Trichoderma Species Problematic to the Commercial Production of Pleurotus in Italy: Characterization, Identification, and Methods of Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation from Oyster Mushroom Production Chain
2.2. Effect of Temperature on Fungal Growth
2.3. Effect of pH on Fungal Growth
2.4. Genetic Characterization
2.5. Dual Confrontation Assays
2.6. Effect of Fungicides on Fungal Growth
2.7. Statistical Analyses
3. Results
3.1. Determination of Microbial Concentration
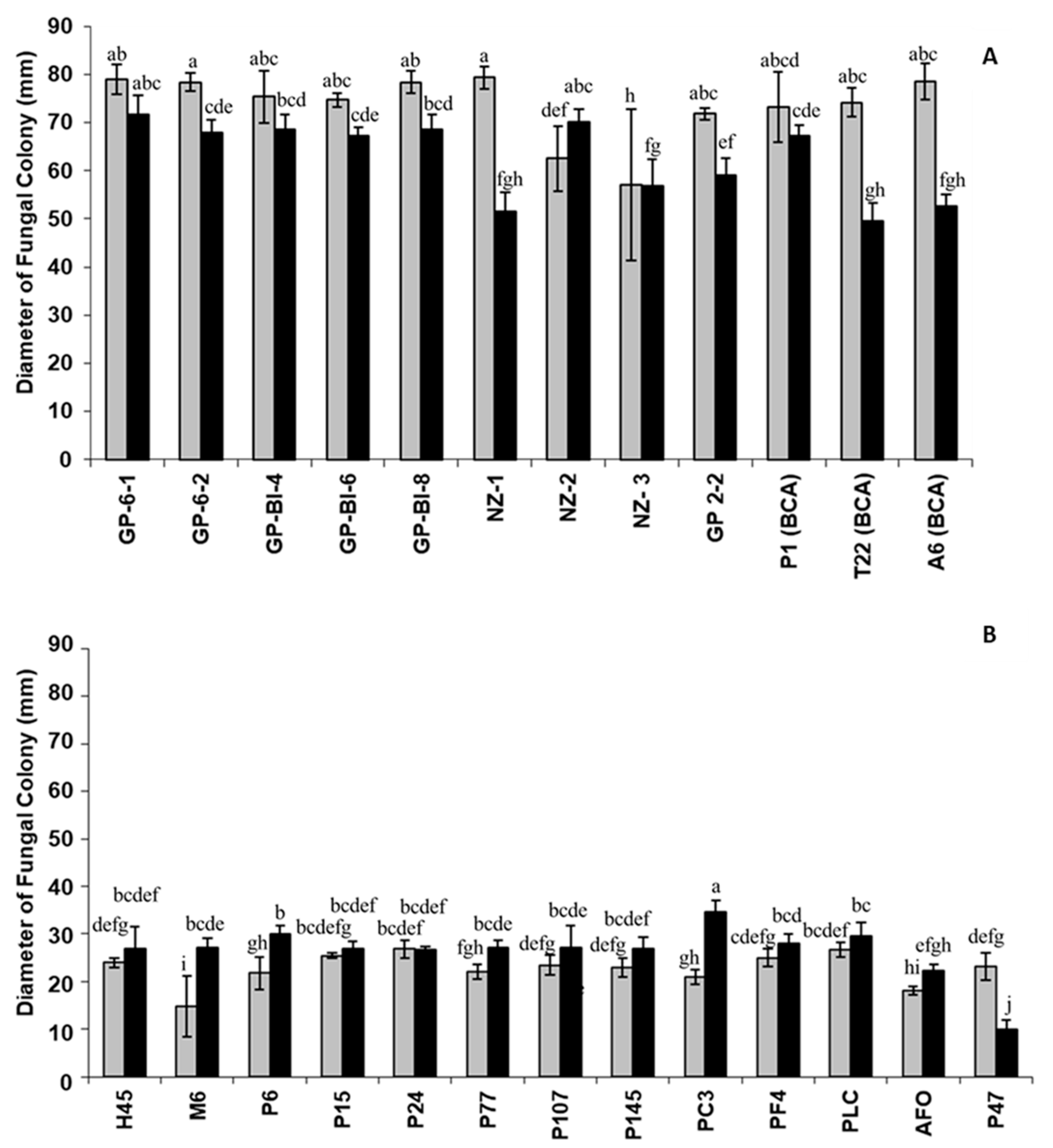
3.2. Fungal Growth and Morphological Characterization
3.3. Genetic and Morphological Characterization of Trichoderma Isolates
3.4. Treatment with Chemical Fungicides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, D.; Eveleigh, D.E. Ecology of Trichoderma. Trichoderma and Gliocladium; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis Ltd.: London, UK, 1998; Volume 1, pp. 57–74. [Google Scholar]
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L. Trichoderma: A Multi-Purpose Tool for Integrated Pest Management. In Principles of Plant-Microbe Interactions; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–353. [Google Scholar]
- Ghazanfar, M.U.; Raza, M.; Raza, W.; Qamar, M.I. Trichoderma as Potential Biocontrol Agent, Its Exploitation in Agriculture: A Review. Plant Prot. 2018, 2, 109–135. [Google Scholar]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological Functions of Trichoderma spp. for Agriculture Applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Seaby, D.A. Differentiation of Trichoderma Taxa Associated with Mushroom Production. Plant Pathol. 1996, 45, 905–912. [Google Scholar] [CrossRef]
- Ospina-Giraldo, M.D.; Royse, D.J.; Chen, X.; Romaine, C.P. Molecular Phylogenetic Analyses of Biological Control Strains of Trichoderma harzianum and Other Biotypes of Trichoderma spp. Associated with Mushroom Green Mold. Phytopathology 1999, 89, 308–313. [Google Scholar] [CrossRef]
- Royse, D.J.; Boomer, K.; Du, Y.; Handcock, M.; Coles, P.S.; Romaine, C.P. Spatial Distribution of Green Mold Foci in 30 Commercial Mushroom Crops. Plant Dis. 1999, 83, 71–76. [Google Scholar] [CrossRef]
- Mamoun, M.L.; Savoie, J.-M.; Olivier, J.-M. Interactions between the Pathogen Trichoderma harzianum Th2 and Agaricus bisporus in Mushroom Compost. Mycologia 2000, 92, 233–240. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Kilpatrick, M.; Ward, F.; Lyons, G.; Burns, L. Colonisation of Phase II Compost by Biotypes of Trichoderma harzianum and Their Effect on Mushroom Yield and Quality. Appl. Microbiol. Biotechnol. 1999, 51, 572–578. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma Species Associated with the Green Mold Epidemic of Commercially Grown Agaricus bisporus. Mycologia 2002, 94, 146–170. [Google Scholar] [CrossRef] [PubMed]
- Seaby, D.; Harman, G.E. Trichoderma and Gliocladium; Harman, G.E., Kubicek, C.P., Eds.; CRC Press: Boca Raton, FL, USA, 1998; Volume 2, ISBN ISBN 9780429079641. [Google Scholar]
- Rinker, D.L.; Alm, G. Investigations of Factors Influencing the Expression of Green Mould. Mushroom World 1997, 8, 25–29. [Google Scholar]
- Seaby, D.A. Infection of Mushroom Compost by Trichoderma Species. Mushroom J. 1987, 179, 355–361. [Google Scholar]
- Woo, S.L.; Di Benedetto, P.; Senatore, M.; Abadi, K.; Gigante, S.; Soriente, I.; Ferraioli, S.; Lorito, M. Identification and Characterization of Trichoderma Species Aggressive to Pleurotus in Italy. J. Zhejiang Univ. Agric. Life Sci. 2004, 30, 469–470. [Google Scholar]
- Allaga, H.; Zhumakayev, A.; Büchner, R.; Kocsubé, S.; Szűcs, A.; Vágvölgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum Species Complex with Mushroom Pathogenic Potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- Hermosa, M.R.; Grondona, I.; Iturriaga, E.A.; Diaz-Minguez, J.M.; Castro, C.; Monte, E.; Garcia-Acha, I. Molecular Characterization and Identification of Biocontrol Isolates of Trichoderma spp. Appl. Environ. Microbiol. 2000, 66, 1890–1898. [Google Scholar] [CrossRef]
- Largentau-Mamoun, M.L.; Mata, G.; Savoie, J.M. Green Mold Disease: Adaptation of Trichoderma harzianum Th2 to Mushroom Compost. In Mushroom Biology and Mushroom Products; Sanchez, E., Huerta, G., Mondel, E., Eds.; Universidad Autonoma Del Estado De Morelos: Cuernavaca, Mexico, 2002; Volume 4. [Google Scholar]
- Elbagory, M.; El-Nahrawy, S.; Omara, A.E.-D.; Eid, E.M.; Bachheti, A.; Kumar, P.; Abou Fayssal, S.; Adelodun, B.; Bachheti, R.K.; Kumar, P.; et al. Sustainable Bioconversion of Wetland Plant Biomass for Pleurotus ostreatus Var. Florida Cultivation: Studies on Proximate and Biochemical Characterization. Agriculture 2022, 12, 2095. [Google Scholar] [CrossRef]
- Werghemmi, W.; Abou Fayssal, S.; Mazouz, H.; Hajjaj, H.; Hajji, L. Olive and Green Tea Leaves Extract in Pleurotus ostreatus Var. Florida Culture Media: Effect on Mycelial Linear Growth Rate, Diameter and Growth Induction Index. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012020. [Google Scholar] [CrossRef]
- Kredics, L.; Naeimi, S.; Hatvani, L.; Vágvölgyi, C.; Cai, F.; Druzhinina, I.S.; Manczinger, L. ‘The Good, the Bad and the Ugly’ in the Shades of Green: The Genus Trichoderma in the Spotlight. Indian Phytopathol. 2021, 74, 403–411. [Google Scholar] [CrossRef]
- Sánchez-Montesinos, B.; Santos, M.; Moreno-Gavíra, A.; Marín-Rodulfo, T.; Gea, F.J.; Diánez, F. Biological Control of Fungal Diseases by Trichoderma aggressivum f. europaeum and Its Compatibility with Fungicides. J. Fungi 2021, 7, 598. [Google Scholar] [CrossRef]
- Muthumeenakshi, S.; Mills, P.R.; Brownd, A.E.; Seaby, D.A. Intraspecific Molecular Variation among Trichoderma harzianum Isolates Colonizing Mushroom Compost in the British Isles. Microbiology 1994, 140, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, M.R.; Grondona, I.; Monte, E. Isolation of Trichoderma harzianum Th2 from Commercial Mushroom Compost in Spain. Plant Dis. 1999, 83, 591. [Google Scholar] [CrossRef]
- Chen, X.; Romaine, C.P.; Ospina-Giraldo, M.D.; Royse, D.J. A Polymerase Chain Reaction-Based Test for the Identification of Trichoderma harzianum Biotypes 2 and 4, Responsible for the Worldwide Green Mold Epidemic in Cultivated Agaricus bisporus. Appl. Microbiol. Biotechnol. 1999, 52, 246–250. [Google Scholar] [CrossRef]
- Chen, X.; Romaine, C.P.; Tan, Q.; Schlagnhaufer, B.; Ospina-Giraldo, M.D.; Royse, D.J.; Huff, D.R. PCR-Based Genotyping of Epidemic and Preepidemic Trichoderma Isolates Associated with Green Mold of Agaricus bisporus. Appl. Environ. Microbiol. 1999, 65, 2674–2678. [Google Scholar] [CrossRef]
- Park, M.S.; Bae, K.S.; Yu, S.H. Two New Species of Trichoderma Associated with Green Mold of Oyster Mushroom Cultivation in Korea. Mycobiology 2006, 34, 111. [Google Scholar] [CrossRef]
- Komoń-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically Closely Related but Phenotypically Divergent Trichoderma Species Cause Green Mold Disease in Oyster Mushroom Farms Worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-S.; Seo, G.-S.; Lee, K.-H.; Bae, K.-S.; Yu, S.-H. Morphological and Cultural Characteristics of Trichoderma spp. Associated with Green Mold of Oyster Mushroom in Korea. Plant Pathol. J. 2005, 21, 221–228. [Google Scholar] [CrossRef]
- Park, M.S.; Seo, G.S.; Lee, K.H.; Bae, K.S.; Yu, S.H. Characterization of Trichoderma spp. Associated with Green Mold of Oyster Mushroom by PCR-RFLP and Sequence Analysis of ITS Regions of rDNA. Plant Pathol. J. 2005, 21, 229–236. [Google Scholar] [CrossRef]
- Nongthombam, J.; Kumar, A.; Ladli, B.; Madhushekhar, M.; Patidar, S. A Review on Study of Growth and Cultivation of Oyster Mushroom. Plant Cell Biotech. Mol. Biol. 2021, 22, 55–65. [Google Scholar]
- Bellettini, M.B.; Bellettini, S.; Fiorda, F.A.; Pedro, A.C.; Bach, F.; Fabela-Morón, M.F.; Hoffmann-Ribani, R. Diseases and Pests Noxious to Pleurotus spp. Mushroom Crops. Rev. Argent. Microbiol. 2018, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Kredics, L.; Kocsubé, S.; Nagy, L.; Komoń-Zelazowska, M.; Manczinger, L.; Sajben, E.; Nagy, A.; Vágvölgyi, C.; Kubicek, C.P.; Druzhinina, I.S.; et al. Molecular Identification of Trichoderma Species Associated with Pleurotus ostreatus and Natural Substrates of the Oyster Mushroom. FEMS Microbiol. Lett. 2009, 300, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gams, W.; Bissett, J. Morphology and Identification of Trichoderma. In Trichoderma and Gliocladium; Volume 1: Basic Biology, Taxonomy and Genetics; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis Ltd.: London, UK, 1998; pp. 3–34. [Google Scholar]
- Manganiello, G.; Nicastro, N.; Caputo, M.; Zaccardelli, M.; Cardi, T.; Pane, C. Functional Hyperspectral Imaging by High-Related Vegetation Indices to Track the Wide-Spectrum Trichoderma Biocontrol Activity Against Soil-Borne Diseases of Baby-Leaf Vegetables. Front. Plant. Sci. 2021, 12, 630059. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, G.; Liu, Y.; Ye, H.; Qi, X.; Wang, Q. Residual Behavior and Risk Assessment of Prochloraz in Bayberries and Bayberry Wine for the Chinese Population. Environ. Monit. Assess. 2019, 191, 644. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Rakhmonov, U.; Soatov, T. Harmful Competitors and Diseases of Pleurotus ostreatus and Their Control Measures. E3S Web Conf. 2023, 389, 03101. [Google Scholar] [CrossRef]
- Rinker, D.L.; Alm, G. Effectiveness of Benomyl-Coated Spawn against Green Mould Disease. Mushroom World 1998, 9, 15–20. [Google Scholar]
- Chang, S.T.; Miles, P.G. Mushrooms Cultivation, Nutritional Value, Medicinal Effect, and Environmental Effect; CRC Press LLC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Gaze, R.H.; Fletcher, J.T. Mushroom Pest and Disease Control: A Color Handbook; Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- Grogan, H.M.; Gaze, R.H. Growth of Trichoderma Harzianum in Traditional and Experimental Composts; Elliott, T.J., Ed.; Balkema: Rotterdam, The Netherlands, 1995. [Google Scholar]
- Luković, J.; Milijašević-Marčić, S.; Hatvani, L.; Kredics, L.; Szűcs, A.; Vágvölgyi, C.; Duduk, N.; Vico, I.; Potočnik, I. Sensitivity of Trichoderma Strains from Edible Mushrooms to the Fungicides Prochloraz and Metrafenone. J. Environ. Sci. Health Part B 2021, 56, 54–63. [Google Scholar] [CrossRef] [PubMed]




 = highest dose,
= highest dose,  = second dose,
= second dose,  = third dose,
= third dose,  = lowest dose tested) of the active ingredient in 1 L of water, on the mycelial growth of Trichoderma isolates three days after treatment with (A) prochloraz (1, 0.7, 0.5, 0.2 mL) and (B) metrafenone (10, 7, 5, 2.5 μL). Fungal growth was measured on a five-value scale, where 0 = no apparent growth of the inoculum, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100% fungal colony development with respect to the control (PDB).
= lowest dose tested) of the active ingredient in 1 L of water, on the mycelial growth of Trichoderma isolates three days after treatment with (A) prochloraz (1, 0.7, 0.5, 0.2 mL) and (B) metrafenone (10, 7, 5, 2.5 μL). Fungal growth was measured on a five-value scale, where 0 = no apparent growth of the inoculum, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100% fungal colony development with respect to the control (PDB).
 = highest dose,
= highest dose,  = second dose,
= second dose,  = third dose,
= third dose,  = lowest dose tested) of the active ingredient in 1 L of water, on the mycelial growth of Trichoderma isolates three days after treatment with (A) prochloraz (1, 0.7, 0.5, 0.2 mL) and (B) metrafenone (10, 7, 5, 2.5 μL). Fungal growth was measured on a five-value scale, where 0 = no apparent growth of the inoculum, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100% fungal colony development with respect to the control (PDB).
= lowest dose tested) of the active ingredient in 1 L of water, on the mycelial growth of Trichoderma isolates three days after treatment with (A) prochloraz (1, 0.7, 0.5, 0.2 mL) and (B) metrafenone (10, 7, 5, 2.5 μL). Fungal growth was measured on a five-value scale, where 0 = no apparent growth of the inoculum, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100% fungal colony development with respect to the control (PDB).
| Production Phase | Bacteria (CFU g−1) | Fungi (CFU g−1) | Presence of Trichoderma | |
|---|---|---|---|---|
| 1 | Uninoculated—crude substrate material | 5.1 × 109 | 5.2 × 106 | Yes |
| 2 | Fermentation tunnel | 9.5 × 1011 | 2.9 × 108 | Yes |
| 3 | After treatment in a pasteurization tunnel | 2.4 × 108 | 0 | No |
| 4 | Seeding of grain spawn, bale packaging | 2.5 × 108 | 0 | No |
| 5 | Inoculated bale, incubation at 25 °C | 6.9 × 108 | 7.1 × 102 | Yes |
| 6 | Fruiting stage, incubation at 25 °C in a mushroom grow house | 7.1 × 108 | 5.8 × 108 | Yes |
| 7 | Recycled water used in production | 7.6 × 107 | 9.2 × 104 | Yes |
| Species (Biotype) | Isolate Code | Source | Geographic Origin | Fungal Source, Compost, Phase of Production | GenBank Number ITS | GenBank Number EF 1α | GenBank Number RPB-II |
|---|---|---|---|---|---|---|---|
| T. atroviride | P1 | NAP | Norway | Biological control strain | OQ360634 | OR146259 | OR146262 |
| T. atroviride | A6 | NAP | Italy | Biological control strain | OR045890 | OR146261 | OR146263 |
| T. afroharzianum | T22 | NAP | USA | Biological control strain (ATCC20847) | OQ360633 | OR146260 | OR146264 |
| T. harzianum | GP-2-2 | This study | Italy | Pleurotus: recycled water | OQ002336 | OQ026348 | OQ026374 |
| T. pleuroticola | GP-6-1 | This study | Italy | Pleurotus: 20 days after seeding | OQ002319 | OQ026371 | OQ026387 |
| T. pleuroticola | GP-6-2 | This study | Italy | Pleurotus: 20 days after seeding | OQ002330 | OQ026370 | OQ026383 |
| T. pleuroticola | GP-BI-4 | This study * | Italy | Pleurotus: 20 days after seeding | OQ002335 | OQ026366 | OQ029696 |
| T. pleuroticola | GP-BI-6 | This study * | Italy | Pleurotus: 20 days after seeding | OQ002331 | OQ026364 | OQ026385 |
| T. pleuroticola | GP-BI-8 | This study | Italy | Pleurotus: 20 days after seeding | OQ002321 | OQ026362 | OQ029698 |
| T. pleuroticola | NZ-1 | This study * | Italy | Pleurotus: infected compost | OQ002327 | OQ026360 | OQ029699 |
| T. pleuroticola | NZ-2 | This study * | Italy | Pleurotus: infected compost | OQ002328 | OQ026359 | OQ029700 |
| T. pleuroticola | NZ-3 | This study * | Italy | Pleurotus: in fruitification | OQ002326 | OQ026358 | OQ029701 |
| Fungal confrontation | Summary | Adjusted p Value | % Inhibition |
|---|---|---|---|
| T22 vs. B. cinerea | ns | 0.3195 | |
| GP-6-2 vs. B. cinerea | ** | 0.0022 | −12% |
| P1 vs. B. cinerea | *** | 0.0002 | −15% |
| NZ-2 vs. B. cinerea | *** | 0.0001 | −16% |
| T22 vs. F. oxysporum | ns | 0.659 | |
| GP-6-2 vs. F. oxysporum | ** | 0.005 | −7% |
| P1 vs. F. oxysporum | ** | 0.0024 | −8% |
| NZ-2 vs. F. oxysporum | *** | 0.0004 | −9% |
| T22 vs. R. solani | ns | 0.6214 | |
| GP-6-2 vs. R. solani | ns | 0.2546 | |
| P1 vs. R. solani | * | 0.0202 | −12% |
| NZ-2 vs. R. solani | **** | <0.0001 | −25% |
| T22 vs. S. sclerotiorum | *** | 0.0004 | −13% |
| GP-6-2 vs. S. sclerotiorum | **** | <0.0001 | −19% |
| P1 vs. S. sclerotiorum | **** | <0.0001 | −26% |
| NZ-2 vs. S. sclerotiorum | **** | <0.0001 | −30% |
| Radial Growth of PC3 on Chemical Fungicide Enriched Media | Summary | Adjusted p Value |
|---|---|---|
| PC3 on prochloraz 1 mL | ** | 0.0019 |
| PC3 on prochloraz 0.7 mL | * | 0.0279 |
| PC3 on prochloraz 0.5 mL | ns | 0.1342 |
| PC3 on prochloraz 0.2 mL | ns | 0.4498 |
| PC3 on metrafenone 10 μL | ** | 0.0019 |
| PC3 on metrafenone 7 μL | ns | 0.1342 |
| PC3 on metrafenone 5 μL | ns | 0.2603 |
| PC3 on metrafenone 2.5 μL | ns | 0.2603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, N.; Pironti, A.; Manganiello, G.; Marra, R.; Vinale, F.; Vitale, S.; Lorito, M.; Woo, S.L. Trichoderma Species Problematic to the Commercial Production of Pleurotus in Italy: Characterization, Identification, and Methods of Control. Microbiol. Res. 2023, 14, 1301-1318. https://doi.org/10.3390/microbiolres14030088
Lombardi N, Pironti A, Manganiello G, Marra R, Vinale F, Vitale S, Lorito M, Woo SL. Trichoderma Species Problematic to the Commercial Production of Pleurotus in Italy: Characterization, Identification, and Methods of Control. Microbiology Research. 2023; 14(3):1301-1318. https://doi.org/10.3390/microbiolres14030088
Chicago/Turabian StyleLombardi, Nadia, Angela Pironti, Gelsomina Manganiello, Roberta Marra, Francesco Vinale, Stefania Vitale, Matteo Lorito, and Sheridan Lois Woo. 2023. "Trichoderma Species Problematic to the Commercial Production of Pleurotus in Italy: Characterization, Identification, and Methods of Control" Microbiology Research 14, no. 3: 1301-1318. https://doi.org/10.3390/microbiolres14030088
APA StyleLombardi, N., Pironti, A., Manganiello, G., Marra, R., Vinale, F., Vitale, S., Lorito, M., & Woo, S. L. (2023). Trichoderma Species Problematic to the Commercial Production of Pleurotus in Italy: Characterization, Identification, and Methods of Control. Microbiology Research, 14(3), 1301-1318. https://doi.org/10.3390/microbiolres14030088









