Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods
Abstract
1. Introduction
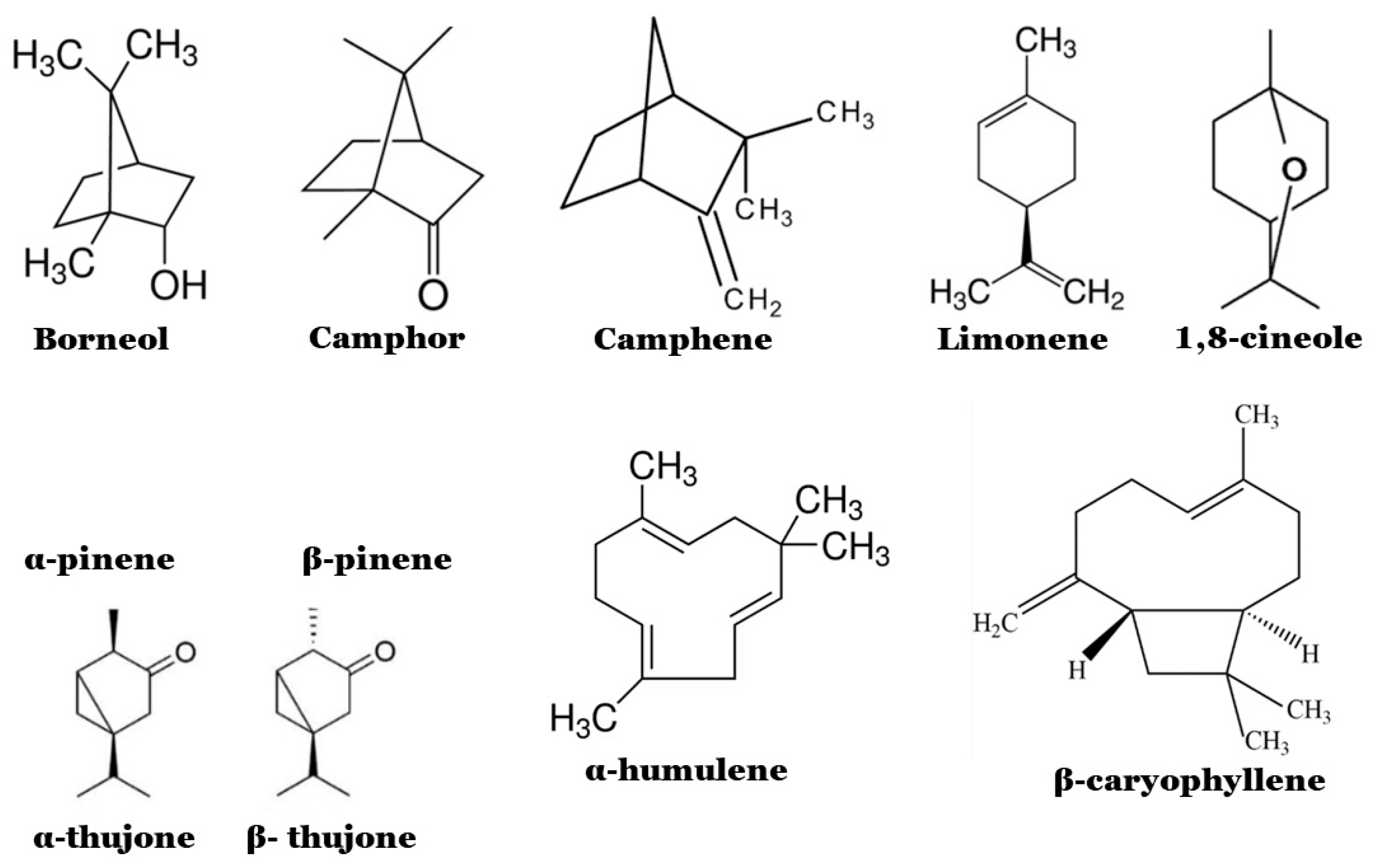
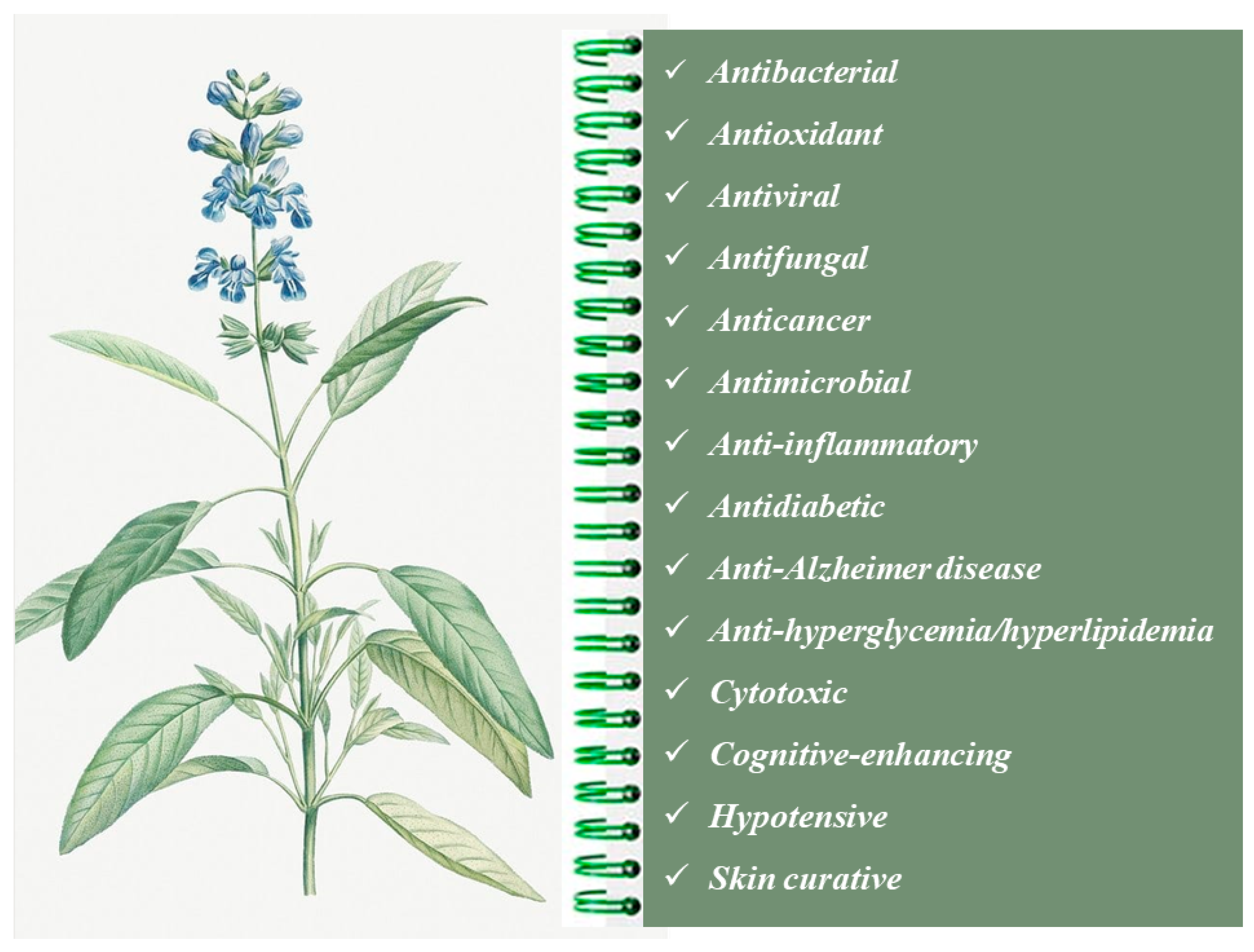
2. Salvia officinalis Essential Oils
2.1. Chemical Composition and Biological Properties
2.2. Antimicrobial Properties
| Salvia EOs Origin | Microorganisms Tested | Method Used | Main Results | Reference |
|---|---|---|---|---|
| Ethanol extract from Salvia officinalis (L.) | Staphylococcus aureus, Listeria monocytogenes, Escherichia coli | Agar disk diffusion test |
| [36] |
| S. officinalis (leave and stem) extracted by maceration (with ethyl acetate), by hydro distillation (with anhydrous sodium sulphate) and by Soxhlet (with hexane) | St. aureus, E. coli | Agar disk diffusion test |
| [37] |
| Ethanol extract of leaves of S. officinalis | Streptococcus mutans, Streptococcus mitis, Streptococcus oralis, Streptococcus salivarius, Streptococcus sanguis, Candida albicans, Candida glabrata, Candida guillermondii, Candida krusei, Candida tropicalis | Broth microdilution method | Ethanolic extract of S. officinalis L. (diluted in alcohol at 40%, at a concentration of 8 mg/mL) showed strong antibacterial activity towards Streptococcus strains (MIC ranged from 0.25 to 1 mg/mL) and moderate antifungal activity towards Candida strains (MIC = 1 mg/mL). | [38] |
| S. officinalis | 9 clinical isolates from the oral cavity of tuberculosis patients, St. aureus, Staphylococcus epidermidis, Str. mutans, Ca. albicans, Ca. tropicalis, Ca. glabrata | Broth microdilution method | 50.0 mg/mL of S. officinalis was effective against all microorganisms. 25.0 and 12.5 mg/mL were partially effective, on 58.3% and 8.3% of strains, respectively) | [39] |
| S. officinalis L. combined with thyme (Tymus vulgaris L.) | E. coli, St. aureus, Bacillus cereus, Salmonella Typhimurium | Agar well diffusion method | The thyme–sage mixture showed the highest antimicrobial activity against B. cereus (inhibition zone diameters, i.z.d. = 31.25), S. aureus (i.z.d. = 28.67), Salm. Typhimurium (i.z.d. = 23.65), E. coli (i.z.d. = 22.13) | [20] |
| Extracted and purchased S. officinalis essential oils and synergistic effect with meropenem (antibiotic) | St. aureus, L. monocytogenes, Streptococcus pyogenes, E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae | Agar well diffusion method |
| [21] |
| Ethanol extract of S. officinalis leaves | St. aureus (2 strains), E. coli (three strains), Ps. aeruginosa, Proteus mirabilis, Kl. pneumoniae, Klebsiella oxytoca, Acinetobacter baumannii, Enterobacter aerogenes, Helicobacter pylori | Agar diffusion method | The highest antimicrobial potentials were observed with the extracts dried at ambient temperature and oven at 45 °C, which inhibited eight of the tested microorganisms: two strains of E. coli, Ps. aeruginosa, two strains of St. aureus, Pr. mirabilis. Ac. baumannii, H. pylori. Salvia extract obtained from oven dried plant at 60 °C exhibited the lowest antibacterial activity | [49] |
| Steam distillate essential oil and corresponding hydrolate from S. officinalis | Isolates from wound swabs of hospitalized patients: St. aureus, Enterobacter cloacae, Ps. aeruginosa, Ca. albicans, Kl. oxytoca, E. coli Isolates from blood cultures: St. aureus, Kl. pneumoniae | Broth microdilution method |
| [50] |
| Essential oils extracted from the stem and leaves of Salvia hispanica L. plant | Two strains of Bacillus megaterium, Bacillus mojavensis, Clavibacter michiganensis, Xanthomonas campestris, Xanthomonas vesicatoria, Pseudomonas syringae pv. phaseolicola, E. coli, Burkholderia gladioli pv. agaricicola, Monilinia laxa, Monilinia fructicola, Monilinia fructigena, Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus, Penicillium digitatum, Penicillium expansum, Sclerotinia sclerotiorum, Fusarium oxysporum. | Disk diffusion method | At the highest concentration (50%) the EOs of S. hispanica showed greater antibacterial activity against Gram-positive bacteria than Gram-negative bacteria. At the highest EOs concentration (40%), the highest inhibition of fungal mycelium growth was observed as follows:
| [43] |
| Methanol extracts from 81 Salvia samples (Salvia africana-lutea; Salvia lanceolata; Salvia chamelaeagnea) | St. aureus, Ac. baumannii, B. cereus, E. coli, Enterococcus faecium, Ps. aeruginosa, Bacillus subtilis | Microdilution assay | The antibacterial activity of S. chamelaeagnea was superior to that of the other two species and was highly effective against all seven pathogens with average MICs 0.23–1.3 mg/mL; then S. africana-lutea (0.52–3.0 mg/mL) and S. lanceolata (0.46–4.2 mg/mL). | [47] |
| Aqueous, ethanolic, and methanolic extracts of Salvia argentea | Enterococcus faecalis, St. aureus, L. monocytogenes, Methicillin-resistant St. aureus (MRSA), B. subtilis, B. cereus, Pr. mirabilis, Pasteurella multocida, Salm. Typhimurium, Campylobacter fetus, E. coli, Kl. pneumoniae, En. cloacae, Citrobacter freundii, Ps. aeruginosa, Salmonella enterica, Ca. albicans (3 strains), Saccharomyces cerevisiae. | Agar diffusion and micro dilution methods |
| [51] |
| EOs obtained from the dried flowering tops of the Salvia rosmarinus Spenn. and Salvia jordanii by hydrodistillation | Yersinia enterocolitica, L. monocytogenes, Enterococcus durans, Ec. faecalis, Ec. faecium, Ca. albicans, Ca. tropicalis, Ca. guilliermondii, Ca. krusei, Ca. parapsilosis, S. cerevisiae | Agar disk diffusion and broth microdilution methods | EOs presented a moderate antibacterial activity on the bacterial strains and were not active towards yeasts. | [52] |
3. Lavandula Essential Oils
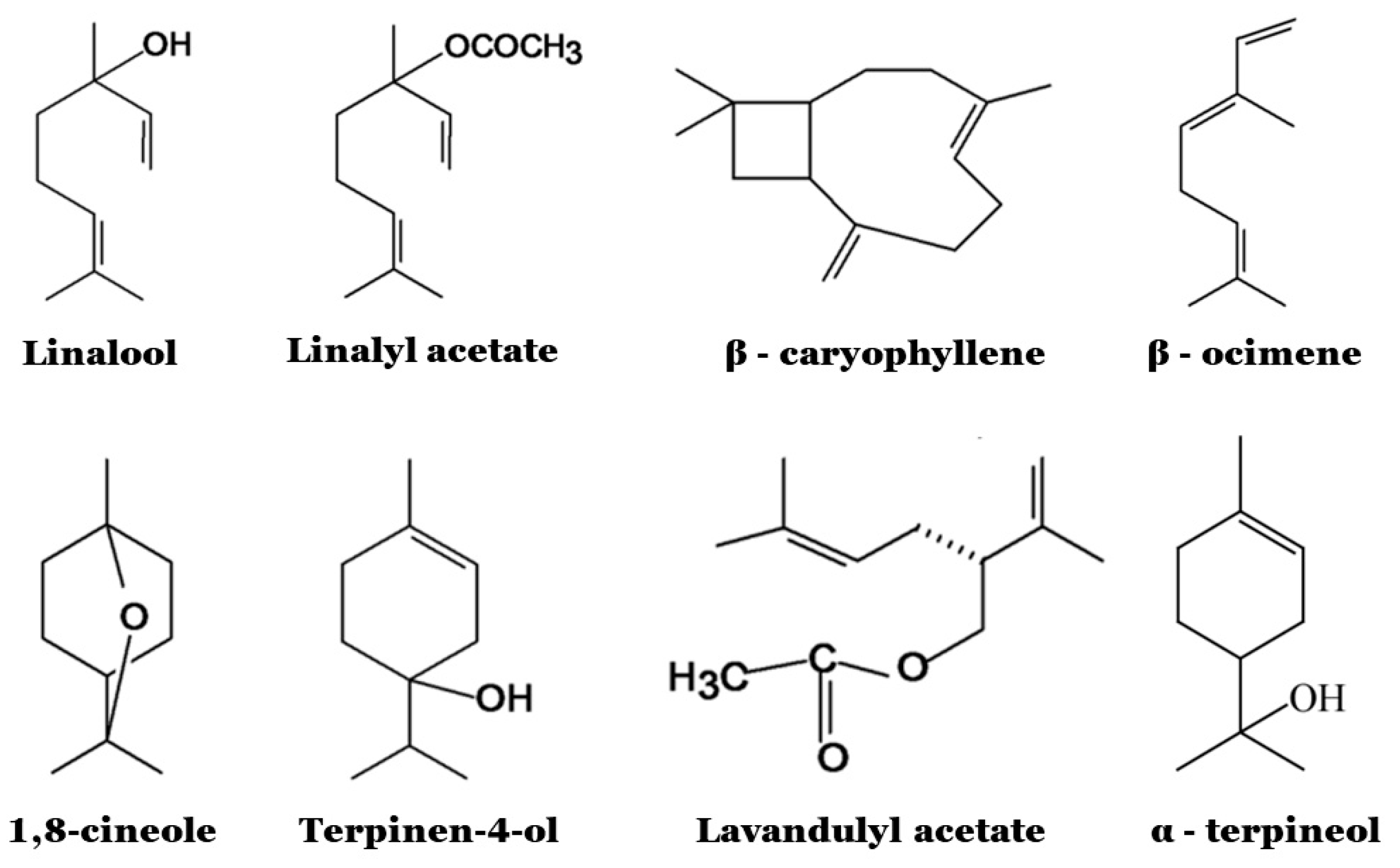
3.1. Chemical Composition and Biological Properties
3.2. Antimicrobial Properties
| LEOs Origin | Microorganisms Tested | Method Used | Main Results | Reference |
|---|---|---|---|---|
| Croatian indigenous cultivar of: Lavandin (L. x intermedia) Lavender (L. angustifolia) | Bacillus cereus, Bacillus pumilus, Enterococcus faecalis, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Kocuria rhizophila, Listeria monocytogenes, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella Enteritidis, Staphylococcus aureus, Streptococcus pyogenes, Yersinia enterocolitica, Candida albicans, Candida glabrata, Candida kefyr, Candida krusei, Candida tropicalis, Cryptococcus neoformans, Hansenula anomala, Saprochaete capitate, Microsporum canis, Microsporum gypseum, Trichophyton mentagrophytes, Trichophyton rubrum, Aspergillus fumigatus, Aspergillus niger, Fusarium oxysporum, Penicillium citrinum | Disk diffusion assay Determination of minimum inhibitory concentration and minimum bactericidal/fungicidal concentration |
| [56] |
| Four cultivars of Lavandula x intermedia | L. monocytogenes (24 strains) Salmonella enterica (10 food strains) | Disk diffusion assay Determination of minimum inhibitory concentration and minimum bactericidal/fungicidal concentration |
| [69] |
| Lavandula stoechas grown in Extremadura (Spain) | St. aureus, B. cereus, L. monocytogenes, Listeria innocua, Salmonella Choleraesuis, E. coli, Candida boidinii, Priceomyces carsonii, Kregervanrija fluxuum, Zygosacharomyces bailii, Aspergillus flavus (2 strains producing aflatoxins) | Disk diffusion assay Determination of inhibition zone diameters |
| [70] |
| L. pedunculata L. angustifolia Lavandula maroccana grown in Morocco | E. coli, Salmonella spp., St. aureus | The EOs were tested separately and in combination, also with ciprofloxacin (antibiotic) The twofold dilution method followed by MIC determination was used |
| [67] |
| Lavandula angustifolia (Spain) | Botrytis cinerea, Sclerotinia sclerotiorum, F. oxysporum, Phytophthora parasitica, Pythium aphanidermatum, Alternaria brassicae, Cladobotryum mycophilum, Trichoderma aggressivum | Disk diffusion method (5–30%, v/v) | The activity was dependent on the concentration, with clear differences in the sensitivity of the fungal isolates, not correlated to the fungal wall composition. | [71] |
| Lavandula pubescens Decne (Arabia) | Ec. faecalis (Vancomycin-resistant), Staphylococcus epidermidis, St. aureus, St. aureus (Methicillin-resistant), Salmonella Typhimurium, Acinetobacter baumannii (Carbapenem-resistant), Shigella sonnei, Kl. pneumoniae, Ps. aeruginosa, Pr. mirabilis, E. coli | Diffusion method | The most sensitive strains were Ac. baumannii, Salm. Typhimurium, Sh. sonnei, Ec. faecalis and St. epidermidis. | [63] |
| Moroccan Lavandula stoechas | Two collection Campylobacter strains: C. jejuni and C. coli Nine wild multidrug resistant Campylobacter strains isolated from food and environment | Broth micro-dilution assay and MIC determination | The results showed that Campylobacter multidrug resistant strains were highly sensitive to Lavandula stoechas EO, with MIC values ranging from 0.063 to 0.25 µg/mL depending on the strain. However, these values were significantly reduced during the combined use with antibiotics: MIC values ranged from 0.004 to 0.003 µg/mL with ampicillin and from 0.004 to 0.125 µg/mL with tetracycline. An impressive inhibitory impact of lavender oil on biofilm formation was also recovered. | [68] |
| Dried and fresh flowers of Lavandula angustifolia L. (lavender) grown in central Italy | Bacillus subtilis, E. coli, Sclerotium rolfsii | Disk diffusion test |
| [66] |
| Five Lavandula stoechas cultivars grown in Thailand: L. stoechas ‘snowman’ L. stoechas ‘white lavender’ L. stoechas ‘major’ L. stoechas ‘avonview’ hybrid L. stoechas × viridis ‘St. Brelade’ | St. aureus, St. epidermidis, Enterococcus faecium, E. coli, Kl. pneumoniae, Ps. aeruginosa, Str. pyogenes, and Salm. Typhimurium | Disk diffusion assay Determination of MIC and inhibition diameters. |
| [72] |
| Moroccan Lavandula atlantica | Methicillin resistant St. aureus, E. coli, En. aerogenes, Ps. aeruginosa, Kl. pneumoniae, Kl. oxytoca, Salmonella spp., Ac. baumannii, and Enterobacter cloacae. | Inhibitory diameters |
| [73] |
| L. stoechas collected in Tunisia | As Gram-negative strains: four E. coli, three Ps. aeruginosa, and one Serratia marcescens. As Gram-positive: three Enterococcus (Ec. faecalis, Ec. aerogenes, and Ec. hirae), two St. aureus, and one Bacillus licheniformis. | Disk diffusion method |
| [74] |
| Lavandula officinalis collected in Turkey | Methicillin-resistant St. aureus, Vancomycin-resistant Enterococcus VRE, Methicilline susceptible St. aureus (MSSA), St. epidermidis, Salmonella Enteriditis, Salm. Typhimurium, E. coli, Kl. pneumoniae, Ps. aeruginosa, Citrobacter freundii, Pr. mirabilis and Ca. albicans. | Agar disk diffusion method | The essential oil was effective on VRE, MSSA, E. coli and St. epidermidis, but not on S. enteritidis, Ps. aeruginosa, and Ca. albicans. | [75] |
| Commercial lavender Lavandula angustifolia | Xanthomonas spp. isolated from tubers and stem of plants growing in Lithuania. Xanthomonas translucens X. arboricola (four strains) | Broth microdilution method and MIC determination | Lavender (2.0%) essential oils inhibited the growth of all Xanthomonas spp. strains: the diameter of the inhibition zones was from 22.8 to 0.9 mm. | [76] |
| Two cultivars of L. angustifolia: ‘Blue River’ and ‘Ellagance Purple’ (Poland). | Ca. albicans | Broth microdilution method and MIC determination |
| [77] |
| Lavandula angustifolia (Bulgaria) | E. coli, Proteus vulgaris, Ps. aeruginosa, St. aureus, Ec. faecalis, L. monocytogenes, Candida utilis, B. subtilis, A. niger, Penicillium chrysogenum, Saccharomyces cerevisiae. | Disk diffusion method | The lavender extract demonstrated antimicrobial activity against all tested pathogens. St. aureus was the most sensitive towards the lavender extract (MIC 60 μg/mL), while for all other pathogens, the MICs were above 600 μg/mL. | [78] |
| Lavandula dentata Lavandula Marrakech | B. cereus, L. monocytogenes, St. aureus, E. coli, Ps. aeruginosa and Salm. enterica. | Agar-well diffusion method and determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) | Only a bacteriostatic effect was observed. | [79] |
4. Food Preservative Applications of Sage and Lavender EOs
5. New Approaches for EOs Application
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some Essential Oils-present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Mérillon, J.-M.; Rivière, C. Natural Antimicrobial Agents; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/essential-oil-market-119674487.html (accessed on 20 May 2023).
- Swamy, M.K.; Sinniah, U.R. Patchouli (Pogostemon cablin Benth.): Botany, agrotechnology and biotechnological aspects. Ind. Crops Prod. 2016, 87, 161–176. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Borges Leal, A.L.A.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Shariatifar, N.; Mostaghim, T.; Afshar, A.; Mohammadpourfard, I.; Sayadi, M.; Rezaei, M. Antibacterial properties of essential oil of Heracleum per-sicum (Golpar) and food-borne pathogens. Int. J. Enteric Pathog. 2017, 5, 41–44. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, H.; Shojaee-Aliabadi, S.; Torbati, M.; Alizadeh, A.M.; Alizadeh, M. Drug Resistance and the Prevention Strategies in Food Borne Bacteria: An Update Review. Adv. Pharm. Bull. 2019, 9, 335–347. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.-M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef] [PubMed]
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella Typhimurium in broth system and on celery. J. Food Process Preserv. 2019, 43, e13888. [Google Scholar] [CrossRef]
- Krasniewska, K.; Gniewosz, M.; Kosakowska, O.; Pobiega, K. Chemical composition and antimicrobial properties of essential oil from lavender (Lavandula angustifolia L.) in commercial available preparation. Postępy Fitoter. 2017, 18, 113–118. [Google Scholar]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462.21. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Carrasco, A.; Thomas, V.; Tudela, J.; Miguel, M.G. Comparative study of GC-MS characterization, antioxidant activity and hyaluronidase inhibition of different species of Lavandula and Thymus essential oils. Flavour Fragr. J. 2016, 31, 57–69. [Google Scholar] [CrossRef]
- de Rapper, S.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid. Based Complement. Alternat. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential oils from wild populations of Algerian Lavandula stoechas L.: Composition, chemical variability, and in vitro biological properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Turgut, A.C.; Emen, F.M.; Canbay, H.S.; Demirdöğen, R.E.; Çam, N.; Kılıç, D.; Yeşilkaynak, T. Chemical characterization of Lavandula angustifolia Mill. as a phytocosmetic species and investigation of its antimicrobia in cosmetic products. J. Turk. Chem. Soc. Sect. Chem. 2017, 4, 283–298. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 2, pp. 329–347. [Google Scholar]
- Fattahi, B.; Nazeri, V.; Kalantari, S.; Bonfill, M.; Fattahi, M. Essential oil variation in wild-growing populations of Salvia reuterana Boiss. collected from Iran, using GC–MS and multivariate analysis. Ind. Crops Prod. 2016, 81, 180–190. [Google Scholar] [CrossRef]
- Beheshti-Rouy, M.; Azarsina, M.; Rezaie-Soufi, L.; Alikhani, M.Y.; Roshanaie, G.; Komaki, S. The antibacterial effect of sage extract (Salvia officinalis) mouthwash against Streptococcus mutans in dental plaque: A randomized clinical trial. Iran. J. Microbiol. 2015, 7, 173–177. [Google Scholar]
- Mokhtari, R.; Fard, M.K.; Rezaei, M.; Moftakharzadeh, S.A.; Mohseni, A. Antioxidant, antimicrobial activities, and characterization of phenolic compounds of Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Thyme–Sage mixture extracts. J. Food. Qual. 2023, 2023, 2602454. [Google Scholar] [CrossRef]
- Sulaiman, A.M.; Abdulaziz, A.S.; Almutawea, A.M.; Alansari, S.A.; Aldoseri, F.A.; Bekhit, S.A.; Al-Thawadi, S.M.; Alqallaf, S.M.; Bekh, A.A. Evaluation of the antibacterial effect of Salvia officinalis Essential Oil and its synergistic effect with Meropenem. Lett. Appl. NanoBioScience 2023, 12, 44. [Google Scholar]
- ISO 9909:1997; Oil of Dalmatian Sage (Salvia officinalis L.). International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Baricevic, D.; Bartol, T. The biological/pharmacological activity of the Salvia genus. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Singapore, 2005; p. 290. [Google Scholar]
- Yilar, M.; Kadioǧlu, I.; Telci, I. Chemical composition and antifungal activity of Salvia officinalis (L.), S. cryptantha (montbret et aucher ex benth.), S. tomentosa (mill.) plant essential oils and extracts. Fresenius Environ. Bull. 2018, 27, 1695–1706. [Google Scholar]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Antienzymatic, and Antimicrobial Bioactivities. Chem. Biodivers. 2017, 14, e1700102. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Dweck, A.C. Introduction. The folklore and cosmetic use of various salvia species. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Singapore, 2005. [Google Scholar]
- Monsen, R.E.; Herlofson, B.B.; Gay, C.; Fjeld, K.G.; Hove, L.H.; Malterud, K.E.; Saghaug, E.; Slaaen, J.; Sundal, T.; Tollisen, A.; et al. A mouth rinse based on a tea solution of Salvia officinalis for oral discomfort in palliative cancer care: A randomized controlled trial. Support. Care Cancer 2021, 29, 4997–5007. [Google Scholar] [CrossRef] [PubMed]
- Lakhal, H.; Ghorab, H.; Chibani, S.; Kabouche, A.; Semra, Z.; Smati, F.; Abuhamdah, S.; Kabouche, Z. Chemical composition and biological activities of the essential oil of Salvia officinalis from Batna (Algeria). Der Pharm. Lett. 2013, 5, 310–314. [Google Scholar]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Albdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordon: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Assessment Report on Salvia fruticosa Mill Folium. EMA/HMPC/599992/2014. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-salvia-fruticosa-mill-folium_en.pdf (accessed on 15 May 2023).
- European Medicines Agency (EMA). Assessment Report on Salvia officinalis L., folium and Salvia officinalis L., Aetheroleum EMA/HMPC/150801/2015. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-report/draft-assessment-report-salvia-officinalis-l-folium-salvia-officinalis-l-aetheroleum_en.pdf (accessed on 15 May 2023).
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Haziri, A.; Faiku, F.; Mehmeti, A.; Kurteshi, K.; Haziri, I.; Rudhani, I. In vitro antibacterial properties of ethanol extract from Salvia officinalis (L.) plant growing wild in Kosovo. Biomed. J. Sci. Tech. Res. 2018, 2, 2578–2580. [Google Scholar]
- Mohamed, A.Y.; Mustafa, A.A. In vitro anti-microbial activity of Essential Oils and other extracts from Salvia officinalis against some bacteria. Plant Sci. 2019; 2019040012, preprints. [Google Scholar]
- Medeiros de Almeida, C.; de Souza Sales Rocha, E.A.L.; Ponchet Alves, É.; de Freitas Lima, R.; Wanderley Cavalcanti, Y.; Barbosa Gomes, R.C.; Vieira Pereira, J.; Melo de Brito Costa, E.M. In vitro evaluation of the antimicrobial potential of Salvia officinalis L. against oral pathogens. J. Health Sci. 2019, 21, 129–133. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Vilela, P.G.D.F.; Almeida, R.B.A.; de Oliveira, F.E.; Carvalho, C.A.T.; Camargo, S.E.A.; Jorge, A.O.C.; de Oliveira, L.D. Antimicrobial activity of noncytotoxic concentrations of Salvia officinalis extract against bacterial and fungal species from the oral cavity. Gen. Dent. 2019, 67, 22–26. [Google Scholar] [PubMed]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea Essential Oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef] [PubMed]
- Divyapriya, G.K.; Veeresh, D.J.; Yavagal, P.C. Evaluation of antibacterial efficacy of Chia (Salvia hispanica) seeds extract against Porphyromonas gingivalis, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans. An in-vitro study. Int. J. Ayurveda Pharm. Res. 2016, 4, 22–24. [Google Scholar]
- Elshafie, H.S.; Aliberti, L.; Amato, M.; De Feo, V.; Camele, I. Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) Essential Oil. Eur. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Osman, M.A.; Yuswan, M.H.; Loh, J.Y.; Lim, W.M.; Lim, S.H.; Lai, K.S. Antibacterial activity and mode of action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020, 69, 1–6. [Google Scholar] [CrossRef]
- Moon, K.; Cha, J. Enhancement of antioxidant and antibacterial activities of Salvia miltiorrhiza roots fermented with Aspergillus oryzae. Foods 2020, 9, 34. [Google Scholar] [CrossRef]
- Chen, B.-C.; Ding, Z.-S.; Dai, J.-S.; Chen, N.-P.; Gong, X.-W.; Ma, L.-F.; Qian, C.-D. New insights into the antibacterial mechanism of cryptotanshinone, a representative diterpenoid quinone from Salvia miltiorrhiza Bunge. Front. Microbiol. 2021, 12, 647289. [Google Scholar] [CrossRef]
- Lim Ah Tock, M.; Combrinck, S.; Kamatou, G.; Chen, W.; Van Vuuren, S.; Viljoen, A. Antibacterial screening, biochemometric and bioautographic evaluation of the non-volatile bioactive components of three indigenous South African Salvia species. Antibiotics 2022, 11, 901. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) Methanolic Extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef]
- Bensebia, O.; Benamani, A.; Issadi, H.M. Antibacterial activity of sage leaves against pathogenic bacteria as affected by different drying Temperature. Arab. J. Med. Aromat. Plants 2021, 7, 74–92. [Google Scholar]
- Aćimović, M.; Pezo, L.; Cabarkapa, I.; Trudić, A.; Stanković Jeremić, J.; Varga, A.; Lončar, B.; Šovljanski, O.; Tešević, V. Variation of Salvia officinalis L. Essential Oil and hydrolate composition and their antimicrobial activity. Processes 2022, 10, 1608. [Google Scholar] [CrossRef]
- Benabdesslem, Y.; Hachem, K.; Kahloula, K.; Belakredar, A.; Slimani, M. In vitro antimicrobial activity of aqueous, ethanolic and methanolic leaves extracts from Salvia argentea. Sys. Rev. Pharm. 2020, 11, 1683–1691. [Google Scholar]
- Pieracci, Y.; Ciccarelli, D.; Giovanelli, S.; Pistelli, L.; Flamini, G.; Cervelli, C.; Mancianti, F.; Nardoni, S.; Bertelloni, F.; Ebani, V.V. Antimicrobial activity and composition of five rosmarinus (Now Salvia spp. and Varieties) Essential Oils. Antibiotics 2021, 10, 1090. [Google Scholar] [CrossRef]
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the Genus Lavandula: From Farm to Pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula × intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of lavandin and lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Buchbauer, G. Handbook of Essential Oils, Science, Technology and Applications, 2nd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Lu, H.; Li, H.; Lu, H.; Li, X.; Zhou, A. Chemical composition of lavender essential oil and its antioxidant activity and inhibition against rhinitis-related bacteria. Afr. J. Microbiol. Res. 2010, 4, 309–313. [Google Scholar]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-beta in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Genotoxicity of lavender oil, linalyl acetate, and linalool on human lymphocytes in vitro. Environ. Mol. Mutagen. 2011, 52, 69–71. [Google Scholar] [CrossRef]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential Oil Analysis and Antimicrobial Evaluation of Three Aromatic Plant Species Growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef] [PubMed]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Heo, H.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.J. Antibacterial activity of essential oil from lavender (Lavandula angustifolia) against pet turtle-borne pathogenic bacteria. Lab. Anim. Res. 2017, 33, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Caprari, C.; Fantasma, F.; Divino, F.; Bucci, A.; Iorizzi, M.; Naclerio, G.; Ranalli, G.; Saviano, G. Chemical Profile, In Vitro Biological Activity and Comparison of Essential Oils from Fresh and Dried Flowers of Lavandula angustifolia L. Molecules 2021, 26, 5317. [Google Scholar] [CrossRef]
- Nafis, A.; Ouedrhiri, W.; Iriti, M.; Mezrioui, N.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Hassani, L. Chemical composition and synergistic effect of three Moroccan lavender EOs with ciprofloxacin against foodborne bacteria: A promising approach to modulate antimicrobial resistance. Lett. Appl. Microbiol. 2021, 72, 698–705. [Google Scholar] [CrossRef]
- El Baaboua, A.; El Maadoudi, M.; Bouyahy, A.; Belmehdi, O.; Kounnoun, A.; Cheyadmi, S.; Ouzakar, S.; Skali Senhaji, N.; Abrini, J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. S. Afr. J. Bot. 2022, 150, 451–465. [Google Scholar] [CrossRef]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula x intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef]
- Boy, F.R.; Benito, M.J.; Córdoba, M.d.G.; Rodríguez, A.; Casquete, R. Antimicrobial Properties of Essential Oils Obtained from Autochthonous Aromatic Plants. Int. J. Environ. Res. Public Health 2023, 20, 1657. [Google Scholar] [CrossRef] [PubMed]
- Dianez, F.; Santos, M.; Parra, C.; Navarro, M.J.; Blanco, R.; Gea, F.J. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Insawang, S.; Pripdeevech, P.; Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Nakham, T.; Artrod, A.; D’Souza, P.E.; Panuwet, P. Essential Oil Compositions and Antibacterial and Antioxidant Activities of Five Lavandula stoechas Cultivars Grown in Thailand. Chem. Biodivers. 2019, 16, e1900371. [Google Scholar] [CrossRef] [PubMed]
- Sayout, A.; Ouarhach, A.; Rabie, R.; Dilagui, I.; Soraa, N.; Romane, A. Evaluation of Antibacterial Activity of Lavandula pedunculata subsp. atlantica (Braun-Blanq.) Romo Essential Oil and Selected Terpenoids against Resistant Bacteria Strains-Structure-Activity Relationships. Chem. Biodivers. 2020, 17, e1900496. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Djebali, K.; Abid, S.; Saada, M.; Ksouri, R. Application of the mixture design for optimum antimicrobial activity: Combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. J. Food Process Preserv. 2019, 43, e14257. [Google Scholar] [CrossRef]
- Aladağ, M.O.; Özcan, M.M.; Ergin, S. Inhibitory effect of some spice essential oils on growth of some gram-negative and gram-positive bacteria and a yeast. J. Food Process Preserv. 2021, 45, 15264. [Google Scholar] [CrossRef]
- Mačionienė, I.; Čepukoit, D.; Šalomskienė, J.; Černauskas, D.; Burokienė, D.; Šalaševičienė, A. Effects of Natural Antimicrobials on Xanthomonas Strains Growth. Horticulturae 2022, 8, 7. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M.; Szczerbińska, D. Lavandula angustifolia Essential Oils as Effective Enhancers of Fluconazole Antifungal Activity against Candida albicans. Molecules 2023, 28, 1176. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of lavender (Lavandula angustifolia) and melissa (Melissa officinalis) waste on quality and shelf life of bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- Btissam, R.; Fatima, E.M.; Kamal, E.; Hassane, G.; Mohamed, N. Composition and Antibacterial Activity of Hydro-Alcohol and Aqueous Extracts Obtained from the Lamiaceae Family. Pharmacog. J. 2018, 10, 81–91. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 30, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.-K.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- Azizkhani, M.; Tooryan, F.; Azizkhani, M. Inhibitory potential of Salvia sclarea and ocimum basilicum aganist chemical and microbial spoilage in cheese. J. Food Saf. 2016, 36, 109–119. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbiological stability of fresh pork sausages. LWT—Food Sci. Technol. 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Kántor, A.; Tokár, M.; Puchalski, C.; Ivanišová, E. Antimicrobial effect of Sage (Salvia officinalis L.) and rosemary (Rosmarinus officinalis L.) Essential Oils on microbiota of chicken breast. Proc. Latv. Acad. Sci. 2017, 71, 461–467. [Google Scholar]
- Moura-Alves, M.; Gouveia, A.R.; de Almeida, J.M.M.M.; Monteiro-Silva, F.; Silva, J.A.; Saraiva, C. Behavior of Listeria monocytogenes in beef Sous vide cooking with Salvia officinalis L. essential oil, during storage at different temperatures. LWT—Food Sci. Technol. 2020, 132, 109896. [Google Scholar] [CrossRef]
- Gál, R.; Čmiková, N.; Prokopová, A.; Kačániová, M. Antilisterial and antimicrobial effect of Salvia officinalis Essential Oil in beef sous-vide meat during storage. Foods 2023, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.R.; Saqueti, B.H.F.; Santos, P.D.S.; Silva, J.M.; Matiucci, M.A.; Feihrmann, A.C.; Mikcha, J.M.G.; Santos, O.O. Effect of Salvia (Salvia officinalis) on the oxidative stability of salmon hamburgers. LWT—Food Sci. Technol. 2022, 154, 112867. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Jafarie, S.; Kaboudari, A. Effect of Salvia officinalis L. extract on chemical, microbial, sensory and shelf life of rainbow trout fillet. Food Sci. Biotechnol. 2019, 28, 1499–1506. [Google Scholar] [CrossRef]
- Dincoglu, A.H.; Caliskan, Z. Investigation of the effect of lavender (Lavandula angustifolia Mill.) essential oil on microbiological, physicochemical, and sensorial properties of meatballs during shelf-life, and its inhibitory effect on Escherichia coli O157:H7. Int. Food Res. J. 2022, 29, 991–1004. [Google Scholar] [CrossRef]
- Šimat, V.; Skroza, D.; Čagalj, M.; Soldo, B.; Generalíc Mekiníc, I. Effect of plant extracts on quality characteristics and shelf-life of cold-marinated shrimp (Parapenaeus longirostris, Lucas, 1846) under refrigerated storage. Food Biosci. 2023, 53, 102673. [Google Scholar] [CrossRef]
- Ramdan, G.M.; Khalafalla, F.A.; Hassan, A.H.A.; Abdel-Atty, N.S. Antibacterial efficacy of chemically and plant-synthesized zinc oxide nanocomposite against Staphylococcus aureus and Escherichia coli inoculated in Tilapia fillet. Malays. J. Microbiol. 2023, 19, 37–46. [Google Scholar]
- Tahir, A.; Ahmad, R.S.; Imran, M.; Ahmad, M.H.; Khan, M.K.; Muhammad, N.; Un Nisa, M.; Nadeem, M.T.; Yasmin, A.; Tahir, H.S.; et al. Recent approaches for utilization of food components as nano-encapsulation: A review. Int. J. Food Prop. 2017, 24, 1074–1096. [Google Scholar] [CrossRef]
- EFSA. Available online: www.efsa.europa.eu/it/topics/topic/nanotechnology. (accessed on 19 July 2023).
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on nutritional, bioactive, and medicinal potential of spices and herbs and their application in food fortifcation and nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar]
- Sana, S.S.; Li, H.; Zhang, Z.; Sharma, M.; Usmani, Z.; Hou, T.; Netala, V.R.; Wang, X.; Gupta, V.K. Recent advances in essential oils-based metal nanoparticles: A review on recent developments and biopharmaceutical applications. J. Mol. Liq. 2021, 333, 115951. [Google Scholar] [CrossRef]
- Velmurugan, P.; Ganeshan, V.; Nishter, N.F.; Jonnalagadda, R.R. Encapsulation of orange and lavender essential oils in chitosan nanospherical particles and its application in leather for aroma enrichment. Surf. Interfaces 2017, 9, 124–132. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of chitosan beads incorporated with lavender or red thyme essential oils in inhibiting Botrytis cinere and their application in strawberry packaging system. LWT—Food Sci. Technol. 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Albuquerque, P.M.; Azevedo, S.G.; de Andrade, C.P.; D’Ambros, N.C.d.S.; Pérez, M.T.M.; Manzato, L. Biotechnological applications of nanoencapsulated Essential Oils: A Review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Katsarov, P. Comparative study on microencapsulation of Lavender (Lavandula angustifolia Mill.) and Peppermint (Mentha piperita L.) Essential Oils via Spray-Drying Technique. Molecules 2021, 26, 7467. [Google Scholar] [CrossRef] [PubMed]
- Doğan, C.; Doğan, N.; Gungor, M.; Eticha, A.K.; Akgul, Y. Novel active food packaging based on centrifugally spun nanofibers containing lavender essential oil: Rapid fabrication, characterization, and application to preserve of minced lamb meat. Food Packag. Shelf Life 2022, 34, 100942. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Roscigno, G.; Pane, C.; Celano, G.; Di Matteo, M.; Mainente, M.; Vuotto, A.; Mencherini, T.; Esposito, T.; Vitti, A.; et al. Essential oils and quality composts sourced by recycling vegetable residues from the aromatic plant supply chain. Ind. Crops Prod. 2021, 162, 113255. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Koidis, A. Methods for Extracting Essential Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Irish Academic Press: San Diego, CA, USA, 2016; pp. 31–38. [Google Scholar]
- Nirmala, M.J.; Sekar, P.C.; Johnson, A.; Kizhuveetil, U.; Shora, S.; Nagarajan, R. A comprehensive review of nanoadditives in Plant-based biodiesels with a special emphasis on essential oils. Fuel 2023, 351, 128934. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, B.; Guerrieri, A.; Racioppo, A.; Bevilacqua, A.; Campaniello, D.; Corbo, M.R. Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods. Microbiol. Res. 2023, 14, 1089-1113. https://doi.org/10.3390/microbiolres14030073
Speranza B, Guerrieri A, Racioppo A, Bevilacqua A, Campaniello D, Corbo MR. Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods. Microbiology Research. 2023; 14(3):1089-1113. https://doi.org/10.3390/microbiolres14030073
Chicago/Turabian StyleSperanza, Barbara, Angela Guerrieri, Angela Racioppo, Antonio Bevilacqua, Daniela Campaniello, and Maria Rosaria Corbo. 2023. "Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods" Microbiology Research 14, no. 3: 1089-1113. https://doi.org/10.3390/microbiolres14030073
APA StyleSperanza, B., Guerrieri, A., Racioppo, A., Bevilacqua, A., Campaniello, D., & Corbo, M. R. (2023). Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods. Microbiology Research, 14(3), 1089-1113. https://doi.org/10.3390/microbiolres14030073








