Abstract
Background: This study aimed to analyze the relevance of different publications about microbiota on ocular diseases and their authors through a citation network analysis. In addition, the different research areas and the most cited publications have been identified. Methods: The bibliographic search was carried out through the Web of Science (WOS) database, using the following search term: “microbiota AND (vision OR eye OR visual)” for the period between 1995 and December 2022. The Citation Network Explorer and the CiteSpace software have been used to analyze the different publications. Results: 705 publications were found in the field of microbiota on ocular diseases, together with 1014 citation networks. The year 2022 was the year with more publications. The first authors with the highest number of publications in the microbiota on the ocular surface field were Chisari G, Chisari CG, and Li Y. This field is multidisciplinary, highlighting “microbiology” and “ophthalmology” as the main research areas. Publications were clustered into three main groups allowing the identification of the main research topics in this field. The principal was the composition and diversity of the bacterial community on the ocular surface of patients with several pathologies. Conclusion: It could be useful for researchers to choose suitable collaborators or projects to promote their research on the role of microbiota on ocular diseases, as well as to know the main research topics that are of major interest today.
1. Introduction
“Ocular microbiota” refers to the collection of microorganisms’ genomes present in the eye [1,2]. Since the launching of the Human Microbiome Project, the understanding of the ocular microbiota has increased not only about the diversity of species but also the composition. The NIH Human Microbiome Projects provide characterization of the human microbiome from different parts of the human body and try to determine its relationship to human disease [3,4]. Between skin, gut, and mucosal surfaces, trillions of microorganisms interact with host cells to develop an adaptive immune system, sometimes triggering autoimmune diseases [5,6,7]. On the other hand, there is evidence that it plays a fundamental role in the defense against the invasion of pathogens, the regulation of the physiology of external agents, and the development of the immune system. However, dysbiosis (unbalanced microbiota) could lead to microbial overgrowth and local or systemic inflammation [8]. Since the completion of the genome of a free-living organism in 1995 [9], new metagenome sequencing tools have made it possible to investigate the possible association between gut microbiota dysbiosis and human diseases [10,11,12,13]. Methods to define the microbiota are usually divided into culture-based techniques and non-culture-based techniques. Culture-based techniques rely on the phenotypic characteristics of microbes to estimate microbial burden, such as the ability of microbes to grow on a specific growth medium under a particular condition [14,15]. Nonculture diagnostic methods include metagenomic sequencing, which targets microbial RNA or DNA, and immunoassays, which target microbial peptides or antigens secreted by microbes [16,17].
The ocular surface is highly vulnerable to microbial contamination as it is exposed directly to the external environment. However, the epithelial cells of the cornea and conjunctiva do not produce an inflammatory response to bacteria in healthy eyes, suggesting that there is an innate immune response. Therefore, commensal microbiota can colonize the ocular epithelium, preventing colonization by pathogenic species and maintaining ocular surface homeostasis [18]. Any imbalance in this delicate ecosystem has provoked several proinflammatory or pathologic states. The ocular surface microbiota may be suffered from environmental conditions such as age, gender, contact lenses, or antibiotics [19].
The principal components of the ocular surface have already been characterized. The genera Staphylococcus, Streptococcus, Micrococcus, Corynebacterium, and Propionibacterium are commensal Gram-positive bacteria that colonize the ocular surface after birth and are localized on conjunctiva, tear film, and eyelids [20]. Regarding Gram-negative bacteria, despite being less common, it has been described that the ocular surface may present genera such as Neisseria, Pseudomonas, and fungi [21].
Different pathologies such as conjunctivitis, blepharitis, or dry eye syndrome have shown alterations in the ocular microbial environment [22,23,24]. In 2012, Lee et al. found that in blepharitis there was an increase in the number of Streptophyta spp., S. aureus, Enterobacter spp., and Corynebacterium spp., and a decrease in Propionibacterium spp. compared to healthy eyes [22]. The genera Corynebacterium and Streptococcus were found to be increased in Chlamydia trachomatis infections, and a reduced bacterial diversity was developed [25].
On the other hand, contact lens wearers are more likely to suffer from inflammatory eye conditions or microbial keratitis. Thus, Zhang et al. found that in contact lens wearers there was an increase in the genera Acinetobacter spp., Methylobacterium spp., and Pseudomonas spp., and a decrease in the genera Streptococcus spp., Haemophilus spp., Corynebacterium spp., and coagulase-negative Staphylococcus. On the other side, nonlens wearers had a higher number of Lactobacillus spp., Haemophilus spp., Streptococcus spp., Neisseria spp., Rothia, and Corynebacterium spp., and coagulase-negative Staphylococci, while a lower number of Methylobacterium spp., Acinetobacter spp., and Pseudomonas spp. All these results suggest that contact lens wear modifies the ocular microbiota, being more similar to the skin microbiota [26,27]. In the case of bacterial keratitis, the predominant pathogen that has been isolated is Pseudomonas aeruginosa, a Gram-negative pathogen [28].
The analysis of citation networks allows, through a publication, to find other relevant additional publications. The aim is to show the connections qualitatively and quantitatively between authors and articles by generating groups [29]. At the same time, it is possible to study the development of a specific area of knowledge, quantify publications in each group, and select the literature on a relevant topic [30,31].
This analysis aims to identify the main research areas on the ocular surface microbiota and to determine the most relevant publication in the field. This study also analyzes the relationships between the publications and the different research groups through the CitNetExplorer software.
2. Materials and Methods
2.1. Database
The bibliographic search was carried out through the Web of Science (WOS) database, using the following search term: “microbiota AND (vision OR eye OR visual)”.
The citation indexes used were Social Sciences Citation Index, Science Citation Index Expanded, and Emerging Sources Citation Index. The time interval selected to search was from January 1995 to December 2022.
The exclusion criteria were articles that had no scientific relevance, which included things such as news, obituaries, projects, and patents. The downloaded information contained:
- -
- The total number of publications;
- -
- The names of the authors and their affiliations, along with the total number of articles, and the counted citations of every author;
- -
- A comprehensive list of the prolific nations and collaborations;
- -
- The overall most frequently cited articles, including the titles, authors, journal details, year of publication, total citations since publication, and specifically, annual citations as well;
- -
- Titles, synopses, and keywords.
2.2. Analysis of Data
2.2.1. Bibliometric Analysis
A series of indicators was used to highlight distributed characteristics and structural patterns of the bibliographic data. The annual number of publications of research results demonstrated an increasing trend of more publications on emerging technologies in neurorehabilitation. The journals with the most publications were identified as those that both published academic articles and likewise contributed to the development of the research field. Both the journal impact factor (IF) and the quartiles in the pertinent categories were derived from the Journal Citation Report (JCR) 2019 and SCImago Journal Rank (2019) and utilized in order to explore the influence of the editorial journal. Data from the quantitative analysis of the evolution of the literature, as well as bibliometric indicators, were used to showcase an illuminating overview of the research during the time of study.
2.2.2. Network Analysis
The analysis of the publications and the visualization of citation networks has been carried out using the Citation Network Explorer software. Thus, it has been possible to go from a citation network made up of millions of publications to delve into a small subnet with 100 publications on the same topic.
The quantitative analysis of the most cited publications was performed using the Citation Score attribute. This made it possible to quantify the internal connections within the Web of Science database as well as other databases (external connections) [31].
Next, the interconnected publications were classified into the same group using the clustering function. To carry out this grouping, the formula developed by Van Eck in 2012 [12] is used.
The identification of publications considered as the nucleus of the citation network was carried out through the identifying core publication’s function. In this study, those publications with four or more citations were considered. The higher this value, the smaller the number of central publications [30]. On the other hand, the drilling down function has been used to deepen and analyze each group at different levels.
The scientometric analysis was performed using the CiteSpace software (5.6.R2), and the following parameter indicators: The H index to analyze the number and level of academic production of researchers and institutions [32]. The degree represents the number of connections between authors (institutions, countries) in the co-occurrence knowledge graph. The higher this value, the greater the communication and co-operation between authors. “Intermediary centrality” measures the significance of nodes in the research co-operation network. Intermediary centrality is a measure of the number of times a node functions as a waypoint along the fastest path between two other nodes, known as the geodetic distance. “Half-life” is a parameter which represents the continuity of institutional research in time and is defined as the number of years a post receives half its citations since its publication. A low citation half-life suggests citation activity which peaks and declines rapidly while a high cited half-life suggests citation activity which peaks and declines more gradually [33].
3. Results
The first articles on vision and microbiota were published in 1995. However, in the last five years, the number of publications has increased exponentially (1995–2017: 29.1%; 2018–2022: 70.9%). After the WoS search, 705 publications were found in all fields and 1014 citation networks.
2022 was the year with the highest number of publications, with 144 articles and 18 citation networks (Figure 1).
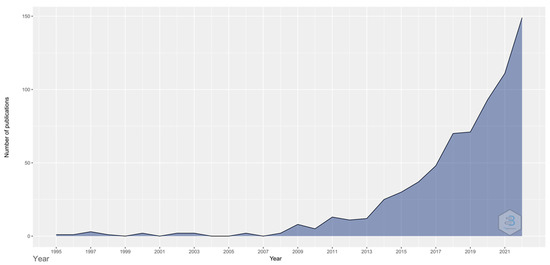
Figure 1.
Number of publications in the different years.
3.1. Description of the Publications
Of all publications, 75.3% were articles, 22.1% were review articles, 1.6% were book chapters, and 1% were editorial materials.
3.2. Language and Countries
Regarding the language of the publications, 97.4% were in English, 1.1% in Portuguese, and 0.7% in Spanish. Therefore, as shown in Figure 2, the countries with the highest number of publications and connections with other countries are the United States (publications: 29; degree: 10; half-life: 6.5; connections: 596), Italy (posts: 11; degree: 5; half-life: 3.5; connections: 103) and France (posts: 10; degree:11; half-life: 8.5; connections: 60).
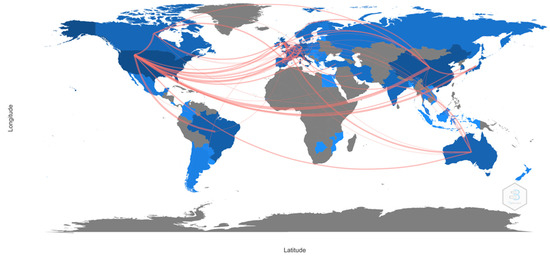
Figure 2.
Collaboration between countries.
3.3. Research Areas
The research area is multidisciplinary. The microbiology (13.3%) and ophthalmology (12.3%) areas should be highlighted (Table 1).

Table 1.
Top 10 research areas.
3.4. Authors and Institutions
As shown in Figure 3, the authors with the highest number of publications are Chisari G (publications: 1.6%; degree: 6; connections: 116), Chisari CG (posts: 1.4%; degree: 4; connections: 71), and Li Y (publications: 1.4%; degree: 8; connections 97).
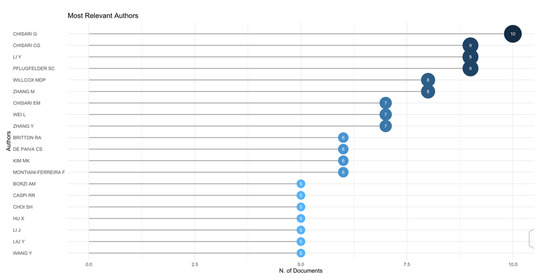
Figure 3.
Top 10 authors.
Regarding the institutions with the highest number of publications (Table 2), they are the League of European Research Universities (LERU) (7.8%), Harvard University (2.7%), and the National Institute for Agricultural Research (INRAE) (2.4%).

Table 2.
Top 10 institutions.
3.5. Journals
Table 3 shows the main journals and the number of publications according to the WoS database.

Table 3.
Top 10 journals.
3.6. Keywords
On the other hand, the most used keywords were “Gut microbiota”, “Microbiota”, and “Disease”. Table 4 shows the most used keywords in the most relevant publications.

Table 4.
The 20 most used keywords.
3.7. Most Cited Publications
As shown in Figure 4, the most cited article has been the one published in 2019, “Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site” by Horai et al. in Immunity [34]. The authors demonstrated a new model of spontaneous uveitis, in which the activation of specific retinal T cells depends on the intestinal microbiota. Thus, these findings not only have implications for the etiology of human uveitis but also raise the possibility that activation of autoreactive T cells by microbes could be a more common trigger of autoimmune disease than previously believed.
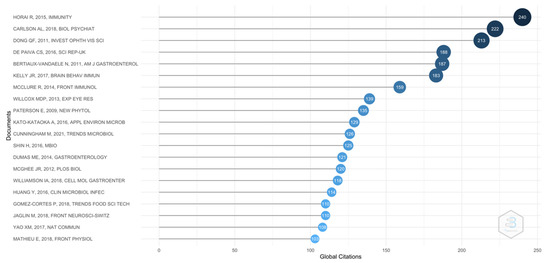
Figure 4.
Top 20 publications.
3.8. Clustering
Through the clustering function three groups, a significant number of publications have been found. The color of the circle indicates which group they are part of and the lines between elements represent bonds. In summary, every group has a different color, and the lines are the connections with other groups. The larger the circle, the greater the total number of citations.
Group 1 is made up of 201 publications and 906 citation networks. The publication by Horai et al. [34] is the one with the highest number of citations. This is also the first publication within the top 20 articles. Therefore, the articles in this group analyze the composition and diversity of the bacterial community on the ocular surface of patients with various ocular pathologies (Figure 5).
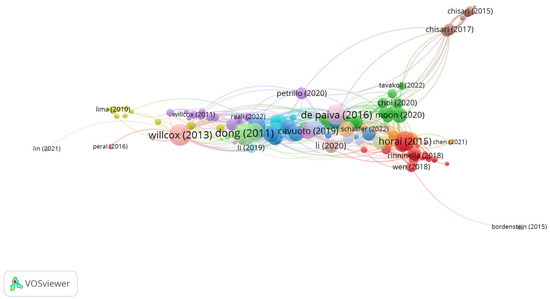
Figure 5.
Group 1 citation network.
Group 2 is made up of 11 publications and 10 citation networks. The publication by Lima et al. [35] in 2010 in Veterinary Ophthalmology is the one with the highest number of citations. In this study, they analyzed the morphological characteristics and the bony orbit of chinchillas. Thus, the most frequent bacteria isolated from the conjunctiva were Streptococcus sp. (27.45%), Staphylococcus aureus (23.52%), and Staphylococcus coagulase negative (19.60%). The articles in this group analyze the ocular characteristics of various animal species to use them as biological models in ophthalmological studies (Figure 6).
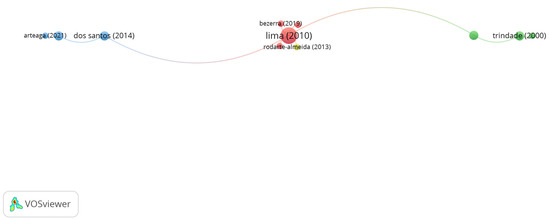
Figure 6.
Group 2 citation network.
Group 3 is made up of 10 publications and 26 citation networks. The Chisari et al. [36] in 2017 in Clinica Terapeutica, is the one with the highest number of citations. The authors showed in this study that the administration of bifidobacterium can represent a successful treatment for dry eye syndrome improvement. The effect of the unbalanced microbiota is not restricted by gastrointestinal abnormalities but could have a systemic impact on immunity. Commensal bacteria or probiotics interact with the endogenous enteric microbiota and intestinal cells, conferring health benefits to the host. The publications of this group analyze how the microbiota can help in the dry eye syndrome treatment (Figure 7).
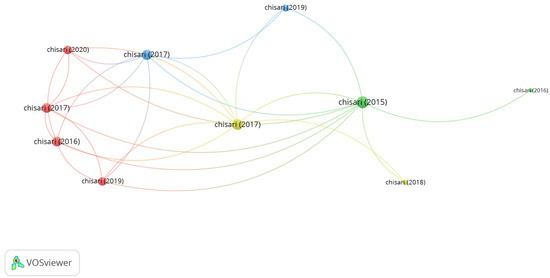
Figure 7.
Group 3 citation network.
4. Discussion
In this study, the role of the microbiota on the ocular surface has been analyzed through a bibliometric and citation network. The bibliometric studies are a quantitative analysis method that utilize mathematical and statistical tools to measure the correlations and impact of publications in a specified area of research. This method allows an overview of massive amounts of academic literature and can likewise determine influential studies, authors, journals, organizations, and nations over time. On top of this, citation network analysis allows for the different research areas to be identified in a more innate way by organizing social networks using coword, coauthorship, and cocitation analysis. It also allows the examination of the relationships between research groups, institutions, and even between nations. In general, the citation network analysis provides a clear outline of the most cited publications in any given research area [37]. The analysis of the microbiota on ocular diseases could prove to be of use for several experts for the identification of opportunities for collaboration among peers and multidisciplinary possibilities of teamwork, and the creation of new groups with a global mindset [38].
An increase in publications since the year 2000 has been observed increasing rapidly since the year 2010. This coincides with the study by Zhu et al. [39] in which they analyzed through a bibliometric analysis the status and development trends of gut microbiota research in the field of depression. This significant increase in publications since 2010 may be related to the fact that the main keywords used in this area of research began to be used for the first time around that year. Thus, today one of the articles with the most citations is from 2011. Dong et al. [40] carried out the first study based on DNA sequencing to identify bacteria in the conjunctiva region. Environmental, commensal, and opportunistic pathogenic bacteria were found in this tissue ubiquitously. The year 2021 was considered a key year, due to its high number of publications. In that year, the review of probiotics and prebiotics trends of Cunningham et al. [41] is a highlight. However, the year with the highest number of citations was 2018, led by the publication of Carlson et al. [42], an article which was the first to demonstrate associations in children between the gut microbiota and cognitive development, and the publication by Williamson et al. [43], in which they proved that a high-throughput microinjection into organoids was a new relevant approach to investigate gastrointestinal microbiota.
The country that published the most articles was the United States [44,45,46]. The same result has been found in most of the bibliometric studies, maybe due to having an advantage in this field, probably due to its better economy and grants on scientific research [47]. Thus, in 2013, they developed a research project on the relationship between the intestinal microbiota and the brain [48]. It should be noted that China has made great progress in this field in the last decade, mainly due to its growing collaboration with the United States, with 24 publications together. However, its influence is still low due to insufficient international co-operation, being the second and third countries in collaboration with China, Japan, and Norway, with five and four publications together. Concerning authors, the top three in the number of articles are from the University of Catania in Italy and the Istituto Superiore di Sanità (Italian National Institute of Health—ISS). This made Italy the second country in the number of publications. Common co-operation has been found between Chisari G and Chisari CG, mainly on dry eye syndrome. Both authors demonstrated that the administration of bifidobacterium represents a successful complete treatment to improve dry eye syndrome. The effect of the unbalanced microbiota is not restricted by gastrointestinal abnormalities but could have a systemic impact on immunity [36]. In turn, they also showed that the administration of probiotic strains was effective in reducing dry eye. Therefore, they identified the integration of probiotic activity (Saccharomyces boulardii MUCL 53837 and Enterococcus faecium LMG S-28935) with the action of tear substitutes, together with the standardization of clinical parameters of the tear film and microbiological activity in the restoration of the ocular surface microbiota of subjects with dry eye [36].
Journals allow researchers to obtain information to select the most suitable ones for publishing their articles. In this study, it has been found that the top 10 journals only published 17.3% of the total number of publications on the microbiota and ocular surface. This indicates a distribution of the literature in various journals, possibly due to the numerous research areas. On one hand, researchers may have many journal options, and, on the other hand, they may have difficulty choosing the most suitable journal due to a lack of knowledge or experience [47]. Furthermore, there was only a 30% concordance rate between the 10 most active journals and the 10 most cited journals, suggesting that the quality of research in this field still needs to be improved. At the same time, international co-operation between researchers should also be strengthened to produce high-quality research [49].
The main keywords found in this analysis were “Gut microbiota”, “microbiota” and “disease”. Most publications study the relationship between the microbiota on the ocular surface and the different pathologies of the eyes. Thus, in uveitis, it has been demonstrated that the imbalance between inflammatory cells Th1/Th17 and Treg is one of the main reasons for this disorder [50]. Intraocular inflammation attracts antigen-specific retinal lymphocytes that migrate to the retina [51]. Recently, it has been shown that the gut microbiota may play an essential role in the development of uveitis [52].
Intestinal dysbiosis has been also described that could occur in people with advanced age-related macular degeneration (AMD). It has been shown that those patients expressed a high number of the bacterial genera Oscillibacter and Anaerotruncus and a lower number of Blautia, Dorea, and Oscillospira compared to healthy older adults [53,54,55]. Studies using high-throughput RNA sequencing have confirmed how gut microbiota regulate relevant retinal genes in AMD [56]. Although certain inflammatory mechanisms associated with innate immunity have been identified in the pathogenesis of AMD, the hypothesis about microbial disorders and the promotion of nutrient absorption are currently open questions in the field [57].
The relationship between glaucoma and microbiota has been poorly investigated. Most studies focused on the presence of mitochondrial DNA in patients with glaucoma [58], as well as the description of factors that may influence the development of the disease, such as age, systemic diseases, diabetes, or genetic mutations [59,60]. Bacteroides and Prevotella are two bacterial variants that are overexpressed and seem to be associated with glaucoma [59,61].
Concerning dry eye, it has been proved that the innate and adaptive immune systems regulate the activation of the ocular surface immune system [62], mainly by T cells and antibodies secreted by plasma cells [63,64]. In 2015, Zeng et al. [65], characterized the ocular surface microbiota, defining all bacterial and viral components as well as immune tolerance and microbial representation. Few modifications in homeostasis alter severely the state of the ocular surface microbiome, triggering dry eye disease. Dry eye patients who wear contact lenses suffer from deterioration of the ocular microbiota more severely due to the alteration of the microbiota. In the study by Kim et al. [66], patients with dry eye expressed a high number of the genera Corynebacterium, Staphylococcus, and Propionibacterium. Kountouras et al. [67], found a direct relationship between reduced IgA production and alteration in the composition of the ocular microbiome [68]. On the other hand, different studies exploring the composition of the ocular microbiome in patients with meibomian gland dysfunction observed an increase in the genera S. aureus and Klebsiella spp. [69,70] and a reduction in coagulase-negative Staphylococcus [71].
These results offer an instructive perspective on the current and future research about the relationship between the microbiota and the ocular surface. It could be useful for researchers to choose suitable collaborators or journals to promote their research. As well as knowing the main research topics that are of most interest today.
5. Conclusions
This study showed a detailed and objective analysis of the different research areas on the microbiota and the ocular surface field. It is an area of expanding research, with growth in the last years and the possibility of broad new lines of research.
The publication with the highest number of citations was related to the analysis of human uveitis.
The main research topic in this field is the composition and diversity of the bacterial community on the ocular surface of patients with various ocular pathologies and their possible treatment.
Author Contributions
Conceptualization, M.A.S.-T., C.A.-P. and C.M.-P.; methodology, M.A.S.-T., C.A.-P. and C.M.-P.; software, M.A.S.-T., C.A.-P. and C.M.-P.; validation, M.A.S.-T., C.A.-P. and C.M.-P.; formal analysis, M.A.S.-T., C.A.-P. and C.M.-P.; investigation B.G.G., M.A.S.-T., C.A.-P. and C.M.-P.; resources, M.A.S.-T., C.A.-P. and C.M.-P.; data curation, M.A.S.-T., C.A.-P. and C.M.-P.; writing—original draft preparation, M.A.S.-T., C.A.-P., B.G.G. and C.M.-P.; writing—review and editing, B.G.G., M.A.S.-T., C.A.-P. and C.M.-P.; visualization, B.G.G., M.A.S.-T., C.A.-P. and C.M.-P.; supervision, B.G.G., C.M.-P., C.A.-P. and M.A.S.-T.; project administration, M.A.S.-T., C.A.-P. and C.M.-P.; funding acquisition, B.G.G., M.A.S.-T., C.A.-P. and C.M.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.J.; Yi, S.; Wei, L. Ocular Microbiota and Intraocular Inflammation. Front. Immunol. 2020, 11, 609765. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Integrative, H.M.P.; Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F., III; et al. The Integrative Human Microbiome Project. Nat. Cell Biol. 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Kahrstrom, C.T.; Pariente, N.; Weiss, U. Intestinal microbiota in health and disease. Nature 2016, 535, 47. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef]
- Berer, K.; Mues, M.; Koutrolos, M.; Al Rasbi, Z.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541. [Google Scholar] [CrossRef] [PubMed]
- McDermott, A.M. Antimicrobial compounds in tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef]
- Fleischmann, R.D.; Adams, M.D.; White, O.; Clayton, A.R.; Kirkness, E.F.; Kerlavage, A.R.; Bult, C.J.; Tomb, J.F.; Dougherty, B.A.; Merrick, J.M.; et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995, 269, 496–512. [Google Scholar] [CrossRef]
- Relman, D.A. Microbial Genomics and Infectious Diseases. N. Engl. J. Med. 2011, 365, 347–357. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Segre, J.A. Microbial growth dynamics and human disease. Science 2015, 349, 1058–1059. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Maeda, N.; Sakamoto, M.; Koh, S.; Inoue, T.; Tano, Y. Bacteriologic profile of the conjunctiva in the patients with dry eye. Am. J. Ophthalmol. 2008, 146, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rubio, M.E.; Rebolledo-Lara, L.; Martinez-Garcia, M.; Alarcon-Tomas, M.; Cortes-Valdes, C. The conjunctival bacterial pattern of diabetics undergoing cataract surgery. Eye 2010, 24, 825–834. [Google Scholar] [CrossRef]
- Schabereiter-Gurtner, C.; Maca, S.; Rölleke, S.; Nigl, K.; Lukas, J.; Hirschl, A.; Lubitz, W.; Barisani-Asenbauer, T. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1164–1171. [Google Scholar]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 9880. [Google Scholar] [CrossRef]
- Ueta, M. Innate immunity of the ocular surface and ocular surface inflammatory disorders. Cornea 2008, 27, S31–S40. [Google Scholar] [CrossRef]
- Lu, L.J.; Liu, J. Human Microbiota and Ophthalmic Disease. Yale J. Biol. Med. 2016, 89, 325–330. [Google Scholar]
- Miller, D.; Iovieno, A. The role of microbial flora on the ocular surface. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 466–470. [Google Scholar]
- Wu, T.; Mitchell, B.; Carothers, T.; Coats, D.; Brady-McCreery, K.; Paysse, E.; Wilhelmus, K. Molecular analysis of the pediatric ocular surface for fungi. Curr. Eye Res. 2003, 26, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, D.H.; Jung, J.Y.; Kim, J.C.; Jeon, C.O. Comparative ocular microbial communities in humans with and without blepharitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5585–5593. [Google Scholar] [CrossRef] [PubMed]
- Kugadas, A.; Christiansen, S.H.; Sankaranarayanan, S.; Surana, N.K.; Gauguet, S.; Kunz, R.; Fichorova, R.; Vorup-Jensen, T.; Gadjeva, M. Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis. PLoS Pathog. 2016, 12, e1005855. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Moore, J.E.; Jiru, X.; Moore, J.E.; Goodall, E.A.; Dooley, J.S.; Hayes, V.E.; Dartt, D.A.; Downes, C.S.; Moore, T.C. Ocular pathogen or commensal: A PCR-based study of surface bacterial flora in normal and dry eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5616–5623. [Google Scholar] [CrossRef]
- Zhou, Y.; Holland, M.J.; Makalo, P.; Joof, H.; Roberts, C.H.; Mabey, D.C.; Bailey, R.L.; Burton, M.J.; Weinstock, G.M.; Burr, S.E. The conjunctival microbiome in health and trachomatous disease: A case control study. Genome Med. 2014, 6, 99. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.; Hutchinson, D.S.; Sun, W.; Ajami, N.J.; Lai, S.; Wong, M.C.; Petrosino, J.F.; Fang, J.; Jiang, J.; et al. Conjunctival Microbiome Changes AssociatedWith Soft Contact Lens and Orthokeratology Lens Wearing. Investig. Ophthalmol. Vis. Sci. 2017, 58, 128–136. [Google Scholar] [CrossRef]
- Shin, H.; Price, K.; Albert, L.; Dodick, J.; Park, L.; Dominguez-Bello, M.G. Changes in the Eye Microbiota Associated with Contact Lens Wearing. mBio 2016, 7, e00198. [Google Scholar] [CrossRef]
- Kugadas, A.; Wright, Q.; Geddes-McAlister, J.; Gadjeva, M. Role of Microbiota in Strengthening Ocular Mucosal Barrier Function through Secretory IgA. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4593–4600. [Google Scholar] [CrossRef]
- Leydesdorff, L. Can Scientific Journals be Classified in terms of Aggregated Journal-Journal Citation Relations using the Journal Citation Reports? J. Am. Soc. Inf. Sci. Technol. 2006, 57, 601–613. [Google Scholar] [CrossRef]
- González, C.M. Análisis de citación y de redes sociales para el estudio del uso de revistas en centros de investigación: An approach to the development of collections. Ciênc. Inf. 2009, 38, 46–55. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. CitNetExplorer: A new software tool for analyzing and visualizing citation networks. J. Informetr. 2014, 8, 802–823. [Google Scholar] [CrossRef]
- Hirsch, J.E. An index to quantify an individual’s scientific research output. Proc. Natl. Acad. Sci. USA 2005, 102, 16569–16572. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J. Am. Soc. Inf. Sci. Technol. 2006, 3, 359–377. [Google Scholar] [CrossRef]
- Horai, R.; Zárate-Bladés, C.R.; Dillenburg-Pilla, P.; Chen, J.; Kielczewski, J.L.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.C.; Yamane, H.; Honda, K.; et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 2015, 43, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.; Montiani-Ferreira, F.; Tramontin, M.; Leigue Dos Santos, L.; Machado, M.; Ribas Lange, R.; Helena Abil Russ, H. The chinchilla eye: Morphologic observations, echobiometric findings and reference values for selected ophthalmic diagnostic tests. Vet. Ophthalmol. 2010, 13, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Chisari, G.; Chisari, E.M.; Francaviglia, A.; Chisari, C.G. The mixture of bifidobacterium associated with fructo-oligosaccharides reduces the damage of the ocular surface. Clin. Ter. 2017, 168, e181–e185. [Google Scholar] [CrossRef]
- Skolarus, T.A.; Lehmann, T.; Tabak, R.G.; Harris, J.; Lecy, J.; Sales, A.E. Assessing citation networks for dissemination and implementation research frameworks. Implement. Sci. 2017, 12, 97. [Google Scholar] [CrossRef]
- Harris, J.K.; Beatty, K.E.; Lecy, J.D.; Cyr, J.M.; Shapiro, R.M., II. Mapping the multidisciplinary field of public health services and systems research. Am. J. Prev. Med. 2011, 41, 105–111. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, J.; Deng, S.; Tan, Y.; Qiu, C.; Zhang, M.; Ni, X.; Lu, H.; Wang, Z.; Li, L.; et al. Bibliometric and Visual Analysis of Research on the Links Between the Gut Microbiota and Depression from 1999 to 2019. Front. Psychiatry 2021, 11, 587670. [Google Scholar] [CrossRef]
- Dong, Q.; Brulc, J.M.; Iovieno, A.; Bates, B.; Garoutte, A.; Miller, D.; Revanna, K.V.; Gao, X.; Antonopoulos, D.A.; Slepak, V.Z.; et al. Diversity of bacteria at healthy human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5408–5413. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant Gut Microbiome Associated with Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Williamson, I.A.; Arnold, J.W.; Samsa, L.A.; Gaynor, L.; DiSalvo, M.; Cocchiaro, J.L.; Carroll, I.; Azcarate-Peril, M.A.; Rawls, J.F.; Allbritton, N.L.; et al. A High-Throughput Organoid Microinjection Platform to Study Gastrointestinal Microbiota and Luminal Physiology. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Lazo, M.; Quispe-Vicuña, C.; Barja-Ore, J.; Fernandez-Giusti, A.; Munive-Degregori, A.; Retamozo-Siancas, Y.; Guerrero, M.E.; Mayta-Tovalino, F. A 10-Year Bibliometric Analysis of Global Research on Gut Microbiota and Parkinson’s Disease: Characteristics, Impact, and Trends. Biomed. Res. Int. 2022, 2022, 4144781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Long, T.; You, J.; Li, P.; Xu, Q. Bibliometric Visualization Analysis of Microbiome-Gut-Brain Axis from 2004 to 2020. Med. Sci. Monit. 2022, 28, e936037. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Wu, J.; Peng, X.; Li, Y.; Tang, L.; Xu, X.; Deng, D.; Zhou, X. Visualized analysis of trends and hotspots in global oral microbiome research: A bibliometric study. Med. Comm. 2020, 3, 351–361. [Google Scholar] [CrossRef]
- Congressional Research Service. U.S. Research and Development Funding and Performance: Fact Sheet. Available online: https://sgp.fas.org/crs/misc/R44307.pdf (accessed on 23 May 2023).
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Wang, S.Q.; Gao, Y.Q.; Zhang, C.; Xie, Y.J.; Wang, J.X.; Xu, F.Y. A Bibliometric Analysis Using CiteSpace of Publications from 1999 to 2018 on Patient Rehabilitation After Total Knee Arthroplasty. Med. Sci. Monit. 2020, 26, e920795. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Caspi, R.R. A look at autoimmunity and inflammation in the eye. J. Clin. Investig. 2010, 120, 3073–3083. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, M.S.; Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Leichtle, A.B.; Largiadèr, C.R.; Fiedler, G.M.; Wolf, S. Association of the Intestinal Microbiome with the Development of Neovascular Age-Related Macular Degeneration. Sci. Rep. 2017, 7, 40826. [Google Scholar] [CrossRef] [PubMed]
- Lin, P. The role of the intestinal microbiome in ocular inflammatory disease. Curr. Opin. Ophthalmol. 2018, 29, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lin, P. Importance of the intestinal microbiota in ocular inflammatory diseases: A review. Clin. Exp. Ophthalmol. 2019, 47, 418–422. [Google Scholar] [CrossRef]
- Nadeem, U.; Xie, B.; Movahedan, A.; D’Souza, M.; Barba, H.; Deng, N.; Leone, V.A.; Chang, E.; Sulakhe, D.; Skondra, D. High Throughput RNA Sequencing of Mice Retina Reveals Metabolic Pathways Involved in the Gut-Retina Axis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Xue, W.; Li, J.J.; Zou, Y.; Zou, B.; Wei, L. Microbiota and Ocular Diseases. Front. Cell Infect. Microbiol. 2021, 11, 759333. [Google Scholar] [CrossRef]
- Baim, A.D.; Movahedan, A.; Farooq, A.V.; Skondra, D. The microbiome and ophthalmic disease. Exp. Biol. Med. 2019, 244, 419–429. [Google Scholar] [CrossRef]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef]
- Challa, P.; Schmidt, S.; Liu, Y.; Qin, X.; Vann, R.R.; Gonzalez, P.; Allingham, R.R.; Hauser, M.A. Analysis of LOXL1 polymorphisms in a United States population with pseudoexfoliation glaucoma. Mol. Vis. 2008, 14, 146–149. [Google Scholar]
- Organisciak, D.; Wong, P.; Rapp, C.; Darrow, R.; Ziesel, A.; Rangarajan, R.; Lang, J. Light-induced retinal degeneration is prevented by zinc, a component in the age-related eye disease study formulation. Photochem. Photobiol. 2012, 88, 1396–1407. [Google Scholar] [CrossRef]
- Gupta, A. Harnessing the microbiome in glaucoma and uveitis. Med. Hypotheses 2015, 85, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Vagge, A.; Pulliero, A.; Izzotti, A. Helicobacter pylori infection and eye diseases: A systematic review. Medicine 2014, 93, e216. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Ridola, L.; Hassan, C.; Bruzzese, V.; Papini, F.; Vaira, D. Glaucoma and Helicobacter pylori: Eyes wide shut? Dig. Liver Dis. 2012, 44, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, H.; Liu, X.; Ding, C. The Relationship Between Helicobacter pylori Infection and Open-Angle Glaucoma: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5238–5245. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.H.; Park, K.H.; Han, S.Y.; Shim, H.S. Investigation of the association between Helicobacter pylori infection and normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 665–668. [Google Scholar] [CrossRef]
- Kountouras, J.; Mylopoulos, N.; Konstas, A.G.; Zavos, C.; Chatzopoulos, D.; Boukla, A. Increased levels of Helicobacter pylori IgG antibodies in aqueous humor of patients with primary open-angle and exfoliation glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2003, 241, 884–890. [Google Scholar] [CrossRef]
- Zavos, C.; Kountouras, J.; Sakkias, G.; Venizelos, I.; Deretzi, G.; Arapoglou, S. Histological presence of Helicobacter pylori bacteria in the trabeculum and iris of patients with primary open-angle glaucoma. Ophthalmic. Res. 2012, 47, 150–156. [Google Scholar] [CrossRef]
- Kulacoǧlu, D.N.; Özbek, A.; Uslu, H.; Sahin, F.; Güllülü, G.; Koçer, I.; Karabela, Y. Comparative lid flora in anterior blepharitis. Turk. J. Med. Sci. 2001, 31, 359–363. [Google Scholar]
- Pinna, A.; Sechi, L.A.; Zanetti, S.; Carta, F. Detection of virulence factors in a corneal isolate of Klebsiella pneumoniae. Ophthalmology 2005, 112, 883–887. [Google Scholar] [CrossRef]
- Jiang, X.; Deng, A.; Yang, J.; Bai, H.; Yang, Z.; Wu, J.; Lv, H.; Li, X.; Wen, T. Pathogens in the Meibomian gland and conjunctival sac: Microbiome of normal subjects and patients with Meibomian gland dysfunction. Infect. Drug. Resist. 2018, 11, 1729–1740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).