate change. However, some authors argue that occlusions are not responsible for symptomatology. Benedictis et al. found a similar number of occlusions in infected branches of Leccino olive trees compared to healthy trees of the same variety [53]. Queiroz-Voltan et al. observed variations in symptom severity for the same variety developed under the same soil and climate conditions, cultural treatments, and management [73]. It was concluded that external symptoms of water deficit cannot be solely attributed to Xf presence or absence. The response and manifestation of symptoms are influenced by various physiological and environmental factors, including differences in plant resistance or tolerance, varying concentrations of the bacteria, different transpiration rates, and occlusion capacity between plant subspecies. Hopkins et al. and Kadel et al. found that less infectious subspecies of the bacteria protect vines from more aggressive subspecies while showing decreased symptom manifestation [74,75].
11. Disease Control Measures
Undoubtedly, Xf is an emerging pathogen and one of the most dangerous pests, with no available treatment. Eradicating the epidemic in an early stage has been successful through the removal of infected plants when the bacteria was only sporadically detected. Currently, there are several attempts to control this disease, including implementing control measures on infected plants and using more sophisticated molecular methods. These control attempts can be grouped into four categories, depending on the target:
- (a)
- The control of infected plants;
- (b)
- The use of tolerant cultivars;
- (c)
- The use of products that affect bacterial development;
- (d)
- The control of insect vectors.
11.1. Control of Infected Plants
Currently, several efforts are being made to develop control measures to restrict the spread of this bacteria by controlling infected plants. In the European Union, quarantine measures are regulated by Regulation (EU) No. 2016/2031 and phytosanitary measures to prevent the introduction and spread of the bacteria within the European territory [35].
In Italy, the legislation mandates the division of the southeastern region into three areas to enhance control of the disease. The infected area, where the disease is prevalent and cannot be eradicated, is designated as a “containment area” where infected plants must be uprooted. Within the “buffer area” (50 m radius around the infected tree), the uprooting of all surrounding plants is required [76]. In Portugal, following the guidelines stipulated in Implementing Regulation (EU) 2020/1201 and Regulation (EU) No. 2016/2031, once the presence of the bacterium is confirmed, measures must immediately be taken to prevent its spread and guarantee eradication [37]. In order to ensure the implementation and compliance with such measures, the national phytosanitary authority (DGAV), under Decree-Law No. 67/2020, of September 15th, establishes the demarcated zones, the measures for the eradication of the bacteria, and the restrictions on the movement of plants intended for planting in the infection zone and buffer zone [77,78].
Thus, as soon as an infected plant is found, a demarcated zone (DZ) is immediately established. A DZ (Figure 4) comprehends the infected zone (IZ) (including all susceptible plants within a 50 m radius around contaminated plants) and a surrounding buffer zone (BZ) (which includes all susceptible plant species within a 2500 m radius around the infected plant). In this demarcated zone (IZ + BZ total of 2550 m radius around the infected plant), the following measures are established [21]: in situ destruction of the infected plants, as well as of others of the same species; in situ destruction of all plants of the species listed in Annexes I and II to Implementing Regulation (EU) 2020/1201; a ban on planting in the infected zone of plants susceptible to the subspecies of the bacteria found in the demarcated area concerned, except under officially approved conditions of physical protection against the introduction of the bacteria by insect vectors; the prohibition of movement out of the demarcated zone and from the infected zone to the buffer zone of any plant, intended for planting, susceptible to the subspecies of the bacteria detected in the demarcated area; the prohibition of commercialization, in the demarcated area, in fairs and markets of any plant, intended for planting, susceptible to the subspecies of the bacteria detected in the demarcated area.
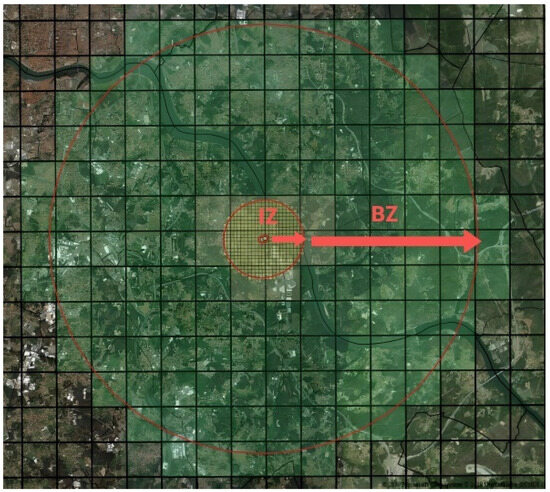
Figure 4.
Demarcated zone, which comprises the infected zone (IZ) 50 m radius around the infected plant and buffer zone (BZ) 2500 m radius around the infected plant (adapted from [21]).
Pereira et al. identified key strategies for controlling the spread of insect vectors, including limiting the mobility of host plants, establishing safety barriers (buffer zones), and implementing mandatory certification and a phytosanitary passport for nurseries transporting plants between internal borders [3]. However, according to Saponari et al., the implementation of containment measures faces significant challenges. These challenges include public resistance toward accepting the control measures, a lack of cooperation from stakeholders, the dissemination of misinformation by certain media outlets, and inadequate responses from government authorities. These difficulties result in delays and limitations in the containment efforts, ultimately allowing the bacterium to spread rapidly across wider areas within the region. This is evidenced by the continuous expansion of the official borders of the infected area [79]. Aside from destroying and uprooting, pruning has also been experimented as a way to control the disease. Since the bacteria typically moves from branch terminals to the plant’s trunk, pruning can eliminate the bacteria and provide temporary protection from reinfection by the vector. Furthermore, it can encourage the growth of new, uninfected branches. This method has already been tested on oleanders [80], citrus [81], coffee [82], grapevine [83,84], almond trees [85], and olive trees [15]. However, according to Bucci et al., there is no conclusive evidence of the effect of pruning on containing any of the diseases caused by Xf [86].
Another method was proposed from early observations of the effects of frost on grapevines affected by Pierce’s disease. As Purcell demonstrated [68], inoculations of Xf conducted throughout the growing season were successful in infected vines. However, infections occurring after May did not result in PD that persisted into the following year. It was noted that the density of live X. fastidiosa cells in vines affected by PD was sufficient to support efficient acquisition by vectors only after early June. Secondary spread of the disease is likely to occur after the time when most infections by vectors do not persist into the following year. They assume that vectors probably transfer X. fastidiosa from vine to vine during the summer months. However, these infections do not result in chronic PD due to overwinter recovery. The question is how vines in locations with high populations of vectors with high infectivity rates (>40%) can escape PD. One possibility is that such vines do become infected by X. fastidiosa but recover if they are inoculated during the summer season [68]. Feil et al. found that the bacteria are sensitive to low temperatures, namely, when infected grapevines are exposed to temperatures between −8 °C and −12 °C [87]. Subsequently, a protocol for cold treatment was tested with promising results on “Pinot Noir”, “Sauvignon”, and “Cabernet” vines affected by PD [88]. The authors took various varieties to four field sites in California with different winter temperatures to create a mathematical model for cold as a curative of vines infected with Xf. They found a direct correlation between control efficiency and cold locations. This simulation could help vineyard owners make management decisions regarding PD by choosing better geographical areas. However, the model has not yet been validated, making predictions or diagnoses speculative. Furthermore, it is uncertain if the results can be extended to other plants since Amanifar et al. were unable to stop the infection by replicating this technique, and the bacteria survived in the roots of infected almond trees [89]. Moreover, the physiological/biochemical mechanism that underlies cold therapy is poorly understood. Identifying the factors responsible for eliminating Xf with this method is crucial to replicating them in other plants and certifying their potential as a new approach to controlling the disease.
11.2. Use of Tolerant Cultivars
Another tested methodology involves screening for cultivars that are resistant and tolerant. The main concept is to study crop varieties that are more resistant, similar to the approach that has been used in the past for various pathogens. Some promising results have been achieved for grapevines [90], citrus [91], and olive trees [92]. However, it is important to note that tolerance to bacterial infection can diverge over time within the same plant, as it is influenced by intrinsic differences in structure, functional relationships, and plant response/defense mechanisms. Additionally, substituting one cultivar with another may not always be feasible without affecting the final product. Some plant varieties have shown resistance or tolerance to the bacteria in various studies. For instance, Sun et al. found that resistant grapevine varieties had a lower degree of xylem occlusion (20%), whereas susceptible varieties had a higher rate of occluded vessels (up to 60%) [54]. The list of tolerant and resistant plant genera and species is already reported. It has been found that tolerant/resistant status is available for 72 plant species (with a total number of 713 records). The most studied genera are Vitis, Citrus, and Prunus (417, 175, and 58 records, respectively), confirming the important economic value of these plant species [16]. Other investigations have been carried out in this context. In 2022, Surano et al. used electron microscopy to observe that Leccino olive trees exhibited greater resistance to infection symptoms compared to the Cellina di Nardo variety [48]. According to Petit et al., the symptoms of OQDs varied significantly among olive varieties. The study found that the less resistant varieties were less effective in producing tilosis, which enabled the bacteria to move within the vessels [4]. Montillon et al. found higher sensitivity in Salentina and Cellina di Nardo olive trees compared to Leccino varieties due to the presence of occlusions containing tyloses, gums, and pectin. However, no bacterial cell aggregates were detected [2]. Similarly, Cardinale et al. reported a low concentration of bacterial cells in vascular occlusions of the stems of Ogliarola and Salentina [58]. Benedictis et al. found a higher number of occlusions in Cellina di Nardo and Ogliarola Salentina olive trees compared to the resistant variety Leccino [53]. Montillon et al. found higher sensitivity in Salentina and Cellina di Nardo olive trees compared to Leccino varieties due to the presence of occlusions containing tyloses, gums, and pectin. However, no bacterial cell aggregates were detected [76]. Mauricio et al. evaluated field resistance to Xf in 264 hybrids of Citrus reticulata × Citrus sinensis and pear orange. Non-infected plants were grafted with Xf-infected grafts. The authors observed that most hybrid progenies did not show symptoms of citrus variegated chlorosis (CVC) or detectable levels of the bacteria, while all pear orange seedlings were infected and showed CVC symptoms. The authors suggest that certain genes may be responsible for the hybrids’ resistance to Xf, as their expression was significantly higher in the hybrid progenies [93]. These are promising studies that highlight the need to develop assays to test for tolerance to the bacteria.
11.3. Use of Products That Affect the Development of the Bacteria
In vitro and field studies have tested various chemicals, such as antibiotics, metal compounds, and natural products, to prevent infection. Benzothiadiazole, tested on tobacco plants, was found to be ineffective. Conversely, N-acetylcysteine, used as a fertilizer by Muranaka et al., showed promising results in improving symptoms, potentially due to its impact on bacterial biofilms [94]. Lacava et al. reported the in vitro antibiotic susceptibility of many Xf subspecies [95]. According to Amanifar et al., tetracyclines were found to be effective when injected into infected almond trees [89]. However, the use of antibiotics in plants has been little studied and remains largely unknown. The use of antibiotics, when mixed with other agrochemicals, can promote a faster development of antibiotic resistance. This, associated with the consumption of raw food, can lead to an increase in antibiotic-resistant bacteria in humans and can cause a major public health problem, as referred to by the “One Health approach”, since it has human, animal, and environmental impacts.
Dentamet, consisting of zinc (4%), copper (2%), and citric acid, has been widely evaluated as an effective treatment. Girelli et al. treated both resistant and susceptible olive trees with this biocomplex, obtaining significant modifications in the leaf metabolic extracts, such as an increase in oleuropein production. This is an important compound for plant protection and resistance against pathogens. The treatment also induced mannitol accumulation in leaves in response to infection, facilitating osmotic regulation. Additionally, endotherapy with Dentamet promoted the release of copper and zinc ions in the foliage, actively promoting the synthesis of the auxin hormone that stimulates plant growth [76]. Blonda et al. replicated the use of this complex. After spraying olive tree canopies once a month from spring to early fall, he found that this fertilizer was able to provide relevant systemic activity, reducing both disease symptoms and the concentration of Xf cells inside the leaves [96]. However, Muranaka argues that bacterial biofilm formation is enhanced with these antimicrobial treatments [97]. The application of copper treatment leads to an upsurge in the prevalence of persistent cells within the biofilm. These bacterial cells exhibit suppressed metabolism and activity, enabling their survival in harsh environments and facilitating their transition into a persistent state [98,99].
Further in vitro investigations demonstrated that cecropin B (CB) exhibited bactericidal properties against multiple phytopathogenic bacteria, such as Erwinia spp., Xanthomonas spp., Pseudomonas spp., and Clavibacter spp. Grapevines that were transgenic and expressed cecropin B had only mild symptoms of infection when inoculated with Xf, and the bacteria spread slowly. The microbial activity and size of Xf colonies were reduced due to the decreased activity of CB [100].
In addition to antibiotics and metals, studies have also been reported that test some natural substances produced by plants in response to Xf. Aldrich et al. and Maddox et al. reported the in vitro inhibitory activity of some compounds, such as polyphenols, azadirachtin A, hesperidin (to a lesser extent), and radicinin [101,102], on the bacteria.
Azevedo et al. and Dourado et al. showed that certain endophytic microorganisms could reduce the virulence of Xf by competing with the pathogen or secreting substances that can modulate its virulence [103,104].
In a different line of research, Ahern et al. and Das et al. used specific phages capable of lysing Xf in vitro. However, their use in the field has not been evaluated [105,106]. Baccari et al. investigated the effectiveness of endophytic bacteria when introduced into vines via stem punching. This method led to significant reductions in disease severity, indicating that these biological agents can reduce disease by inducing the expression of disease resistance. The strain used showed high efficacy in controlling Pierce’s disease and can be easily applied through spray treatment as an eradication measure [107].
Research on essential oils (EOs) has also shown to be potentially useful in controlling this pathogen, as their efficacy against a wide range of pathogens and pests has been confirmed in vitro by several authors [108,109]. Santiago et al. investigated the action of sandalwood and patchouli essential oils and obtained promising results; the oils exhibited antibacterial activity and may, therefore, be potentially used as natural sources for developing new pesticides [110]. Montesinos et al. tested the efficacy of eucalyptus essential oil against 11 phytopathogenic bacteria belonging to 6 different species. The study found that all phytopathogenic bacteria were susceptible to the oil, with Xf and Xanthomonas fragariae being the most affected. The bactericidal effect was particularly strong, with a lytic effect observed in three subspecies of Xf used in the study [111].
The application of the plant growth regulator abscisic acid (ABA) to infected “Pinot Noir” and “Cabernet Sauvignon” grapevines was described, including a foliar application. Pinot Noir vines treated with ABA showed a significant increase in the production of xylem sap phenolic compounds and healing effects when compared to control plants. The results demonstrated a positive correlation between ABA treatment and xylem sap phenolic compounds, indicating the antimicrobial properties of this compound [112].
11.4. Control of Insect Vectors
In Portugal, a group of methods that involve the application of plant protection products that ensure safety for human health and the environment has been used for the control of insect vectors. Recently, an extraordinary authorization was granted for the application of plant protection products containing acetamiprid, rape seed oil, and orange oil, which are expected to be effective in controlling insect vectors [113]. According to Altamura et al., acetamiprid is highly toxic against Philaenus spumarius [114]. However, some authors have suggested that this neonicotinoid does not have a significant impact on bacterial inoculation, as the vectors treated with this insecticide showed less vulnerability to it compared to other insecticides [40]. Bethke et al. also reported the effective insecticidal action of a neonicotinoid in reducing insect vectors in California [115]. However, the overuse of contact insecticides leads to the development of resistance in many pest species and the suppression of natural enemy populations [41]. According to Carolo, citrus oil showed a good effect when applied in high volumes. However, it only worked on insects that were in nymphal states [116].
According to Dongiovanni et al., the use of orange oil significantly reduced the number of nymphs, indicating its potential to control juvenile populations [114]. However, its effectiveness may be limited to nymphs present in herbaceous plants and weeds, and it may not be usable in areas where the control of undergrowth plants is challenging or when the insects are in the adult stage. Additionally, Domenico et al. found that P. spumarius males and females were attracted or repelled by different concentrations of the same oil [117]. Research conducted by Lago et al. demonstrated the potential of using kaolin to serve as a protective barrier against insects, such as Homalodisca vitripennis, that can cause disease progression. The use of kaolin is known to repel insects and reduce oviposition, leading to death. Furthermore, natural predators like birds and small lizards have been observed preying on Cicadellinae nymphs and adults, while larvae coccinellids and lacewings attack postures. Taking this into consideration, it appears that an appropriate timing for testing new formulations, as well as determining the volume of grout and the number of applications, may be necessary to construct an effective integrated pest management strategy that is also sustainable [118].
According to the findings of Avosani et al., to effectively disrupt the transmission and spread of X. fastidiosa, a successful and sustainable strategy should focus on minimizing contacts between vectors and host plants, as well as reducing the suitability of host plants for the bacterium. In their research, a multimodal control approach called behavioral manipulation (BM) can be used to disrupt insect perception and interaction with their surroundings. BM involves interfering with the stimuli that insects use to perceive and interact with their environment. This can be achieved by applying chemical repellents or vibrating deterrent compounds on the olive canopy, for example. By manipulating these stimuli, it is possible to influence insect behaviors, such as mating, permanence on hosts, oviposition, and feeding. BM plays a crucial role in short-range communication and host acceptance by insects, making it a valuable strategy for controlling pests [119]. In French Polynesia, Hoddle et al. tested the release of natural enemies of insect vectors, namely, Gonatocerus sp. parasitoid eggs. In this trial, they observed that after 7 months, the vector population decreased by 95% [120,121]. They also explored the isolation of specific viruses capable of decreasing bacterial adhesion to the insect, being potentially useful as biopesticides.
12. Conclusions
Understanding Xf, its vectors, and their relationship with the plant hosts, as well as recognizing symptoms in different hosts, is crucial to obtaining sustainable protection of plants. This review discussed the infection process, symptom developments, current distribution in Portugal, and actions taken to control the disease. Multiple solutions should be followed to reduce infection, namely, reducing insect vectors and using resistant plant varieties, as mentioned. The most effective control methods involve a combination of approaches, such as cultural measures and the removal of host plants and insects. However, due to climate change, the populations of insect vectors have become more extensive, resulting in the consequent dissemination of the bacteria over longer periods. The destruction of these insects raises questions regarding their role in nature. Simultaneously, there are no specific products against them. Insects are the center of trophic chains, maintaining and regulating the population of most plants through pollinations and phytophagy and, among other functions, are also involved in the recycling of organic matter. Therefore, insects are fundamental pieces for the maintenance of life. There is a need to deepen the knowledge about the consequences and effects of a decrease in the numbers of these insects on the ecosystem.
Methods based on the introduction of endophytic microorganisms into the interior of plants have also been reported. One of the main benefits caused by endophytes in the host plant is the promotion of growth. Simultaneously, they can provide biological control by diminishing or preventing the deleterious effects caused by pests and phytopathogens, reducing the use of pesticides. The literature studied indicates that endophytic microorganisms play an important role in plant development; thus, such research indicates a promising future for agriculture and vegetable cultivation. There is a need to deepen the knowledge regarding these methods since it was observed that they have an inhibitory effect on Xf development.
Despite the promising results in its control, Xf continues to spread and impact Europe’s landscape, society, and cultural heritage. It is essential to have a clearer understanding of the interaction between host plants, pathogens, and favorable environments and establish the epidemiological significance, at a national level, of the infected plants that do not show symptoms and that present normal development. The detection of several species of asymptomatic plants shows the difficulty of knowing the time of infection. And, because of that, these plants can be hosts of this bacterium without it causing any damage. This fact raises the question “how long can these plants live with Xf without any damages?”. At the moment, the control of this bacterium is carried out by applying the measures of EU Regulation No. 2016/2031, which consist of the destruction of infected plants. Considering the variability in plant responses to infection, it is necessary to implement these measures to effectively reduce the risk of spread. The non-existence of symptoms in bacteriologically positive plants may result, within the national survey, in the existence of false negative results, which can cause the dissemination of the bacteria. Likewise, obtaining systematically negative samples can lead to the underestimation of the expansion of the disease and cause its dispersion.
It is important to note that the symptoms of the disease are severe, causing high plant mortality. This fact entails significant economic losses, for producers and, consequently, for our country. Urgent action is needed, including the creation of knowledge networks and research institutes, to facilitate knowledge transfer and develop sustainable solutions for different crops, soil, and climate conditions.
Author Contributions
Conceptualization, T.L., I.C., M.M.M. and P.P.; methodology, T.L., I.C., M.M.M. and P.P.; validation, T.L., I.C., and M.M.M.; formal analysis, Â.M., I.C., P.P., M.d.L.E.D. and M.M.M.; investigation, T.L. and L.S.; data curation, T.L. and L.S.; writing—original draft preparation, T.L. and L.S.; writing—review and editing, T.L., I.C., P.P., Â.M. and M.d.L.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the projects UIDP/00772/2020 and LA/P/0059/2020 funded by the Portuguese Foundation for Science and Technology (FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Northern Regional Directorate of Agriculture and Fisheries of the Ministry of Agriculture and Food and, especially, Maria Manuel Mesquita and all colleagues involved in this work. Without their effort, this could not have been achieved.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov., sp. nov: Gram-Negative, xylem-limited, fastidious plant bacteria related to Xanthomonas spp. Int. J. Syst. Evol. Microbiol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- Montilon, V.; De Stradis, A.; Saponari, M.; Kubaa, R.A.; Giampetruzzi, A.; D’Attoma, G.; Saldareli, P. Xylella fastidiosa subsp. pauca ST53 exploits pit membranes of susceptible olive cultivars to spread systemically in the xylem. Plant Pathol. 2023, 72, 144–153. [Google Scholar] [CrossRef]
- Pereira, P.S. Xylella fastidiosa—A new menace for Portuguese agriculture and forestry. Rev. Ciências Agrárias 2015, 38, 149–154. [Google Scholar]
- Petit, G.; Bleve, G.; Gallo, A.; Mita, G.; Montanaro, G.; Nuzzo, V.; Zambonini, D.; Pitacco, A. Susceptibility to Xylella fastidiosa and functional xylem anatomy in Olea europaea: Revisiting a tale of plant-pathogen interaction. AoB Plants 2021, 13, plab027. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; van der Werf, W.; Cendoya, M.; Lasink, A.O. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [PubMed]
- Trkulja, V.; Tomić, A.; Iličić, R.; Nožinić, M.; Milovanović, T.P. Xylella fastidiosa in Europe: From the introduction to the current status. Plant Pathol. J. 2022, 38, 551. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 7/24 (4) Xylella fastidiosa. EPPO Bull. 2019, 49, 175–227. [Google Scholar] [CrossRef]
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Fatmi, M.; Chang, C.J. Xylella fastidiosa subspecies: X. fastidiosa subsp. piercei, subsp. nov., X. fastidiosa subsp. multiplex subsp. nov., and X. fastidiosa subsp. pauca subsp. nov. Syst. Appl. Microbiol. 2004, 27, 290–300. [Google Scholar] [CrossRef]
- Schuenzel, E.L.; Scally, M.; Stouthamer, R.; Nunney, L. A multigene phylogenetic study of clonal diversity and divergence in North American strains of the plant pathogen Xylella fastidiosa. Appl. Environ. Microbiol. 2005, 71, 3832. [Google Scholar] [CrossRef]
- Nunney, L.; Schuenzel, E.L.; Scally, M.; Bromley, R.E.; Stouthamerc, R. Large-scale intersubspecific recombination in the plant-pathogenic bacterium Xylella fastidiosa is associated with the host shift to mulberry. Appl. Environ. Microbiol. 2014, 80, 3025–3033. [Google Scholar] [CrossRef]
- Randall, J.J.; Goldberg, N.P.; Kemp, J.D.; Radionenko, M.; French, J.M.; Olsen, M.W.; Hanson, S.F. Genetic analysis of a novel Xylella fastidiosa subspecies found in the Southwestern United States. Appl. Environ. Microbiol. 2009, 75, 5631–5638. [Google Scholar] [CrossRef] [PubMed]
- Janse, J.D.; Obradovic, A. Xylella fastidiosa: Its biology, diagnosis, control and risks on JSTOR. J. Plant Pathol. 2010, 92, 1.35–1.48. Available online: https://www.jstor.org/stable/41998754 (accessed on 15 April 2023).
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P.P. Xylella fastidiosa: Insights into an emerging plant pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Baù, A.; Delbianco, A.; Stancanelli, G.; Tramontini, S. Susceptibility of Olea europaea L. varieties to Xylella fastidiosa subsp. pauca ST53: Systematic literature search up to 24 March 2017. EFSA J. 2017, 15, e04772. [Google Scholar] [CrossRef]
- Martelli, G.P.; Boscia, D.; Porcelli, F.; Saponari, M. The olive quick decline syndrome in south-east Italy: A threatening phytosanitary emergency. Eur. J. Plant Pathol. 2016, 144, 235–243. [Google Scholar] [CrossRef]
- Delbianco, A.; Gibin, D.; Pasinato, L.; Boscia, D.; Morelli, M. Update of the Xylella spp. host plant database—Systematic literature search up to 30 June 2022. EFSA J. 2023, 21, e07726. [Google Scholar] [CrossRef]
- Martinetti, D.; Soubeyrand, S. Identifying lookouts for epidemio-surveillance: Application to the emergence of Xylella fastidiosa in France. Phytopathology 2019, 109, 265–276. [Google Scholar] [CrossRef]
- Hernández, O.G.; García, L.V. Incidencia de Xylella fastidiosa en las Islas Baleares y distribución potencial en la península ibérica. Investig. Geográficas 2018, 69, 55–72. [Google Scholar] [CrossRef]
- Gilioli, G.; Simonetto, A.; Colturato, M.; Bazarra, N.; Férnandez, J.R.; Naso, M.G.; Donato, B.; Bosco, D.; Dongiovanni, C.; Maiorano, A.; et al. An eco-epidemiological model supporting rational disease management of Xylella fastidiosa. An application to the outbreak in Apulia (Italy). Ecol. Modell. 2023, 476, 110226. [Google Scholar] [CrossRef]
- Olmo, D.; Nieto, A.; Borràs, D.; Montesinos, M.; Adrover, F.; Pascual, A.; Gost, P.A.; Quetglas, B.; Urbano, A.; García, J.D.; et al. Landscape epidemiology of Xylella fastidiosa in the Balearic Islands. Agronomy 2021, 11, 473. [Google Scholar] [CrossRef]
- DGAV. Plano de Contingência Xylella fastidiosa e Seus Vetores; Direção-Geral da Alimentação e Veterinária: Lisbon, Portugal, 2022. [Google Scholar]
- DGAV. Xylella fastidiosa. 2023. Available online: https://www.dgav.pt/plantas/conteudo/sanidade-vegetal/inspecao-fitossanitaria/informacao-fitossanitaria/xylella-fastidiosa/ (accessed on 25 March 2023).
- Godefroid, M.; Morente, M.; Schartel, T.; Cornara, D.; Purcell, D.; Gallego, A.; Moreno, A.; Pereira, J.A.; Fereres, A. Climate tolerances of Philaenus spumarius should be considered in risk assessment of disease outbreaks related to Xylella fastidiosa. J. Pest Sci. 2004, 95, 855–868. [Google Scholar] [CrossRef]
- EFSA; Vos, S.; Camilleri, M.; Diakaki, M.; Lázaro, E.; Parnell, S.; Schenck, M.; Schrader, G.; Vincent, A. Pest survey card on Xylella fastidiosa. EFSA Support. Publ. 2019, 16, 1667E. [Google Scholar] [CrossRef]
- Di Serio, F.; Bodino, N.; Cavalieri, V.; Demichelis, S.; Di Carolo, M.; Dongiovanni, C.; Fumarola, G.; Gilioli, G.; Guerrieri, E.; Picciotti, U.; et al. Collection of data and information on biology and control of vectors of Xylella fastidiosa. EFSA J. 2019, 16, 1–102. [Google Scholar] [CrossRef]
- Purcell, A.H.; Saunders, S.R.; Hendson, M.; Grebus, M.E.; Henry, M.J. Causal role of Xylella fastidiosa in oleander leaf scorch disease. Phytopathology 2007, 89, 53–58. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Lázaro, E.; Parnell, S.; Civera, A.V.; Schans, J.; Schenck, M.; Schrader, G.; Abrahantes, J.C.; Zancanaro, G.; Vos, S. Guidelines for statistically sound and risk-based surveys of Xylella fastidiosa. EFSA Support. Publ. 2020, 17, EN-1873. [Google Scholar] [CrossRef]
- Almeida, R.P.P. (Ed.) Chapter 12: Xylella fastidiosa vector transmission biology. In Vector-Mediated Transmission of Plant Pathogens; The American Phytopathological Society: Saint Paul, MN, USA, 2016; pp. 165–173. [Google Scholar]
- Chuche, J.; Sauvion, N.; Thiéry, D. Mixed xylem and phloem sap ingestion in sheath-feeders as normal dietary behavior: Evidence from the leafhopper Scaphoideus titanus. J. Insect Physiol. 2017, 102, 62–72. [Google Scholar] [CrossRef]
- Pompon, J.; Quiring, D.; Goyer, C.; Giordanengo, P.; Pelletier, Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 2011, 57, 1317–1322. [Google Scholar] [CrossRef]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa subspecies pauca sequence type 53 by different insect species. Insects 2019, 10, 324. [Google Scholar] [CrossRef]
- Li, W.B.; Pria, W.D.; Lacava, P.M.; Qin, X.; Hartung, J.S. Presence of Xylella fastidiosa in sweet orange fruit and seeds and its transmission to seedlings. Phytopathology 2003, 93, 953–958. [Google Scholar] [CrossRef]
- Dalla, W.; Magalhães, P.L.; Li, W.; Miranda, V.S.; Costa, P.I.; Farias, P.R.S.; Hartung, V.S.; Pereira, E.O.; Francischini, F.J.B. Xylella fastidiosa em frutos e sementes de laranja-doce afetados pela clorose dos variegada dos citros. Laranja 2002, 23, 183–202. [Google Scholar]
- Cordeiro, A.B.; Sugahara, V.H.; Stein, B.; Leite Junior, R.P. Evaluation by PCR of Xylella fastidiosa subsp. pauca transmission through citrus seeds with special emphasis on lemons (Citrus limon (L.) Burm. f). Crop Prot. 2014, 62, 86–92. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/2031 of the European Parliament and of the Council on protective measures against pests of plants, amending Regulations (EU) No. 228/2013, (EU) No. 652/2014 and (EU) No. 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/PDF/?uri=CELEX:32020R1201&from=EN (accessed on 6 November 2022).
- Gibin, D.; Pasinato, L.; Delbianco, A. Update of the Xylella spp. host plant database—Systematic literature search up to 31 December 2022. EFSA J. 2023, 21, e08061. [Google Scholar] [CrossRef]
- DGAV. Plano de Ação pra Erradicação de Xylella fastidiosa e controlo dos seus vetores. Zona Demarcada da Área Metropolitana do Porto; Direção-Geral da Alimentação e Veterinária: Lisbon, Portugal, 2022. [Google Scholar]
- Carvalho, C.; Rodrigues, J.; Martins, L. Dispersion of the bacterium Xylella fastidiosa in Portugal. J. Agric. Sci. Technol. A 2022, 12, 35–41. [Google Scholar] [CrossRef]
- Selcuk, Y. On the meadow spittlebug Philaenus spumarius. Turk. J. Zool. 2000, 24, 447–459. [Google Scholar]
- Lago, C.; Cornara, D.; Minutillo, S.A.; Moreno, A.; Fereres, A. Feeding behaviour and mortality of Philaenus spumarius exposed to insecticides and their impact on Xylella fastidiosa transmission. Pest Manag. Sci. 2022, 78, 4841. [Google Scholar] [CrossRef]
- Dietrich, C.H. Keys to the families of Cicadomorpha and subfamilies and tribes of Cicadellidae (Hemiptera: Auchonorrhyncha). Fla. Enthomol. 2005, 88, 502–517. [Google Scholar] [CrossRef]
- Simionato, A.V.C.; Da Silva, D.S.; Lambais, M.R.; Carrilho, E. Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC-EI-MS. J. Mass Spectrom. 2007, 42, 490–496. [Google Scholar] [CrossRef]
- Killiny, N.; Almeida, R.P.P. Factors affecting the initial adhesion and retention of the plant pathogen Xylella fastidiosa in the foregut of an insect vector. Appl. Environ. Microbiol. 2014, 80, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.L.; Almeida, R.P.P.; Purcell, A.H.; Lindow, S.E. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. USA 2004, 101, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Prado, S.S.; Almeida, R.P.P. Chitin utilization by the insect-transmitted bacterium Xylella fastidiosa. Appl. Environ. Microbiol. 2010, 76, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.S.; Blua, M.J.; Mizell, R.F.; Andersen, P.C. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Rapicavoli, J.N.; Blanco-Ulate, B.; Muszynski, A.; Figueroa-Balderas, R.; Morales-Cruz, A.; Azadi, P.; Doburchowska, J.M.; Castro, C.; Cantu, D.; Roper, M.C. Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nat. Commun. 2018, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Surano, A.; Abou Kubaa, R.; Nigro, F.; Altamura, G.; Losciale, P.; Saponari, M.; Saldarelli, P. Susceptible and resistant olive cultivars show differential physiological response to Xylella fastidiosa infections. Front. Plant Sci. 2022, 13, 3603. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.K.; Kirkpatrick, B.C. The PhoP/Q two-component regulatory system is essential for Xylella fastidiosa survival in Vitis vinifera grapevines. Physiol. Mol. Plant Pathol. 2015, 89, 55–61. [Google Scholar] [CrossRef]
- Silva, F.R.; Vettore, A.L.; Kemper, E.L.; Leite, A.; Arruda, P. Fastidian gum: The Xylella fastidiosa exopolysaccharide possibly involved in bacterial pathogenicity. FEMS Microbiol. Lett. 2001, 203, 165–171. [Google Scholar] [CrossRef]
- Killiny, N.; Hernandez Martinez, R.; Korsi Dumenyo, C.; Cooksey, D.A.; Almeida, R.P.P. The exopolysaccharide of Xylella fastidiosa is essential for biofilm formation, plant virulence, and vector transmission. Mol. Plant-Microbe Interact. MPMI 2013, 26, 1044–1053. [Google Scholar] [CrossRef]
- Cursino, L.; Athinuwat, D.; Patel, K.R.; Galvani, C.D.; Zaini, P.A.; Li, Y.; De La Fuente, L.; Hoch, H.C.; Burr, T.J.; Mowery, P. Characterization of the Xylella fastidiosa PD1671 gene encoding eegenerate c-di-GMP GGDEF/EAL domains, and its role in the development of Pierce’s Disease. PLoS ONE 2015, 10, e0121851. [Google Scholar] [CrossRef]
- De Benedictis, M.; De Caroli, M.; Baccelli, I.; Marchi, G.; Bleve, G.; Gallo, A.; Ranaldi, F.; Falco, V.; Pasqualli, V.; Piro, G.; et al. Vessel occlusion in three cultivars of Olea europaea naturally exposed to Xylella fastidiosa in open field. J. Phytopathol. 2017, 165, 589–594. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, Y.; Walker, M.A.; Labavitch, J.M. Vascular occlusions in grapevines with Pierce’s disease make disease symptom development worse. Plant Physiol. 2013, 161, 1529–1541. [Google Scholar] [CrossRef]
- Pérez-Donoso, A.G.; Sun, Q.; Caroline Roper, M.; Carl Greve, L.; Kirkpatrick, B.; Labavitch, J.M. Cell wall-degrading enzymes enlarge the pore size of intervessel pit membranes in healthy and Xylella fastidiosa-infected grapevines. Plant Physiol. 2010, 152, 1748–1759. [Google Scholar] [CrossRef]
- Chatterjee, S.; Wistrom, C.; Lindow, S.E. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. USA 2008, 105, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.; Zaini, P.A.; Baccari, C.; Tran, S.; Da Silva, A.M.; Lindow, S.E. Xylella fastidiosa outer membrane vesicles modulate plant colonization by blocking attachment to surfaces. Proc. Natl. Acad. Sci. USA 2014, 111, E3910–E3918. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Luvisi, A.; Meyer, J.B.; Sabella, E.; De Bellis, L.; Cruz, A.C.; Ampatzidis, Y.; Cherubini, P. Specific fluorescence in situ hybridization (Fish) test to highlight colonization of xylem vessels by Xylella fastidiosa in naturally infected olive trees (Olea europaea L.). Front. Plant Sci. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; Li, W.B.; Ayres, A.J.; Hartung, J.S.; Miranda, V.S.; Teixeira, D.C. Distribution of Xylella fastidiosa in citrus rootstocks and transmission of citrus variegated chlorosis between sweet orange plants through natural root grafts. Plant Dis. 2007, 84, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.P.; Pereira, E.F.; Purcell, A.H.; Lopes, J.R.S. Multiplication and movement of a citrus strain of Xylella fastidiosa within sweet orange. Plant Dis. 2007, 85, 382–386. [Google Scholar] [CrossRef]
- Lechthaler, S.; Kiorapostolou, N.; Pitacco, A.; Anfodillo, T.; Petit, G. The total path length hydraulic resistance according to known anatomical patterns: What is the shape of the root-to-leaf tension gradient along the plant longitudinal axis? J. Theor. Biol. 2020, 502, 110369. [Google Scholar] [CrossRef]
- Gerlin, L.; Cottret, L.; Cesbron, S.; Taghouti, G.; Jacques, M.A.; Genin, S.; Baroukh, C. Genome-scale investigation of the metabolic determinants generating bacterial fastidious growth. mSystems 2020, 5, e00698-19. [Google Scholar] [CrossRef]
- Zhang, Y.; Carmesin, C.; Kaack, L.; Klepsch, M.M.; Kotowska, M.; Matei, T.; Schenk, H.J.; Weber, M.; Walther, P.; Schmidt, V.; et al. High porosity with tiny pore constrictions and unbending pathways characterize the 3D structure of intervessel pit membranes in angiosperm xylem. Plant Cell Environ. 2020, 43, 116–130. [Google Scholar] [CrossRef]
- Van Ieperen, W.; Van Meeteren, U.; Van Gelder, H. Fluid ionic composition influences hydraulic conductance of xylem conduits. J. Exp. Bot. 2000, 51, 769–776. [Google Scholar] [CrossRef]
- Goodwin, P.H.; DeVay, J.E.; Meredith, C.P. Physiological responses of Vitis vinifera cv. ‘Chardonnay’ to infection by the Pierce’s disease bacterium. Physiol. Mol. Plant Pathol. 1988, 32, 17–32. [Google Scholar] [CrossRef]
- Caruso Machado, E. Trocas gasosas e relações hídricas de laranjeira “Valência” enxertada sobre quatro porta-enxertos e submetida à deficiências hídrica. Bragantia 1998, 57, 15–22. [Google Scholar] [CrossRef]
- Purcell, A.H.; Saunders, S.R. Fate of Pierce’s Disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis. 2007, 83, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A. Paradigms: Examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 2013, 51, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Bragard, C.; Dehnen-Scmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.-A.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; Milonas, P.; et al. Update of the scientific ppinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, e05665. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.H.; Hopkins, D.L. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 2003, 34, 131–151. [Google Scholar] [CrossRef]
- Román-Écija, M.; Navas-Cortés, J.A.; Velasco-Amo, M.P.; Arias-Giraldo, L.F.; Gómez, L.M.; Fuente, L.; Landa, B.B. Two Xylella fastidiosa subsp. multiplex strains isolated from almond in Spain differ in plasmid content and virulence traits. Phytopathology 2022, 113, 960–974. [Google Scholar] [CrossRef]
- McElrone, A.J.; Sherald, J.L. Effects of water stress on symptomatology and growth of Parthenocissus quinquefolia infected by Xylella fastidiosa. Plant Dis. 2001, 85, 1160–1164. [Google Scholar] [CrossRef]
- Queiroz-Voltan, R.B.; Perosin Cabral, L.; Paradela Filho, O. Severidade do sintoma da bactéria Xylella fastidiosa em cultivares de cafeeiro. Bragantia 2004, 63, 395–404. [Google Scholar] [CrossRef]
- Hopkins, D.L. Biological control of Pierce’s Disease in the vineyard with strains of Xylella fastidiosa benign to grapevine. Plant Dis. 2005, 89, 1348–1352. [Google Scholar] [CrossRef]
- Kandel, P.P.; Almeida, R.P.P.; Cobine, P.A.; De La Fuente, L. Natural competence rates are variable among Xylella fastidiosa strains and homologous recombination occurs in vitro between subspecies fastidiosa and multiplex. Mol. Plant-Microbe Interact.–MPMI 2017, 30, 589–600. [Google Scholar] [CrossRef]
- Girelli, C.R.; Hussain, M.; Verweire, D.; Oehl, M.; Massana-Codina, J.; Avendaño, S.; Migoni, D.; Scortichini, M.; Fanizzi, F.P. Agro-active endo-therapy treated Xylella fastidiosa subsp. pauca-infected olive trees assessed by the first 1H-NMR-based metabolomic study. Scient. Rep. 2022, 12, 5973. [Google Scholar] [CrossRef] [PubMed]
- DRE. Decreto-Lei n.o 67/2020, de 15 de Setembro. Available online: https://dre.pt/dre/detalhe/decreto-lei/67-2020-142870334 (accessed on 23 April 2023).
- DRE. Portaria n.o 243/2020, de 14 de Outubro. Available online: https://dre.pt/dre/detalhe/portaria/243-2020-145359683 (accessed on 23 April 2023).
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in olive in Apulia: Where we stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Grebus, M.E.; Henry, J.M. Evaluation of pruning as a method to reduce damage by oleander leaf scorch. Slosson Rep. 1999, 98–99, 1–3. [Google Scholar]
- Beretta, M.J.G.; Rodas, V.; Junior, A.G.; Derrick, K.S. Control of citrus variegated chlorosis by pruning. Int. Organ. Citrus Virol. Conf. Proc. (1957–2010) 1996, 13, 378–379. [Google Scholar] [CrossRef]
- Queiroz-Voltan, R.B.; Cabral, L.P.; Filho, O.P.; Fazuoli, L.C. Eficiência da poda em cafeeiros no controle da Xylella fastidiosa. Bragantia 2006, 65, 433–440. [Google Scholar] [CrossRef]
- Feil, H.; Feil, W.S.; Purcell, A.H. Effects of date of inoculation on the within-plant movement of Xylella fastidiosa and persistence of Pierce’s Disease within field grapevines. Phytopathology 2007, 93, 244–251. [Google Scholar] [CrossRef]
- Alves, E.; Kitajima, E.W.; Leite, B. Interaction of Xylella fastidiosa with different cultivars of Nicotiana tabacum: A comparison of colonization patterns. J. Phytopathol. 2003, 151, 500–506. [Google Scholar] [CrossRef]
- Haviland, D.R.; Viveros, M.A. Surveys for Almond Leaf Scorch in Kern County, CA, and Implications on Pruning as a Tool for Management. Annual Report to the Almond Board of California. 2004. Available online: https://cekern.ucanr.edu/files/98498.pdf (accessed on 25 March 2023).
- Bucci, E.M. Xylella fastidiosa, a new plant pathogen that threatens global farming: Ecology, molecular biology, search for remedies. Biochem. Biophys. Res. Commun. 2018, 502, 173–182. [Google Scholar] [CrossRef]
- Feil, H.; Purcell, A.H. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 2007, 85, 1230–1234. [Google Scholar] [CrossRef]
- Lieth, J.H.; Meyer, M.M.; Yeo, K.H.; Kirkpatrick, B.C. Modeling cold curing of Pierce’s Disease in Vitis vinifera ‘Pinot Noir’ and ‘Cabernet Sauvignon’ grapevines in California. Phytopathology 2011, 101, 1492–1500. [Google Scholar] [CrossRef]
- Amanifar, N.; Taghavi, M.; Salehi, M. Xylella fastidiosa from almond in Iran: Overwinter recovery and effects of antibiotics. Phytopathol. Mediterr. 2016, 55, 337–345. [Google Scholar] [CrossRef]
- Krivanek, A.F.; Walker, M.A. Vitis resistance to Pierce’s Disease is characterized by differential Xylella fastidiosa populations in stems and leaves. Phytopathology 2005, 95, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Alves De Souza, A.; Aurélio Takita, M.; Morais Do Amaral, A.; Coletta-Filho, H.D.; Machado, M.A. Citrus responses to Xylella fastidiosa infection, the causal agent of citrus variegated chlorosis. Tree Forest. Sci. Biotechnol. 2009, 3, 73–80. Available online: www.fundecitrus.com (accessed on 25 March 2023).
- Boscia, D.; Altamura, G.; Saponari, M.; Tavano, D.; Zicca, S.; Pollastro, P.; Silletti, M.R.; Savino, V.N.; Martelli, G.P.; Delle Done, A.; et al. Incidenza di Xylella in oliveti con disseccamento rapido. L’Informatore Agrar. 2017, 27, 47–51. [Google Scholar]
- Mauricio, F.N.; Soratto, T.A.T.; Diogo, J.A.; Boscariol-Camargo, R.L.; De Souza, A.A.; Coletta-Filho, H.D.; Silva, J.A.A.; Medeiros, A.H.; Machado, M.A.; Cristofani-Yaly, M. Analysis of defense-related gene expression in citrus hybrids infected by Xylella fastidiosa. Phytopathology 2019, 109, 301–306. [Google Scholar] [CrossRef]
- Muranaka, L.S.; Giorgiano, T.E.; Takita, M.A.; Forim, M.R.; Silva, L.F.C.; Coletta-Filho, H.D.; Machado, M.A.; de Souza, A.A. N-Acetylcysteine in agriculture, a novel use for an old molecule: Focus on controlling the plant-pathogen Xylella fastidiosa. PLoS ONE 2013, 8, e72937. [Google Scholar] [CrossRef]
- Lacava, P.T.; Araújo, W.L.; Maccheroni, W.; Azevedo, J.L. RAPD profile and antibiotic susceptibility of Xylella fastidiosa, causal agent of citrus variegated chlorosis. Lett. Appl. Microbiol. 2001, 33, 302–306. [Google Scholar] [CrossRef]
- Blonda, P.; Tarantino, C.; Scortichini, M.; Tarantino, M.; Adamo, M. Monitoring the effectiveness of a bio-fertilizer restoration technique using multi-resolution satellite and meteo-data: The case of Xylella fastidiosa subsp. pauca. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Muranaka, L.S.; Takita, M.A.; Olivato, J.C.; Kishi, L.T.; de Souza, A.A. Global expression profile of biofilm resistance to antimicrobial compounds in the plant-pathogenic bacterium Xylella fastidiosa reveals evidence of persister cells. J. Bacteriol. 2012, 194, 4561–4569. [Google Scholar] [CrossRef]
- Lee, M.W.; Tan, C.C.; Rogers, E.E.; Stenger, D.C. Toxin-antitoxin systems mqsR/ygiT and dinJ/relE of Xylella fastidiosa. Physiol. Mol. Plant. Pathol. 2014, 87, 59–68. [Google Scholar] [CrossRef]
- Merfa, M.V.; Niza, B.; Takita, M.A.; De Souza, A.A. The MqsRA toxin-antitoxin system from Xylella fastidiosa plays a key role in bacterial fitness, pathogenicity, and persister cell formation. Front. Microbiol. 2016, 7, 904. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.L.; Andersen, P.C.; Leite, B. Effect of Vitis vinifera L. cv. Chardonnay xylem fluid on cecropin B activity against Xylella fastidiosa. Physiol. Mol. Plant Pathol. 2004, 64, 73–81. [Google Scholar] [CrossRef]
- Aldrich, T.J.; Rolshausen, P.E.; Roper, M.C.; Reader, J.M.; Steinhaus, M.J.; Rapicavoli, J.; Vosburg, D.A.; Maloney, K.N. Radicinin from Cochliobolus sp. inhibits Xylella fastidiosa, the causal agent of Pierce’s Disease of grapevine. Phytochemistry 2015, 116, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.L.; Araújo, W.L.; Lacava, P.T. The diversity of citrus endophytic bacteria and their interactions with Xylella fastidiosa and host plants. Genet. Mol. Biol. 2016, 39, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.N.; Santos, D.S.; Nunes, L.R.; da Costa de Oliveira, R.L.B.; de Oliveira, M.V.; Araújo, W.L. Differential gene expression in Xylella fastidiosa 9a5c during co-cultivation with the endophytic bacterium Methylobacterium mesophilicum SR1.6/6. J. Basic Microbiol. 2015, 55, 1357–1366. [Google Scholar] [CrossRef]
- Ahern, S.J.; Das, M.; Bhowmick, T.S.; Young, R.; Gonzalez, C.F. Characterization of novel virulent broad-host-range phages of Xylella fastidiosa and Xanthomonas. J. Bacteriol. 2014, 196, 459–471. [Google Scholar] [CrossRef]
- Das, M.; Bhowmick, T.S.; Ahern, S.J.; Young, R.; Gonzalez, C.F. Control of Pierce’s Disease by Phage. PLoS ONE 2015, 10, e0128902. [Google Scholar] [CrossRef]
- Baccari, C.; Antonova, E.; Lindow, S. Biological control of Pierce’s disease of grape by an endophytic bacterium. Phytopathology 2019, 109, 248–256. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Dung, N.T.; Suh, H.J.; Kang, S.C. Antibacterial activity of essential oil and extracts of Cleistocalyx operculatus buds against the bacteria of Xanthomonas spp. J. Am. Oil Chem. Soc. 2010, 87, 1341–1349. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.B.; Moraes, T.S.; Massuco, J.E.; Silva, L.O.; Lucarini, R.; da Silva, D.F.; Vieira, T.M.; Crotti, A.E.M.; Martins, C.H.G. In vitro evaluation of essential oils for potential antibacterial effects against Xylella fastidiosa. J. Phytopathol. 2018, 166, 790–798. [Google Scholar] [CrossRef]
- Montesinos, L.; Baró, A.; Gascón, B.; Montesinos, E. Bactericidal and plant defense elicitation activities of Eucalyptus oil decrease the severity of infections by Xylella fastidiosa on almond plants. Front. Plant Sci. 2023, 14, 868. [Google Scholar] [CrossRef]
- Meyer, M.M.; Kirkpatrick, B.C. Exogenous applications of abscisic acid increase curing of Pierce’s disease-affected grapevines growing in pots. Plant Dis. 2011, 95, 173–177. [Google Scholar] [CrossRef]
- DGAV. Autorização Excecional de Emergência N.º 2023/12. Art.º 53 do Regulamento (CE) n.º 1107/2009, de 21 de outubro, para utilização de produtos fitofarmacêuticos no controlo de potenciais vetores da bactéria Xylella fastidiosa em plantas hospedeiras, no contexto do Plano de contingência para a Xylella fastidiosa e seus vetores. 3 pp. Available online: https://www.dgav.pt/plantas/conteudo/sanidade-vegetal/inspecao-fitossanitaria/informacao-fitossanitaria/xylella-fastidiosa (accessed on 26 June 2023).
- Dongiovanni, C.; Di Carolo, M.; Fumarola, G.; Tauro, D.; Altamura, G.; Cavalieri, V. Evaluation of insecticides for the control of juveniles of Philaenus spumarius L., 2015–2017. Arthropod Manag. Tests 2018, 43, tsy073. [Google Scholar] [CrossRef]
- Bethke, J.A.; Blua, M.J.; Redak, R.A. Effect of selected insecticides on Homalodisca coagulata (Homoptera: Cicadellidae) and transmission of oleander leaf scorch in a greenhouse study. J. Econ. Entomol. 2001, 94, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, C.; Cavalieri, V.; Altamura, G.; Di Carolo, M.; Fumarola, G.; Saponari, M.; Porcelli, F. Preliminary results of comparative efficacy evalutation trials against Philaenus spumarius L., vector of Xylella fastidiosa. In Xylella fastidiosa & the Olive Quick Decline Syndrome (OQDS). A Serious Worldwide Challenge for the Safeguard of Olive Trees; D’Onghia, A.M., Brunel, S., Valentini, F., Eds.; Options Méditerranéennes–Série A Séminaires Méditerranéens; CHIEAM: Montpellier, France, 2017; Volume 121, pp. 79–80. Available online: https://om.ciheam.org/article.php?IDPDF=00007219 (accessed on 21 September 2023).
- di Domenico, C.; Ganassi, C.S.; Delfine, S.; Pistillo, M.; Germinara, G.S.; de Cristofaro, A. Biological activities of some essential oils towards Philaenus spumarius adults. IOBC-WPRS Bull. 2019, 141, 88–90. [Google Scholar]
- Lago, C.; Morente, M.; Heras-Bravo, D.; Martí-Campoy, A.; Rodríguez-Ballester, F.; Plaza, M.; Moreno, A.; Fereres, A. Dispersal ability of Neophilaenus campestris, a vector of Xylella fastidiosa, from olive groves to over-summering hosts. J. Appl. Entomol. 2021, 145, 648–659. [Google Scholar] [CrossRef]
- Avosani, S.; Nieri, R.; Mazzoni, V.; Anfora, G.; Hamouche, Z.; Zippari, C.; Vitale, M.L.; Verrastro, V.; Tarasco, E.; D’Isita, I.; et al. Intruding into a conversation: How behavioral manipulation could support management of Xylella fastidiosa and its insect vectors. J. Pest Sci. 2023, 1, 1–17. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Van Driesche, R.G. Biological control of insect pests. In Encyclopedia of Insects, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 91–101. [Google Scholar] [CrossRef]
- Stenger, D.C.; Sisterson, M.S.; Krugner, R.; Backus, E.A.; Hunter, W.B. A new Phytoreovirus infecting the glassy-winged sharpshooter (Homalodisca vitripennis). Virology 2009, 386, 469–477. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).