Abstract
Glaesserella parasuis (G. parasuis) can elicit meningitis in pigs; however, the pathogenic mechanisms of meningitis induced by G. parasuis remain unclear. Long non-coding RNAs (lncRNAs) have been proven to play key roles in a variety of physiological and pathological processes. However, whether lncRNAs are involved in meningitis triggered by G. parasuis has not been investigated. In this study, we performed an integrative analysis of lncRNAs expression profiles in the porcine brain infected with G. parasuis using RNA-seq. The results showed that lncRNA expressions in G. parasuis-induced meningitis were modified, and a total of 306 lncRNAs exhibited significant differential expression, in which 176 lncRNAs were up-regulated and 130 lncRNAs were down-regulated. KEGG enrichment analysis demonstrated that the differentially expressed target mRNAs of affected lncRNAs in G. parasuis-infected porcine brain were mainly involved in the cell adhesion molecules (CAMs), Jak-STAT signaling pathway, PI3k-Akt signaling pathway, and TNF signaling pathway. The expression relationship between the most affected differential lncRNAs and their differential target mRNAs was visualized by a co-expression network. A protein-protein interaction network consisting of 12 differential targets was constructed using STRING analysis. In addition, differential expressions of important lncRNAs were validated by qRT-PCR. lncRNA ALDBSSCT0000007362, ALDBSSCT0000001959, ALDBSSCT0000005529, MSTRG.2939.1, and MSTRG.32374.1 showed the same expression pattern with the lncRNA sequencing data. Our results demonstrated that G. parasuis could modify the lncRNA expression profiles in the porcine brain. To the best of our knowledge, this is the first report revealing the integrative analysis of lncRNA expression profiles in G. parasuis-induced meningitis, which could enhance important information to understand the inflammatory functions of lncRNAs involved in swine meningitis, and also provide a foundation for finding out novel strategies to prevent and treat meningitis in piglets triggered by G. parasuis.
1. Introduction
Glaesserella parasuis (G. parasuis), a Gram-negative bacterium, colonizes the upper respiratory tract of swine and is the etiological agent of Glässer’s disease [1]. The typical characteristic of this disease is fibrinous, meningitis, and polyserositis [2]. G. parasuis can induce serious inflammatory responses and contributes to big economic losses in the swine industry worldwide. To date, 15 serotypes of G. parasuis have been identified based on the Kielstein-Rapp-Gabrielson serotyping scheme [3]. Serotype 5 is the most prevalent serotype, and can be used as a virulence marker for G. parasuis infection [4]. Previous research has reported that G. parasuis can invade the central nervous system through the blood-brain barrier (BBB), resulting in meningitis [5]. However, the pathogenesis of meningitis caused by G. parasuis is not clear.
Long non-coding RNAs (lncRNAs), part of non-coding RNAs, are larger than 200 nucleotides and lack protein-coding ability [6]. It has been documented that lncRNAs are involved in epigenetic regulation, transcription modulation, and post-transcription modulation [7]. lncRNAs mainly function by regulating the expression of genes in the same chromosome (cis-acting lncRNAs) or genes in other chromosomes (trans-acting lncRNAs) [8]. Recent studies have shown that lncRNAs play key roles in a variety of physiological and pathological processes, including mammalian embryo development, bacteria-induced inflammatory responses, as well as tumorigenesis and metastasis [9,10,11,12]. lncRNA-MEG3 can modulate miR-210/TLR4 expression, leading to inflammatory responses and apoptosis of porcine alveolar macrophages infected with G. parasuis [10]. lncRNA SNHG8 promoted tumorigenesis and metastasis by sponging miR-149-5p in hepatocellular carcinoma [11]. In addition, lncRNA C11orf54-1 modulated the neuroinflammatory responses by activating the NF-κB signaling pathway during meningitic Escherichia coli infection [12]. However, whether lncRNA is involved in meningeal inflammation during G. parasuis infection remains unclear.
In the present study, the lncRNA expression profiles in the porcine brain were explored in a meningitis model induced by G. parasuis-infection. An integrated analysis of the differentially expressed lncRNAs and their putative target genes was conducted. Our results will help to better understand the functions and roles of lncRNAs in G. parasuis-induced meningitis, and might also provide some novel therapeutic targets by which to control swine meningitis.
2. Materials and Methods
2.1. Ethics Approval
This study was conducted in strict accordance with the recommendations of the China Regulations for the Administration of Affairs Concerning Experimental Animals 1988 and the Hubei Regulations for the Administration of Affairs Concerning Experimental Animals 2005. The protocol was approved by the Department of Science and Technology of Hubei Province (permit number SYXK (ER) 2010-0029). All experimental animals were euthanized before dissection. The animal study was reviewed and approved by the Animal Care and Use Committee of Wuhan Polytechnical University, Hubei Province, China (EM316, 15 October 2019).
2.2. Bacterial and Animals
The G. parasuis SH0165 strain, serotype 5, a high virulent isolate, was isolated from a commercial pig lung with arthritis, fibrinous polyserositis, hemorrhagic pneumonia, and meningitis [1]. The SH0165 strain was grown in tryptic soy broth (TSB) (Difco laboratories, Detroit, MI, USA) or tryptic soy agar (TSA) (Difco laboratories, Detroit, MI, USA) at 37 °C, with 10 μg/mL of nicotinamide adenine dinucleotide (NAD) (Sigma, St.Louis, MO, USA) and 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA).
Six 28-day-old naturally farrowed early-weaned piglets (Duroc × Landrace × Large White), weighing 8–10 kg, were purchased from Wuhan Wannianqing Animal Husbandry Co., Ltd. (Wuhan, China) for in vivo experiments.
2.3. Experimental Design
The piglets were randomly divided into two groups, which were the control group and the infection group. The infection group was intraperitoneally injected with 1 mL of normal saline, including 2 × 109 CFU G. parasuis. The control group was intraperitoneally injected with the equivalent amount of normal saline. Following infection, the piglets from both groups were monitored for 7 days. The cerebrum tissues from both groups were obtained and immediately frozen in liquid nitrogen and used for RNA isolation.
2.4. RNA Extraction and Illumina Sequencing
Total RNA was extracted from porcine cerebrum tissues using a miRNeasy Mini Kit (Qiagen, Dusseldorf, Germany). First, the frozen samples were ground in liquid nitrogen and added with QIAzol lysis solution (supplied in miRNeasy Mini Kit, Qiagen, Dusseldorf, Germany). Then, the lysates were processed following the manufacturer’s instructions. Qualified total RNA was further purified using an RNAClean XP Kit (Beckman Coulter, Inc. Kraemer Boulevard Brea, CA, USA) and a RNase-Free DNase Set (QIAGEN, Hilden, Germany). The quality of total RNA was verified using a NanoDrop ND-2000 spectrophotometer and an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). Only RNA with an RIN of ≧7.0 and a 28S/18S ratio of ≧0.7 were selected for deep sequencing. Libraries were generated using the VAHTS Total RNA-seq Library PrepKit for Illumina (Vazyme Biotech Co., Ltd., Nanjing, China) and were subsequently sequenced by using the Illumina HiSeq X-Ten platform.
2.5. Transcriptome Data Analysis
The Raw Reads were filtered using the Seqtk sequence processing tool (https://github.com/lh3/seqtk, accessed on 15 March 2022) to obtain the clean reads for data analysis. Genome mapping was performed for the clean reads by using the spliced mapping algorithm of Hisat2 [13]. The number of fragments per gene after the Hisat2 alignment was counted using Stringtie [14], and then was normalized using the TMM (trimmed mean of M values) [15]. The FPKM (fragments per kilobase of exon model per million mapped fragments) value for each gene was calculated using the Perl script. FPKM was used to calculate the fold change. A read count was used to perform differential gene analysis by using edgeR [16]. A gene with a fold change of >2 and an adjusted p-value of <0.05 (q < 0.05) was thought to be significantly differentially expressed.
2.6. lncRNA Prediction
The splicing results of the Stringtie [17] were compared with the reference annotation using gffcompare (version: 0.9.8). The transcription length ≤ 200 bp and exon ≤ 2 or the predicted ORF > 300 bp were removed [18]. The non-coding potentials of lncRNAs were predicted using the combination of Pfam Scan [19], the Coding Potential Calculator (CPC) [20], and the Coding-Non-Coding Index (CNCI) [21]. The transcripts with a CPC score < 0, a CNCI score < 0, and an insignificant Pfam Scan alignment were selected as the potential lncRNAs.
2.7. Prediction of lncRNA Targets
Trans regulation and cis regulation were used to predict target genes. For the trans-acting prediction, in the adoption database, blast was first used to select the sequences with complementary or similar sequences, and then RNAplex [22] was utilized to calculate the complementary energy between the two sequences and select the sequences above the threshold (identity > 85%, e value < 1 × 10−20). For the cis-acting prediction, coding genes with a distance of <100 bp to lncRNAs were screened.
2.8. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
The target gene function was determined by Gene Ontology (GO) and Kyoto Gene Genome Encyclopedia (KEGG) enrichment analysis. The selected genes were mapped to each term in the GO database. GO terms with a q-value ≦ 0.05 were defined as significantly enriched by query genes. The KEGG pathways with a q-value ≦ 0.05 were thought to be significantly enriched.
2.9. Protein-Protein Interaction Network Construction
The interactions between differentially expressed target genes of DElncRNAs were explored by STRING software (https://cn.string-db.org, accessed on 23 March 2022). After the input of a gene list to the website, interaction scores ranging from 0 to 1 were generated. An interaction score > 0.15 was considered the threshold for interaction. Cytoscape software was used to construct protein-protein interaction (PPI) networks (https://cytoscape.org, accessed on 22 March 2022).
2.10. Co-Expression Analysis of lncRNA and mRNA
lncRNAs regulate gene expression by interacting with the target mRNAs. A co-expression network was constructed based on the integrated analysis of the expression levels of DElncRNAs and their differential target genes. We detected similar expression patterns of DElncRNAs and the differentially expressed targets by screening the transcript levels from RNA-seq data. The Pearson’s correlation coefficient and corresponding p-value were calculated using the WGCNA R software package (https://horvath.genetics.ucla.edu/html/CoexpressionNetwork/Rpackages/WGCNA/index.html, accessed on 23 March 2022), and only the strongest correlations (correlation coefficient > 0.9 or < −0.9, p < 0.05) were retained and visualized by Cytoscape software 3.10.1 (https://cytoscape.org, accessed on 23 March 2022).
2.11. Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from porcine cerebrum tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The frozen samples were ground in liquid nitrogen and lysed with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA was reverse transcribed into cDNA by utilizing reverse transcriptase (TaKaRa, Dalian, China). The obtained cDNA was further quantified by using a SYBR Green PCR Kit (TaKaRa, Dalian, China) following the manufacturer’s protocol. For the amplification of individual transcripts, three technical repeats were set for each biological sample, with β-actin as the reference gene. The primer sequences used in this study are shown in Table 1.

Table 1.
Primers used for quantitative RT-PCR.
3. Results
3.1. Statistics of lncRNA Sequencing Data
The Illumina HiSeq X-Ten platform was utilized to profile the transcriptome in G. parasuis-infected porcine brain. An average of 69,720,059 ± 5,589,663 raw reads were obtained from the infection group, compared to 64,959,443 ± 4,370,318 raw reads from the control group (Table 2). The raw reads were filtered through quality control to obtain the clean reads ratio, which ranged from 95.95% to 96.94% (Table 2). In addition, 56,760,761 ± 4,147,585 and 52,534,850 ± 3,556,276 uniquely mapped reads were gained from the infection group and the control group, respectively (Table 2). The unique mapping ratio ranged from 86.38% to 86.73% after rRNA trimming (Table 2), which indicated that the sequencing data would be qualified for further analysis.

Table 2.
Statistical summary of lncRNA sequencing datasets.
3.2. Differentially Expressed lncRNAs
Transcriptome analysis was performed to profile the global changes occurring in meningitis induced by G. parasuis-infection (Supplemental Figure S1). Through unsupervised hierarchical clustering, porcine brain samples were clustered into two unique groups with distinct signatures (Figure 1A), indicating the reliability of the data for the following analysis. Transcriptome differential expression analysis showed that 306 lncRNAs were significantly differentially expressed in G. parasuis-infected porcine brain, with a fold change > 2 (q < 0.05) (Figure 1B). Among these differential lncRNAs, 176 lncRNAs were up-regulated and 130 lncRNAs were down-regulated following G. parasuis infection, compared to the control group (q < 0.05) (Figure 1B, Supplemental Table S1).

Figure 1.
Statistical summary of the differentially expressed lncRNAs. (A) Heat map. (B) Volcano plot showing the differentially expressed lncRNAs in the infection group compared to the control group. The infection group: H.1, H.2, H.3; The control group: C.1, C.2, C.3.
3.3. Target Prediction and Functional Enrichment
In order to reveal the potential function of lncRNAs involved in the pathogenesis of G. parasuis-induced meningitis, target genes of lncRNAs were predicted by cis- and trans-acting, respectively. A total of 2846 targets were revealed by cis-prediction, and 299,618 targets were revealed by trans-prediction for all the lncRNAs in our data (Supplemental Tables S2 and S3). For differentially expressed lncRNAs (DElncRNAs), a total of 17,575 target genes were revealed, of which 107 were predicted by cis and 17,468 by trans (Supplemental Table S4).
These 17,575 candidate target genes of DElncRNAs were utilized for the subsequent GO and KEGG enrichment analysis. GO analysis showed that cellular processes, metabolic processes, and individual organism processes were dominant terms in the category of biological processes (Figure 2A). In the cellular component category, cell and cell part were the most abundant terms, while binding was the top term in the molecular function category (Figure 2A). KEGG enrichment revealed that the TNF signaling pathway, platelet activation, B cell receptor signaling pathway, and N-glycan biosynthesis were the predominant pathways, in which the potential targets of the DElncRNAs were involved (Figure 2B).
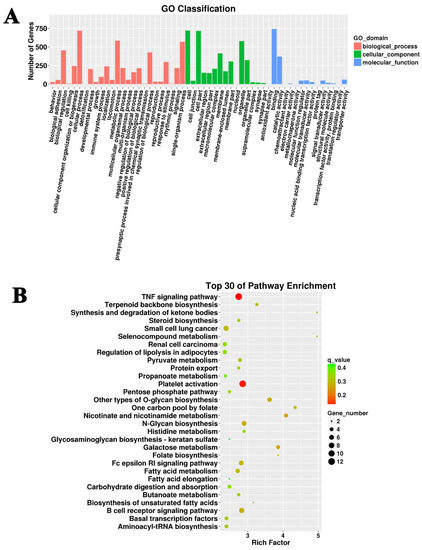
Figure 2.
GO and KEGG analysis of the target genes of differentially expressed lncRNAs. (A) Cellular process, cell part, and binding were the most dominant terms in the G. parasuis-infected piglet brain. (B) The top 30 KEGG pathways in the infection group compared to the control group.
Furthermore, the 17,575 candidate target genes of DElncRNAs were narrowed down to 116 differential mRNAs, based on their differential expression between the GPS group vs. the control group (Supplemental Table S5). These 116 differentially expressed target genes were subsequently used for GO and KEGG enrichment analysis. GO analysis identified similar affected processes (Figure 3A). KEGG enrichment showed that the differentially expressed targets of DElncRNAs were mainly enriched in the cell adhesion molecules (CAMs), the Jak-STAT signaling pathway, the PI3k-Akt signaling pathway, and the TNF signaling pathway (Figure 3B).
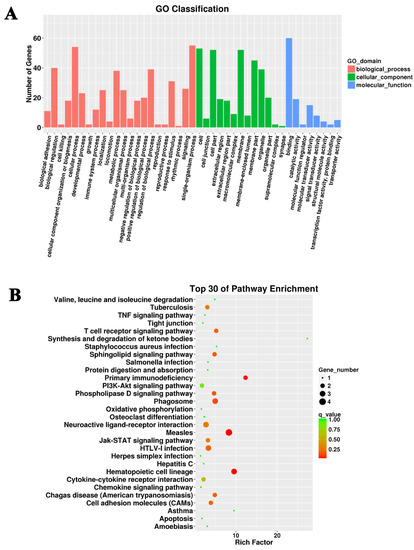
Figure 3.
GO and KEGG analysis of the differentially expressed target genes of differentially expressed lncRNAs. (A) Cellular process, single-organism process, and binding were the most abundant terms in the G. parasuis-infected piglet brain. (B) The top 30 KEGG pathways in the infection group compared to the control group.
3.4. Protein-Protein Interaction Network
To reveal the protein interactions between protein-coding genes regulated by G. parasuis, a PPI network was constructed using differentially expressed target genes of the most affected DElncRNAs (ALDBSSCT0000007362, ALDBSSCT0000001959, ALDBSSCT0000008196, and ALDBSSCT0000005529). The PPI network consisted of 12 differentially expressed targets (node) and 13 interactions (edges) (Figure 4A, Supplemental Table S6). The node degree was utilized to evaluate the crucial roles of proteins in the network and the top connected proteins were FCGR2B and IL7R (Figure 4B). Then, KEGG pathway analysis was performed on these interacted protein-coding genes, indicating that these genes were mainly involved in the primary immunodeficiency, Jak-STAT signaling pathway, HTLV-Ⅰ infection, and cytokine-cytokine receptor interaction (Figure 4C).
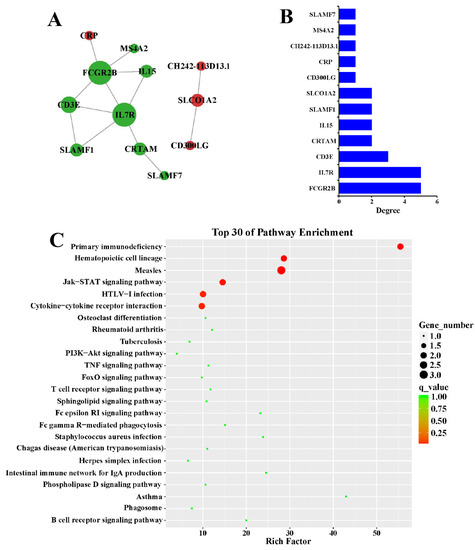
Figure 4.
Construction of the PPI network. (A) The interactions of the differentially expressed targets of differential lncRNAs in the infection group were analyzed by STRING. Nodes stand for genes and edges stand for interactions. The node size stands for the node degree. (B) The node degrees of the hub genes were displayed, with FCGR2B and IL7R as the most interacted genes. (C) The top KEGG pathways were enriched using the hub genes. The primary immunodeficiency, Jak-STAT signaling pathway, HTLV-Ⅰ infection, and cytokine-cytokine receptor interaction were highly enriched.
3.5. Co-Expression Analysis of DElncRNAs and DEmRNAs
We performed co-expression analysis to further explore the potential functions and regulatory mechanisms of DElncRNAs, and also to determine the associations between DElncRNAs and their differentially expressed targets. Under the stimulation of G. parasuis, correlations between the two gene sets (DElncRNAs and DEmRNAs) were calculated using WGCNA R, and only the strongest correlations were utilized to construct a co-expression network (Figure 5), in which four DElncRNAs and thirty-six differential targets were preserved and correlated with each other.
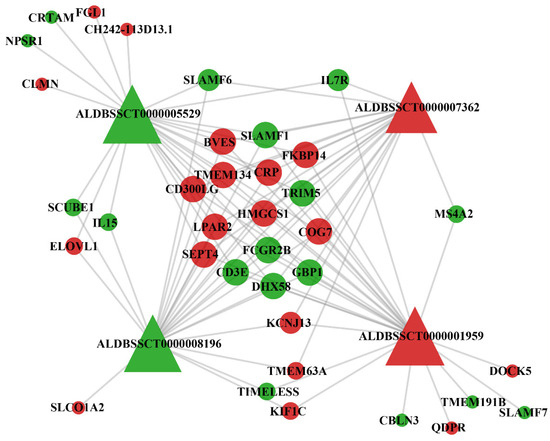
Figure 5.
Co-expression network of differentially expressed target genes and differentially expressed lncRNAs in the infection group compared to the control group. Triangles stand for differentially expressed lncRNAs and circular nodes stand for differentially expressed target genes. Red represents up-regulation and green represents down-regulation.
3.6. Validation of the Expression of lncRNAs
The expression of selected lncRNAs was validated by qRT-PCR. The results showed that the expression of the selected lncRNAs was similar to the lncRNA sequencing results (Figure 6). Compared with the control group, the expression of ALDBSSCT0000001959 and ALDBSSCT0000005529 were significantly up-regulated, whereas ALDBSSCT0000007362, MSTRG.2939.1, and MSTRG.32374.1 were significantly down-regulated in the infection group (Figure 6). It is known that most of lncRNAs lack species conservation. Therefore, we searched the sequences of these validated lncRNAs in the NONCODE database (http://www.noncode.org/index.php, accessed on 12 April 2022), but failed to find any homology in humans or mice.
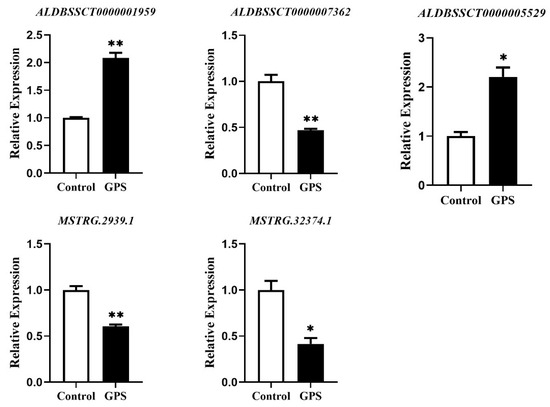
Figure 6.
Validation of the expression levels of the selected lncRNAs by qRT-PCR. The relative expression levels of ALDBSSCT0000001959, ALDBSSCT0000005529, ALDBSSCT0000007362, MSTRG.2939.1, and MSTRG.32374.1 were determined by qRT-PCR. * p < 0.05 and ** p < 0.01 vs. the control.
4. Discussion
G. parasuis infection can cause severe inflammatory responses. Meningitis is one of the most common complications of G. parasuis infection [23]. However, the pathogenesis of meningitis caused by G. parasuis remains unclear. Therefore, the key to Glässer’s disease treatment is to understand the pathogenic mechanism of G. parasuis infection with meningitis. In recent years, high-throughput lncRNAs sequencing has been able to quickly screen out potential therapeutic targets for disease [24]. In this study, the aim was to identify important lncRNAs and genes involved in meningitis during G. parasuis infection. The results suggested that lncRNAs were potentially involved in the regulatory mechanism of bacterial meningitis and this study could provide some new insights into the better prevention and treatment of meningitis.
Meningitis refers to inflammation of the meninges surrounding the brain and the spinal cord [25]. The blood-brain barrier (BBB) consists of brain microvascular endothelial cells (BMECs), astrocyte, pericytes, and microglia that maintain the homeostasis of the central nervous system [26]. G. parasuis was proven to adhere to and invade porcine BMEC contributing to bacterial meningitis [5]. A previous study showed that lncRNAs play important roles in epigenetic regulation, transcriptional regulation, and post-transcriptional regulation, as well as diseases [27]. It has been reported that some lncRNAs could ameliorate ischemic/reperfusion (I/R) injury by interacting with the nuclear factor erythroid 2-related factor 2 (Nrf2) and could serve as important therapeutic targets for I/R injury [28]. In this study, lncRNA ALDBSSCT0000001959 and ALDBSSCT0000005529 were significantly up-regulated in brains infected with G. parasuis, while lncRNA ALDBSSCT0000007362, MSTRG.2939.1, and MSTRG.32374.1 were significantly down-regulated. There has been increasing evidence that shows that lncRNAs are involved in inflammatory processes and regulating central nervous system-related diseases [12]. lncRNA DDIT4-AS1 could regulate neuroinflammation induced by meningitic Escherichia coli through promoting DDIT4 mRNA stability [29]. lncRNA SPH9-4 could promote BBB disruption caused by meningitic Escherichia coli via the miR-17-5p/MMP3 axis [30]. In addition, lncRNA C11orf54-1 modulated neuroinflammatory responses by activating NF-κB signaling during meningitic Escherichia coli infection [12]. Therefore, we speculated that dysfunction of lncRNAs in pig brains might be involved in the process of meningeal inflammation caused by G. parasuis, but special mechanisms need to be further studied.
It has been documented that the protein-protein interaction network could be used to screen potential associations in the molecular mechanisms of the biological processes of proteins [31]. Thus, in this study, the STRING database was used to construct protein-protein interaction (PPI) networks using the differentially expressed genes, and then was visualized using Cytoscape software. According to the network analysis, the top hub genes were FCGR2B, IL7R, CD3E, CRTAM, IL15, SLAMF1, and SLCO1A2, most of which were important immunological factors. Fc gamma receptor 2b (FCGR2B) is the only inhibitory Fc gamma receptor implicated in both antibody production and effector responses to antibody complexes [32], and has been reported to participate in many autoimmune diseases as an anti-inflammatory IgG receptor [33,34]. Previous research has reported that inhibiting the expression of the inflammatory cytokine receptor IL7R reduced the inflammatory responses in mouse colonic epithelial cells [35]. The class I-restricted T cell-associated molecule (CRTAM), an activation marker expressed on the cell surface of activated invariant natural killer T (iNKT) cells, CD8+, T cells, and a small subset of CD4+ T cells, has been proven to be associated with a proinflammatory profile in murine CD4+ T cells and the production of IFN-γ in human iNKT cells [36]. Through a pro-pathogen inflammatory response, CRTAM increases susceptibility to salmonella in mice [37]. The signaling lymphocytic activation molecule family 1 (SLAMF1) is an Ig-like receptor and initiates signal transduction networks in a variety of immune cells [38]. According to research, SLAMF1 regulated inflammatory reactions and was up-regulated in preclinical ulcerative colitis compared to the controls [39].
Further KEGG analysis of these top hub genes demonstrated that the primary immunodeficiency, Jak-STAT signaling pathway, HTLV-I infection, and cytokine-cytokine receptor interaction were the main affected pathways. Studies have demonstrated that inhibiting the activation of the Jak-STAT signaling pathway is implicated in the pathogenesis of inflammation and autoimmune diseases, and can reduce neurological damage, lessen brain edema and blood-brain barrier permeability, and enhance the production of tight junction proteins [40,41]. Human T-cell lymphotropic virus (HTLV) infection is the first discovered retrovirus to cause malignancy in humans, and it is also a main cause of encephalitis [42,43]. The interplay in cytokine-cytokine receptor interaction is crucial for a number of inflammatory processes [44]. Therefore, we inferred that these core genes and signaling pathways might be involved in the meningitis caused by G. parasuis, but the specific functions and mechanisms need to be further explored.
Identifying the target genes of lncRNAs is very important to explore the functions of lncRNAs in biological processes, which may be achieved via the interaction of lncRNAs and their relevant target genes [45]. Thus, the co-expression interaction network was constructed to assess the correlation between lncRNAs and their target genes. Furthermore, a total of 17,575 DElncRNA target genes in G. parasuis-induced porcine meningitis were identified and we will choose some important target genes to study their function in meningitis in our future study. The co-expression network was constructed with four DElncRNAs and thirty-six differential targets. Among the 36 differential targets, we found that FCGR2B, CD3E, SLAMF1, IL15, IL 7R, SLCO1A2, CRP, MS4A2, SLAMF7, and CH242-113D13.1 were overlapped with genes presented in the PPI network. FCGR2B, CD3E, CRP, and SLAMF1 were at the core position of the network, with the highest connection being with the four DElncRNAs. As mentioned above, FCGR2B and SLAMF1 are important immune molecular-regulating inflammatory responses. FCGR2B participates in many autoimmune diseases as an anti-inflammatory IgG receptor [33,34] and SLAMF1 can initiate signal transduction networks in a variety of immune cells [38]. In addition, Fcgr2b, as an inhibitory receptor in humans and mice, is expressed throughout B cell development [46]. A study of Fcgr2b-conditional KO mice demonstrated that Fcgr2b regulates autoantibody responses by limiting marginal zone B cell activation [46]. Fcgr2b has also been reported to control antibody-mediated target cell depletion through three therapeutically relevant surface receptors (CD20, CD25, and OX40), rather than immunoreceptor tyrosine-based inhibition motif (ITIM) signaling [47]. For SLAMF1, it has been demonstrated that human neutrophils express SLAMF1 upon Mycobacterium tuberculosis (Mtb)-stimulation and the activation of SLAM1 induces neutrophil autophagy, which indicates that SLAMF1 participates in neutrophil autophagy during active tuberculosis induced by Mtb [48]. It has been reported that a depletion of SLAMF1 suppressed autophagy and induced apoptosis in methotrexate (MTX)-treated JEG3/MTX and JAR/MTX cells, indicating that SLAMF1 might promote MTX resistance via activating protective autophagy in choriocarcinoma cell lines [49]. The CD3 epsilon subunit of the T-cell receptor complex (CD3E), which, together with CD3-gamma, -delta and -zeta, and the T-cell receptor alpha/beta and gamma/delta heterodimers, forms the T-cell receptor-CD3 complex. This complex plays an important role in coupling antigen recognition with several intracellular signal-transduction pathways [50]. The C-reactive protein (CRP) is an acute-phase protein that can be used as an early diagnostic marker for inflammation, which is evolutionarily conserved and has been identified in a range of organisms, from arthropods to mammals [51,52]. A study of Nile tilapia (Oreochromis niloticus) indicated that the recombinant protein of CRP improved the phagocytic activity of monocytes/macrophages, and possessed a bacterial agglutination activity in a calcium-dependent manner [52]. In our future study, we will focus on confirmation of the interactions between the DElncRNAs and these core target genes, as well as a functional validation of FCGR2B, SLAMF1, CD3E, and CRP in the pathogenesis of G. parasuis-induced meningitis.
5. Conclusions
Taken together, our results suggest that G. parasuis could modulate lncRNA expression profiles in porcine meningitis triggered by G. parasuis. Key molecular signatures and associated signaling pathways were identified. Our results enhance the knowledge of the functions of lncRNAs during meningitis elicited by G. parasuis, which might lay a theoretical foundation for finding novel therapeutic targets for bacterial meningitis treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres14030097/s1, Table S1: Differentially expressed lncRNAs in G. parasuis-infected porcine brain; Table S2: Predicted target genes of lncRNAs by cis; Table S3: Predicted target genes of lncRNAs by trans; Table S4: Predicted target genes of DElncRNAs in G. parasuis-infected porcine brain; Table S5: Differentially expressed target genes of DElncRNAs in G. parasuis-infected porcine brain; Table S6: Genes in protein-protein interactions.
Author Contributions
L.G. and Y.Q. conceived and designed the experiments; P.S., Y.Y. and H.C. (Hongxing Cheng) performed the experiments; Y.Y., S.F., Y.L., Y.Q., H.C. (Hongbo Chen), J.Z., H.Z., L.S., H.R. and Z.C. analyzed the data; P.S., Y.Y. and L.G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Hubei Provincial Natural Science Funding (No. 2022CFB418), the National Natural Science Foundation of China (grant no. 32273067), Hubei Key Laboratory of Animal Nutrition and Feed Science, Wuhan Polytechnic University (No. 202301), Key Laboratory of Tropical Animal Breeding and Disease Research (HKL202303), and Key Laboratory of Animal Embryo Engineering and Molecular Breeding of Hubei Province (KLAEMB-2023-02).
Institutional Review Board Statement
The animal experiments and animal care were approved by the Animal Care and Use Committee of Wuhan Polytechnical University, Hubei Province, China (EM316, 15 October 2019).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
References
- Guo, L.; Cheng, H.; Fu, S.; Liu, J.; Zhang, Y.; Qiu, Y.; Chen, H. Methylome and Transcriptome-Based Integration Analysis Identified Molecular Signatures Associated with Meningitis Induced by Glaesserella parasuis. Front. Immunol. 2022, 13, 840399. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Rafiee, M.; Blackall, P.J. Establishment, validation and use of the Kielstein-Rapp-Gabrielson serotyping scheme for Haemophilus parasuis. Aust. Vet. J. 2000, 78, 172–174. [Google Scholar] [CrossRef]
- Qi, B.; Li, F.; Chen, K.; Ding, W.; Xue, Y.; Wang, Y.; Wang, H.; Ding, K.; Zhao, Z. Comparison of the Glaesserella parasuis Virulence in Mice and Piglets. Front. Vet. Sci. 2021, 8, 659244. [Google Scholar] [CrossRef]
- Vanier, G.; Szczotka, A.; Friedl, P.; Lacouture, S.; Jacques, M.; Gottschalk, M. Haemophilus parasuis invades porcine brain microvascular endothelial cells. Microbiology 2006, 152, 135–142. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, Y.; Chen, C.; Yang, D.; Ding, T.; Zhu, L.; Deng, J.; Xu, Z. The Triangle Relationship Between Long Noncoding RNA, RIG-I-like Receptor Signaling Pathway, and Glycolysis. Front. Microbiol. 2021, 12, 807737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Yang, D.; Xie, F.; Wang, Z. The role of long non-coding RNAs in angiogenesis and anti-angiogenic therapy resistance in cancer. Mol. Ther. Nucleic Acids 2022, 28, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Zhang, Y.; Yang, J.; Li, Z.; Wu, L.; Wu, J.; Wu, N.; Liu, L.; Liu, Z.; et al. Dynamic characteristics and functional analysis provide new insights into long non-coding RNA responsive to Verticillium dahliae infection in Gossypium hirsutum. BMC Plant Biol. 2021, 21, 68. [Google Scholar] [CrossRef]
- Huang, W.; Zhong, W.; He, Q.; Xu, Y.; Lin, J.; Ding, Y.; Zhao, H.; Zheng, X.; Zheng, Y. Time-series expression profiles of mRNAs and lncRNAs during mammalian palatogenesis. Oral Dis. 2023, 29, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.H.; Guo, Z.B.; Zhou, Y.Y.; Wang, C.; Yin, R.L.; Bai, W.L. LncRNA-MEG3 Regulates the Inflammatory Responses and Apoptosis in Porcine Alveolar Macrophages Infected with Haemophilus parasuis Through Modulating the miR-210/TLR4 Axis. Curr. Microbiol. 2021, 78, 3152–3164. [Google Scholar] [CrossRef]
- Dong, J.; Teng, F.; Guo, W.; Yang, J.; Ding, G.; Fu, Z. lncRNA SNHG8 Promotes the Tumorigenesis and Metastasis by Sponging miR-149-5p and Predicts Tumor Recurrence in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2018, 51, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, R.; Yang, B.; Li, L.; Chen, J.; Fu, J.; Qu, X.; Huo, D.; Tan, C.; Chen, H.; et al. Long non-coding RNA lncC11orf54-1 modulates neuroinflammatory responses by activating NF-κB signaling during meningitic Escherichia coli infection. Mol. Brain 2022, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Akavaram, S.; Bayles, D.O. Genomewide transcriptional response of Escherichia coli O157:H7 to norepinephrine. BMC Genom. 2022, 23, 107. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.M.; Zhang, Z.; Liu, J.B.; Li, N.; Yang, G.W.; Luo, D.; Zhang, Y.; Yuan, B.; Jiang, H.; Zhang, J.B. Genome-wide identification and analysis of long noncoding RNAs in longissimus muscle tissue from Kazakh cattle and Xinjiang brown cattle. Anim. Biosci. 2021, 34, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Transcriptome dataset of Babesia bovis life stages within vertebrate and invertebrate hosts. Data Brief 2020, 33, 106533. [Google Scholar] [CrossRef] [PubMed]
- Osabe, T.; Shimizu, K.; Kadota, K. Differential expression analysis using a model-based gene clustering algorithm for RNA-seq data. BMC Bioinform. 2021, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Li, J.; Bao, H.; Wu, C. Genome-Wide Association Study and Transcriptome Differential Expression Analysis of the Feather Rate in Shouguang Chickens. Front. Genet. 2020, 11, 613078. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q.; Fan, X. Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. PLoS ONE 2017, 12, e0172389. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, Y.; Li, Y.; Cheng, Z.M.; Zhong, Y. Genome-wide identification of the HKT genes in five Rosaceae species and expression analysis of HKT genes in response to salt-stress in Fragaria vesca. Genes Genom. 2019, 41, 325–336. [Google Scholar] [CrossRef]
- Liu, X.Q.; Li, B.X.; Zeng, G.R.; Liu, Q.Y.; Ai, D.M. Prediction of Long Non-Coding RNAs Based on Deep Learning. Genes 2019, 10, 273. [Google Scholar] [CrossRef]
- Guo, J.C.; Fang, S.S.; Wu, Y.; Zhang, J.H.; Chen, Y.; Liu, J.; Wu, B.; Wu, J.R.; Li, E.M.; Xu, L.Y.; et al. CNIT: A fast and accurate web tool for identifying protein-coding and long non-coding transcripts based on intrinsic sequence composition. Nucleic Acids Res. 2019, 47, W516–W522. [Google Scholar] [CrossRef]
- Feng, X.; Han, H.; Guo, Y.; Feng, X.; Guo, S.; Zhou, W. LncRNA ENST869 Targeting Nestin Transcriptional Region to Affect the Pharmacological Effects of Chidamide in Breast Cancer Cells. Front. Oncol. 2022, 12, 874343. [Google Scholar] [CrossRef]
- He, L.; Yan, X.; Dai, K.; Wen, X.; Cao, S.; Huang, X.; Wu, R.; Zhao, Q.; Huang, Y.; Yan, Q.; et al. Comparative transcriptome analysis reveals that deletion of CheY influences gene expressions of ABC transports and metabolism in Haemophilus parasuis. Funct. Integr. Genom. 2021, 21, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Iannello, A.; Ciarrocchi, A.; Fragliasso, V.; Vaisitti, T. Lift the curtain on long non-coding RNAs in hematological malignancies: Pathogenic elements and potential targets. Cancer Lett. 2022, 536, 215645. [Google Scholar] [CrossRef] [PubMed]
- Espinal, E.R.; Matthews, T.; Holder, B.M.; Bee, O.B.; Humber, G.M.; Brook, C.E.; Divyapicigil, M.; Sharp, J.; Kim, B.J. Group B Streptococcus-Induced Macropinocytosis Contributes to Bacterial Invasion of Brain Endothelial Cells. Pathogens 2022, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, P.; Satchell, S.C.; Ramnath, R. Cerebral microvascular endothelial glycocalyx damage, its implications on the blood-brain barrier and a possible contributor to cognitive impairment. Brain Res. 2022, 1780, 147804. [Google Scholar] [CrossRef]
- Ghahramani Almanghadim, H.; Ghorbian, S.; Khademi, N.S.; Soleymani Sadrabadi, M.; Jarrahi, E.; Nourollahzadeh, Z.; Dastani, M.; Shirvaliloo, M.; Sheervalilou, R.; Sargazi, S. New Insights into the Importance of Long Non-Coding RNAs in Lung Cancer: Future Clinical Approaches. DNA Cell Biol. 2021, 40, 1476–1494. [Google Scholar] [CrossRef]
- Sadrkhanloo, M.; Entezari, M.; Orouei, S.; Zabolian, A.; Mirzaie, A.; Maghsoudloo, A.; Raesi, R.; Asadi, N.; Hashemi, M.; Zarrabi, A.; et al. Targeting Nrf2 in ischemia-reperfusion alleviation: From signaling networks to therapeutic targeting. Life Sci. 2022, 300, 120561. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, B.; Yang, R.; Fu, J.; Li, L.; Huo, D.; Chen, J.; Yang, X.; Tan, C.; Chen, H.; et al. Long Non-coding Antisense RNA DDIT4-AS1 Regulates Meningitic Escherichia coli-Induced Neuroinflammation by Promoting DDIT4 mRNA Stability. Mol. Neurobiol. 2022, 59, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, R.; Fu, J.; Yang, B.; Chen, J.; Tan, C.; Chen, H.; Wang, X. LncRSPH9-4 Facilitates Meningitic Escherichia coli-Caused Blood-Brain Barrier Disruption via miR-17-5p/MMP3 Axis. Int. J. Mol. Sci. 2021, 22, 6343. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Xie, X.; Zhou, J.; Fang, X.; Wang, F.; Wang, M. Identification of TAF1, SAT1, and ARHGEF9 as DNA methylation biomarkers for hepatocellular carcinoma. J. Cell Physiol. 2020, 235, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.E.; Martin-Ramirez, J.; Boross, P.; Mangsbo, S.M.; Reynolds, J.; Moss, J.; Pusey, C.D.; Cook, H.T.; Tarzi, R.M.; Verbeek, J.S. Increased incidence of anti-GBM disease in Fcgamma receptor 2b deficient mice, but not mice with conditional deletion of Fcgr2b on either B cells or myeloid cells alone. Mol. Immunol. 2012, 50, 49–56. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, J.; Luo, H.; Urbonaviciute, V.; Xu, Z.; He, C.; Holmdahl, R. Two major genes associated with autoimmune arthritis, Ncf1 and Fcgr2b, additively protect mice by strengthening T cell tolerance. Cell Mol. Life Sci. 2022, 79, 482. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.; Glass, L.J.; Rothmond, D.A.; Purves-Tyson, T.; Sweeney, A.; Kondo, Y.; Kubo, S.; Matsumoto, M.; Weickert, C.S. Increased levels of a pro-inflammatory IgG receptor in the midbrain of people with schizophrenia. J. Neuroinflamm. 2022, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, J.; Li, Y.; Zhao, R.; Du, S.; Lv, C.; Wu, W.; Liu, R.; Sheng, X.; Song, Y.; et al. MicroRNA-31 Reduces Inflammatory Signaling and Promotes Regeneration in Colon Epithelium, and Delivery of Mimics in Microspheres Reduces Colitis in Mice. Gastroenterology 2019, 156, 2281–2296.e2286. [Google Scholar] [CrossRef] [PubMed]
- Beristain-Covarrubias, N.; Canche-Pool, E.B.; Ramirez-Velazquez, C.; Barragan-Galvez, J.C.; Gomez-Diaz, R.A.; Ortiz-Navarrete, V. Class I-Restricted T Cell-Associated Molecule Is a Marker for IFN-γ-Producing iNKT Cells in Healthy Subjects and Patients with Type 1 Diabetes. J. Interferon Cytokine Res. 2017, 37, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, A.; Nuccio, S.P.; Ushach, I.; Edwards, R.A.; Pahu, R.; Silva, S.; Zlotnik, A.; Raffatellu, M. CRTAM Shapes the Gut Microbiota and Enhances the Severity of Infection. J. Immunol. 2019, 203, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, M.; Skjesol, A.; Ryan, L.; Richard, G.M.; Kandasamy, R.K.; Wang, N.; Terhorst, C.; Husebye, H.; Espevik, T. SLAMF1 is required for TLR4-mediated TRAM-TRIF-dependent signaling in human macrophages. J. Cell Biol. 2018, 217, 1411–1429. [Google Scholar] [CrossRef]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; Kalla, R.; Adams, A.T.; Keita, Å.V.; D’Amato, M.; Gomollon, F.; et al. Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021, 161, 1526–1539.e1529. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1602–1616.e1606. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, Z.; Zou, Y.; Tian, Q.; Han, S.; Xu, Z.; Liao, J.; Gao, L.; Chen, Q.; Li, M. Tetramethylpyrazine attenuates blood-brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway. Eur. J. Pharmacol. 2019, 854, 289–297. [Google Scholar] [CrossRef] [PubMed]
- King-Robson, J.; Hampton, T.; Rosadas, C.; Taylor, G.P.; Stanton, B. HTLV-1 encephalitis. Pract. Neurol. 2022, 22, 60–63. [Google Scholar] [CrossRef]
- Moles, R.; Sarkis, S.; Galli, V.; Omsland, M.; Artesi, M.; Bissa, M.; McKinnon, K.; Brown, S.; Hahaut, V.; Washington-Parks, R.; et al. NK cells and monocytes modulate primary HTLV-1 infection. PLoS Pathog. 2022, 18, e1010416. [Google Scholar] [CrossRef] [PubMed]
- Oppegaard, K.; Harris, C.S.; Shin, J.; Paul, S.M.; Cooper, B.A.; Chan, A.; Anguera, J.A.; Levine, J.; Conley, Y.; Hammer, M.; et al. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine 2021, 148, 155653. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lu, Y.; Sun, W.; Han, M.; Zhang, Y.; Zhang, J. Changing expression profiles of lncRNAs, circRNAs and mRNAs in esophageal squamous carcinoma. Oncol. Lett. 2019, 18, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Barlev, A.N.; Malkiel, S.; Kurata-Sato, I.; Dorjée, A.L.; Suurmond, J.; Diamond, B. FcγRIIB regulates autoantibody responses by limiting marginal zone B cell activation. J. Clin. Investig. 2022, 132, e157250. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.P.; Roghanian, A.; Oldham, R.J.; Chan, H.T.C.; Penfold, C.A.; Kim, H.J.; Inzhelevskaya, T.; Mockridge, C.I.; Cox, K.L.; Bogdanov, Y.D.; et al. FcγRIIB controls antibody-mediated target cell depletion by ITIM-independent mechanisms. Cell Rep. 2022, 40, 111099. [Google Scholar] [CrossRef]
- Pellegrini, J.M.; Sabbione, F.; Morelli, M.P.; Tateosian, N.L.; Castello, F.A.; Amiano, N.O.; Palmero, D.; Levi, A.; Ciallella, L.; Colombo, M.I.; et al. Neutrophil autophagy during human active tuberculosis is modulated by SLAMF1. Autophagy 2021, 17, 2629–2638. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, Y.; Tian, Y. SLAMF1 promotes methotrexate resistance via activating autophagy in choriocarcinoma cells. Cancer Manag. Res. 2021, 12, 13427–13436. [Google Scholar] [CrossRef]
- Nieves, D.J.; Pandzic, E.; Gunasinghe, S.D.; Goyette, J.; Owen, D.M.; Justin Gooding, J.; Gaus, K. The T cell receptor displays lateral signal propagation involving non-engaged receptors. Nanoscale 2022, 14, 3513–3526. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, A. Evolution of C-reactive protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Cai, J.; Huang, Y.; Jian, J. CRP involved in Nile tilapia (Oreochromis niloticus) against bacterial infection. Biology 2022, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).