Abstract
Aims: Mutans streptococci include Streptococcus mutans and Streptococcus sobrinus, which can cause tooth decay. The current study aimed to compare their virulence genes with each other and to correlate them with the clinical data of patients. Materials and methods: Altogether 21 S. mutans and 19 S. sobrinus strains were investigated, originating from 24 children (age 2.7 ± 0.4 years) and 13 mothers (27.3 ± 3.7). The PCR method was applied to detect 11 virulence genes. Caries indices (dmf, decayed/missing/filled; DMFT, decayed/missing/filled teeth) and SM score (Mutans streptococci amount in saliva) were recorded. Results: Most of the S. mutans strains harbored all the virulence genes studied, while S. sobrinus had significantly fewer genes. The genes gbpA, gbpB, wapA and ftf were present in all isolates of S. sobrinus, the spaP, gtfB, vicR, SMU.1037c and SMU.105 genes were present in 41–88% of the isolates, while gtfD and SMU.104 genes were absent in S. sobrinus strains studied. A positive correlation appeared between the biofilm-related vicR and polysaccharide-production-related gtfD genes. In contrast, another polysaccharide-production-related gtfB gene was present in some cases in strains lacking the vicR or gtfD gene. Positive association was found between the presence of adhesion-related spaP gene in pediatric-derived S. sobrinus strains and an increase in SM score. Conclusions: Differences exist between the two common species of mutans streptococci: strains of S. mutans have more virulence genes than that of S. sobrinus, both crucial and virulence enhancing. Deeper research is needed to clarify the mechanisms behind the increased cariogenicity in cohabitation.
1. Introduction
Dental caries is a common disease in both adults and children—about 70–90% of the world’s population is affected by caries [1,2]. The prevalence of dental caries in toddlers varies within Europe, ranging from 8% in Finland [3] to 56% in Poland [4]. A study conducted in Estonia revealed that caries occurred in 29% of 3-year-old children, 72% in 6-year-old children and 68% in 12-year-olds [5].
The oral cavity contains highly diverse normal microbiota, which performs various useful functions. In total, about 1000 different species of microorganisms have been found in the oral cavity [6], of which about 50–100 species reside in the mouth of one person. Streptococci make up about 50% of the oral microbiota [7,8]. The development of dental caries is associated with numerous factors, such as oral cleaning and eating habits, salivation and dentition, but it is also significantly associated with changes in the composition of the oral microbiota, in particular, the excessive proliferation of mutans streptococci (MS). The most common species among MS in humans are Streptococcus mutans and Streptococcus sobrinus [1].
The environmental conditions in the mouth and focal dental caries are complex and constantly changing. This highlights the remarkable adaptability of MS, which is attributable to a wide range of virulence factors, including biofilm formation and adhesion, polysaccharide production, and carbohydrate cleavage with acid production [8,9]. A biofilm is an aggregate of bacteria attached to a surface, usually covered with a matrix of exopolysaccharides [10]. Bacteria living in biofilms are significantly more tolerant to antibiotics and biocides [11]. Biofilm formation by MS is associated with the genes wapA [12] and vicR, and the latter helps the bacterium detect environmental changes and respond to stress conditions [13]. Glycan-binding proteins (Gbps—gbpA and gbpB), glycosyltransferases (GTF—gtfB and gtfD) [14], as well as a surface protein called antigen I/II (coded by spaP, also known as pac, P1 and Ag I/II) are involved in MS adhesion [15]. Gbps and GTF-producing genes are closely related because S. mutans synthesizes glycans from sucrose (a substrate for GTFs) using glycosyltransferases [14]. The function of spaP is to mediate S. mutans adhesion to saliva-coated tooth surfaces [16]. In addition, streptococci have the ability to produce extracellular polysaccharides (EPS) from sucrose [17], involving gtfB, gtfD, and ftf genes [1,18]. EPS are important components in biofilm [19] and contribute to the cariogenicity, stress tolerance and antimicrobial resistance of S. mutans [20]. EPS also serves to provide a supply of substrates for the bacterium to promote adhesion and aggregation between microorganisms, and to increase the thickness and density of plaque [21].
Another important virulence factor for MS is acidogenicity [1]. MSs ferment carbohydrates and produce organic acids, especially lactic acid, by changing the pH of the external environment [22]. The environment of the dental plaque becomes acidic, resulting in the demineralization of tooth enamel and later dentin. Caries bacteria themselves are acid tolerant [2,23].
Although caries and its causes have been studied for decades, there are few studies comparing the virulence genes of both major caries pathogens and associating them with oral health indicators. Therefore, this investigation aimed to compare the cariogenicity-related virulence genes and clinical impact of S. mutans and S. sobrinus isolated from 2- to 4-year-old children and their mothers.
2. Materials and Methods
2.1. Bacterial Strains
Forty MS strains were included in the study, including 21 S. mutans and 19 S. sobrinus. The strains originated from a former study where the oral health of mothers and children was assessed [24], with permission of the Ethics Committee for Human Research of the University of Tartu (protocol no. 166/T-7). The strain donors included 24 children (aged 24 to 41 months) and 13 mothers (aged 22 to 31 years) (Table 1). All donors had dental caries. Background data included the DMF index [25] and SM score measured using the commercial kit Dentocult SM Strip mutans (Orion Diagnostica Oy, Espoo, Finland). The strains are stored in the HUMB collection (Human Microbial Biobank) at the University of Tartu (http://eemb.ut.ee/humb, accessed on 3 November 2022).

Table 1.
Clinical parameters (mean ± SD) of strain donors.
2.2. Molecular Methods
A QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used to isolate DNA.
To determine if all mutans streptococci strains were different, genotyping was performed by pulsed field gel electrophoresis (Supplementary Figure S1). Based on the genotyping data, 38 bacterial strains were included in the analysis.
The PCR method was used to reveal virulence genes. The known virulence genes of MS are presented in Table 2; of them, 11 virulence genes were selected for the study: gbpA, gbpB and spaP genes, responsible for adhesion; wapA and vicR, involved in biofilm formation; ftf, gtfB and gtfD, involved in the production of the polysaccharides needed for biofilm; SMU.104 and SMU.105, responsible for acid production, and SMU.1037c contributing to acid tolerance.

Table 2.
Virulence mechanisms and their genes in mutans streptococci (adapted from [26]).
More details of molecular methods are presented in Supplementary Table S1. The primers and the most suitable primer annealing temperatures are presented in Supplementary Table S2.
2.3. Statistical Analysis
Data were stored and analyzed in MS Excel software. Spearman’s rs correlation test (p < 0.05) was used to find the assocation of virulence genes with caries markers (p < 0.05). Pearson’s Chi square test (p < 0.05) was used to find differences between the groups.
3. Results
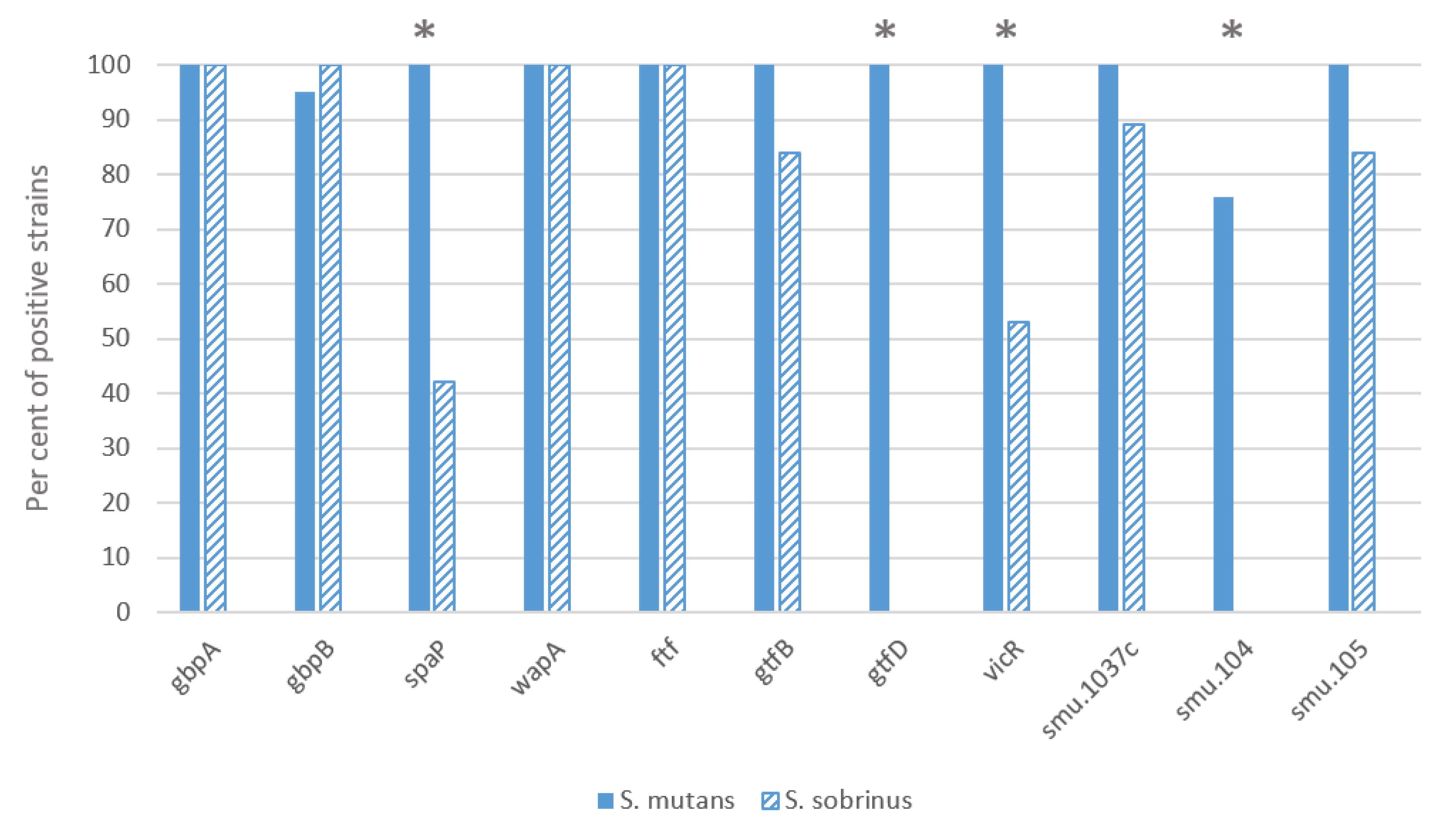
Most of the investigated S. mutans strains had all the virulence genes studied, both essential and virulence-enhancing (Figure 1). Only the SMU.104 gene, which is involved in acid production, was absent in a quarter of the strains of this species. In contrast, several virulence genes were significantly less represented in the S. sobrinus strains: gbpA, gbpB, wapA and ftf genes were present in all strains, and spaP, gtfB, vicR, SMU.1037c and SMU.105 genes in 41–88% strains, while gtfD and SMU.104 genes were not found in the S. sobrinus strains. There was a statistically significant difference between the two species for the genes spaP, vicR, gtfD and SMU.104 (p < 0.01). A correlation appeared between the vicR and gtfD genes (r2 = 0.574; p < 0.01), the latter of which was absent from strains lacking the vicR gene. In contrast, the gtfB gene was present in some cases in strains lacking the vicR or gtfD gene (vicR and gtfB r2 = 0.328; p < 0.05 and gtfD and gtfB r2 = 0.325; p < 0.05).
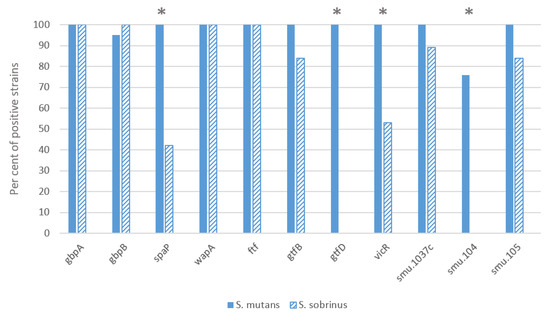
Figure 1.
Distribution of virulence genes between two mutans streptococci species. Pearson chi-square test was applied, the asterisk indicates difference p < 0.01.
Table 3 and Table 4 show correlations between clinical parameters and virulence genes. We found a positive association between the spaP gene and the SM score in children (r2 = 0.643, p = 0.033).

Table 3.
Correlation between SM score and virulence genes between mutans streptococci and mothers/children. The level of each person’s SM score (0–3) is compared to the specific gene present/absent in their bacterial strain. Results indicating positive correlation and statistical significance (p < 0.05) are marked in bold.

Table 4.
Relationship between DMFT index and virulence genes between mutans streptococci in mothers and dmf index and virulence genes between mutans streptococci in children. Each mother’s DMFT level (0–32)/children’s dmf level (0–20) is compared to the specific gene present/absent in the bacterial strain.
4. Discussion
This study confirmed that both SM species have multiple virulence genes but differences detected between the species and strains. Only the adhesion genes gbpA, gbpB, ftf and wapA were present in all investigated strains. Significantly more virulence genes were found in S. mutans than in S. sobrinus. Analysis also showed a positive association between the presence of spaP in S. sobrinus and an increase in the SM score in children.
All of the investigated genes play important roles in the physiology of MS. If the genes are turned off, the strain may be less resistant to environmental conditions, as changes may occur in the formation and structure of the biofilm [12,27,28], in adhesion to the tooth surface [29,30,31] or a decrease in EPS production [21,32]. Current research revealed that most of the S. mutans strains carried all the virulence genes studied, both essential and virulence-enhancing, related to biofilm formation, adhesion, acid production and tolerance, and interactions with the environment. In contrast, several virulence genes occurred less frequently in S. sobrinus strains. Previous studies have also shown some differences between these species. For example, de Soet et al. [33] and Igarashi et al. [34] found that S. sobrinus has a higher acid production capacity than S. mutans. In addition, previous research [34,35] has shown that S. sobrinus is rich in GTF-producing genes, and the most important is gtfB. In our study, this gene was found in 82% of the S. sobrinus strains studied, but the gtfD gene was completely absent in our strains. Other genes not studied in this work may compensate for the production of different glycotransferases.
Vic genes regulate the expression of several other virulence-related genes (gtfB, gtfC, gtfD, ftf, and gbpB) by acting on their promoter regions. The null-mutation of vicR is probably lethal to S. mutans [13] and is therefore present in all strains studied. In a study of Zhuang et al. [36], 121 vicR genes were isolated and purified from S. mutans isolated from the children with and without caries and were found to be conserved in all isolates. Although vicR has been found to be essential for S. mutans, this may be different for S. sobrinus, which corresponds to the current study, where nearly half of the S. sobrinus strains lacked this gene. The prevalence of vicR varies among streptococci while the cause is not yet clear [13,37]. The VicRK signaling system is known to affect GTF expression in S. mutans. In the absence of the vicRK system in mutant strains of S. mutans, a decrease in gtfD gene expression and an increase in gtfB gene expression were observed [13,38]. The current study did not examine the expression level but revealed a positive correlation between the presence of the vicR and gtfD genes.
The SMU.1037c gene was found in 88% of S. sobrinus strains, and it helps to adapt the bacterium to changing environmental conditions. Conrads et al. [35] found that S. sobrinus lacked the SMU.1037c and TCS-7 systems, but they compared only two strains of this species. The SMU.104 encodes the protein α-glucosidase glycosyl hydrolase, whose biological role is involved in carbohydrate metabolism and whose molecular function is involved in the hydrolysis of glycosyl bonds [39]. According to Banas [40], acid production varies between MS strains. SMU.104 and SMU.105 genes are not necessarily essential for the bacterium but may increase the competitive advantage over other strains by contributing to faster acid production. In our study, a quarter of the S. mutans strains and all S. sobrinus strains lacked the SMU.104 gene.
S. mutans and S. sobrinus strains may have different mechanisms of perception and response, and because they often symbiotically coexist in the biofilm, S. sobrinus may not need to have all the genes. The main virulence traits of S. mutans are controlled or modulated by quorum sensing and thus depend on its own cell number but maybe also on cell numbers of cohabitants [35]. Although the genes selected for the study are important in the development of virulence, not all genes required for virulence were identified in the study. On the other hand, MS may have more virulence genes that were overlooked in this study.
Caries markers of children in our study were similar to a previous Estonian study where caries was diagnosed in 42% of the children at the age of 41 months, and the average dmft index was 1.6 ± 2.5 [41] as well as to studies conducted in other countries—1.64 ± 3.84 in Greece, 1.25 ± 2.47 in China [42,43]. DMFT index in mothers was also nearly similar to studies conducted worldwide (11.02 ± 6.3 in Brasil, 14.45 in the Philippines) [44,45]. Here, the clinical markers of the strain donors with the genetic information of the strains were compared. Although the spaP gene was detected to a much lesser extent in S. sobrinus than in S. mutans, a positive relationship between this gene and SM score in children was observed. This may be a prerequisite for further increased cariogenicity in these children. Because spaP is responsible for adhesion, bacteria with this gene may have a better ability to attach to the tooth and, ultimately, colonize the tooth surface better [30,46]. Our result corresponds to some previous studies showing that children with caries tend to carry spaP-positive mutans streptococci [45,47].
The other correlations between the virulence genes and clinical markers were not statistically significant. It should be taken into consideration that one person may have both S. mutans and S. sobrinus in the mouth, and they have been found to increase cariogenicity when living together [48,49,50,51]. At the same time, the set of strains was not large in our study, and the donor group was heterogeneous, which can be considered a limitation of the study. On the other hand, scarce studies have been performed on S. sobrinus so far, and only a few strains have been investigated in these studies; therefore, our study can be used as reference work for further studies.
5. Conclusions
Differences exist between the virulence gene patterns of the mutans streptococci: strains of S. mutans have more virulence genes than that of S. sobrinus, both crucial and virulence enhancing. The clinical significance of different virulence genes needs further investigation. Deeper research is needed to clarify the mechanisms behind the increased cariogenicity in cohabitation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres13040065/s1, Table S1: Details of molecular methods; Table S2: Primers of virulence genes studied; Figure S1: A—Genotyped S. sobrinus strains. M1—SmaI enzyme; all strains are identified by their last three digits from their HUMB code; strains with similar gene patterns of mother and child are marked in blue (child 011-HUMB_13011, 012-HUMB_13012 and mother 105-HUMB_13105). Strain 018 is similar to latter strains, but the strain has been isolated from another individual. Strains 038 and 039 are isolated from the same caries site and also have the same gene pattern. The gene pattern of the previous strains are also similar to strain 104, but it is also isolated from another individual. Among the strains were three pairs of bacterial strains from the same individuals. Since the two pairs of strains also had a similar gene pattern, the two replicates were removed from further analysis. Two strains of S. sobrinus with different gene patterns were isolated from a third person, so both strains were included in the analysis. Thus, 37 human information but 38 bacterial strains were included into the study. B—Genotyped S. mutans strains. M2—ApaI enzyme; all strains are identified by their last three digits from their HUMB code.
Author Contributions
Conceptualization, R.M. and J.Š.; methodology, J.Š. and G.M.; software, M.J. and J.Š.; formal analysis, G.M., J.Š., M.J., J.O. and R.M.; investigation, G.M. and J.Š.; resources, R.M. and J.O.; data curation, J.Š., M.J. and R.M.; writing—original draft preparation, G.M.; writing—review and editing, J.Š., M.J., J.O. and R.M.; visualization, G.M. and R.M.; supervision, J.Š., M.J. and R.M.; project administration, R.M.; funding acquisition, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Enterprise Estonia (grant No. EU48695) and the Estonian Ministry of Education and Research (grant No. KOGU-HUMB).
Institutional Review Board Statement
The strains originated from a former study where the oral health of mothers and children was assessed [24], with permission of the Ethics Committee for Human Research of the University of Tartu (protocol no. 166/T-7).
Data Availability Statement
All the data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank Tiiu Rööp for excellent technical help.
Conflicts of Interest
The authors report no conflict of interest regarding the publication of this paper.
References
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, S.; Söderling, E.; Sewón, L.; Lapinleimu, H.; Simell, O. A prospective study on sucrose consumption, visible plaque and caries in children from 3 to 6 years of age. Community Dent. Oral Epidemiol. 2001, 29, 136–142. [Google Scholar] [CrossRef]
- Szatko, F.; Wierzbicka, M.; Dybizbanska, E.; Struzycka, I.; Iwanicka-Frankowska, E. Oral health of Polish three-year-olds and mothers’ oral health-related knowledge. Community Dent. Health 2004, 21, 175–180. [Google Scholar] [PubMed]
- Olak, J.; Nõmmela, R.; Lilleberg, M.; Murakas, R. Ülevaade 3-, 6- ja 12-Aastaste Laste Suutervise Uuringust. Eesti Hambaarstide Liit. 2019. Available online: https://ehl.ee/artikkel/ulevaade-3-6-ja-12-aastaste-laste-suutervise-uuringust/ (accessed on 3 November 2022). (In Estonian).
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Ojcius, D.M.; Yilmaz, Ö. The Oral Microbiota: Living with a Permanent Guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbiology of Dental Plaque Biofilms and Their Role in Oral Health and Caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef]
- Lemos, J.A.; Burne, R.A. A model of efficiency: Stress tolerance by Streptococcus mutans. Microbiology 2008, 154 Pt 11, 3247–3255. [Google Scholar] [CrossRef]
- Costerton, J.W. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Zhu, L.; Kreth, J.; Cross, S.E.; Gimzewski, J.K.; Shi, W.; Qi, F. Functional characterization of cell-wall-associated protein WapA in Streptococcus mutans. Microbiology 2006, 152, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, M.D.; Guggenheim, B.; Spatafora, G.A.; Huang, Y.C.C.; Choi, J.; Hung, D.C.; Treglown, J.S.; Goodman, S.D.; Ellen, R.P.; Cvitkovitch, D.G. A VicRK Signal Transduction System in Streptococcus mutans Affects gtfBCD, gbpB, and ftf Expression, Biofilm Formation, and Genetic Competence Development. J. Bacteriol. 2005, 187, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, H.K. Molecular genetic analysis of the virulence of oral bacterial pathogens: An historical perspective. Crit. Rev. Oral Biol. Med. 2003, 14, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Hirota, K.; Nemoto, K.; Fernandez, E.J.; Ota, F.; Fukui, K. Detection of Streptococcus mutans by PCR amplification of spaP gene. J. Med. Microbiol. 1994, 41, 231–235. [Google Scholar] [CrossRef][Green Version]
- Kelly, C.G.; Todryk, S.; Kendal, H.L.; Munro, G.H.; Lehner, T. T-cell, adhesion, and B-cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect. Immun. 1995, 63, 3649–3658. [Google Scholar] [CrossRef]
- Bowden, G.H.; Hamilton, I.R. Survival of oral bacteria. Crit. Rev. Oral Biol. Med. 1998, 9, 54–85. [Google Scholar] [CrossRef]
- Sato, S.; Kuramitsu, H.K. Isolation and characterization of a fructosyltransferase gene from Streptococcus mutans GS-5. Infect. Immun. 1986, 52, 166–1670. [Google Scholar] [CrossRef]
- Rölla, G. Why is sucrose so cariogenic? The role of glucosyltransferase and polysaccharides. Scand. J. Dent. Res. 1989, 97, 115–119. [Google Scholar] [CrossRef]
- Lei, L.; Yang, Y.; Mao, M.; Li, H.; Li, M.; Yang, Y.; Yin, J.; Hu, T. Modulation of Biofilm Exopolysaccharides by the Streptococcus mutans vicX Gene. Front. Microbiol. 2015, 6, 1432. [Google Scholar] [CrossRef]
- Shellis, R.P.; Dibdin, G.H. Analysis of the Buffering Systems in Dental Plaque. J. Dent. Res. 1988, 67, 438–446. [Google Scholar] [CrossRef]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. The continuum of dental caries—Evidence for a dynamic disease process. J. Dent. Res. 2004, 83, C39–C42. [Google Scholar] [CrossRef] [PubMed]
- Olak, J. Dental Health in Preschool and Schoolchildren in Relation to Dental Fear and Some Fear-Related Factors, and the Outcome of a Caries Prevention Program in Offspring of Fearful Mothers. Ph.D. Thesis, University of Turku, Turku, Finland, 2013. [Google Scholar]
- Honkala, E.; Runnel, R.; Honkala, S.; Olak, J.; Vahlberg, T.; Saag, M.; Mäkinen, K.K. Measuring dental caries in the mixed dentition by ICDAS. Int. J. Dent. 2011, 2011, 15024. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.-M.; Klein, C.; Schwindt, D.; Von Ohle, C. Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int. J. Oral Sci. 2014, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.J.; Michalek, S.M.; Zhu, M.; Drake, D.; Qian, F.; Banas, J.A. Cariogenicity of Streptococcus mutans Glucan-Binding Protein Deletion Mutants. Oral Health Dent. Manag. 2013, 12, 191–199. [Google Scholar]
- Matsumi, Y.; Fujita, K.; Takashima, Y.; Yanagida, K.; Morikawa, Y.; Matsumoto-Nakano, M. Contribution of glucan-binding protein A to firm and stable biofilm formation by Streptococcus mutans. Mol. Oral Microbiol. 2015, 30, 217–226. [Google Scholar] [CrossRef]
- Hazlett, K.R.O.; Mazurkiewicz, J.E.; Banas, J.A. Inactivation of the gbpA Gene of Streptococcus mutans Alters Structural and Functional Aspects of Plaque Biofilm Which Are Compensated by Recombination of the gtfB and gtfC Genes. Infect. Immun. 1999, 67, 3909–3914. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Demuth, D.R. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 1997, 23, 183–190. [Google Scholar] [CrossRef]
- Rozen, R.; Steinberg, D.; Bachrach, G. Streptococcus mutans fructosyltransferase interactions with glucans. FEMS Microbiol. Lett. 2004, 232, 39–43. [Google Scholar] [CrossRef]
- Yamashita, Y.; Bowen, W.H.; Burne, R.A.; Kuramitsu, H.K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 1993, 61, 3811–3817. [Google Scholar] [CrossRef]
- De Soet, J.J.; Van Loveren, C.; Lammens, A.J.; Pavičić, M.J.A.M.P.; Homburg, C.H.E.; Ten Cate, J.M.; De Graaff, J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Yamamoto, A.; Goto, N. PCR for detection and identification of Streptococcus sobrinus. J. Med. Microbiol. 2000, 49, 1069–1074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conrads, G.; de Soet, J.J.; Song, L.; Henne, K.; Sztajer, H.; Wagner-Döbler, I.; Zeng, A.-P. Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J. Oral Microbiol. 2014, 6, 26189. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.L.; Yu, L.X.; Liao, J.K.; Zhou, Y.; Lin, H.C. Relationship between the genetic polymorphisms of vicR and vicK Streptococcus mutans genes and early childhood caries in two-year-old children. BMC Oral Health 2018, 18, 39. [Google Scholar] [CrossRef]
- Mattos-Graner, R.O.; Porter, K.A.; Smith, D.J.; Hosogi, Y.; Duncan, M.J. Functional Analysis of Glucan Binding Protein B from Streptococcus mutans. J. Bacteriol. 2006, 188, 3813–3825. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- EMBL-EBI. Gene Ontology and GO Annotations. Available online: https://www.ebi.ac.uk/QuickGO/annotations?geneProductId=Q8DWF5 (accessed on 2 June 2020).
- Banas, J.A. Virulence properties of Streptococcus mutans. Front. Biosci. 2004, 9, 1267–1277. [Google Scholar] [CrossRef]
- Olak, J.; Mändar, R.; Karjalainen, S.; Söderling, E.; Saag, M. Dental health and oral mutans streptococci in 2–4-year-old Estonian children. Int. J. Paediatr. Dent. 2007, 17, 92–97. [Google Scholar] [CrossRef]
- Bourgeois, D.M.; Llodra, J.C. Global burden of dental condition among children in nine countries participating in an international oral health promotion programme, 2012–2013. Int. Dent. J. 2014, 64, 27–34. [Google Scholar] [CrossRef]
- Fan, C.C.; Wang, W.H.; Xu, T.; Zheng, S.G. Risk factors of early childhood caries (ECC) among children in Beijing—A prospective cohort study. BMC Oral Health 2019, 19, 34. [Google Scholar] [CrossRef]
- Lukacs, J.R. Sex differences in dental caries experience: Clinical evidence, complex etiology. Clin. Oral Investig. 2011, 15, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Pieralise, F.J.S.; Maciel, S.M.; de Andrade, F.B.; Garcia, J.E.; Poli-Frederico, R.C. Detection of Streptococcus mutans of the spaP gene and dental caries in mother/child pairs. RGO Rev. Gaúcha Odontol. 2013, 61, 205–211. [Google Scholar]
- Crowley, P.J.; Brady, L.J.; Michalek, S.M.; Bleiweis, A.S. Virulence of a spaP Mutant of Streptococcus mutans in a Gnotobiotic Rat Model. Infect. Immun. 1999, 67, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Durán-Contreras, G.L.; Torre-Martínez, H.H.; De La Rosa, E.I.; Hernández, R.M.; De La Garza-Ramos, M.A. spaP gene of Streptococcus mutans in dental plaque and its relationship with early childhood caries. Eur. J. Paediatr. Dent. 2011, 12, 220–224. [Google Scholar] [PubMed]
- Hirose, H.; Hirose, K.; Isogai, E.; Mima, H.; Ueda, I. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 1993, 27, 292–297. [Google Scholar] [CrossRef]
- Okada, M.; Soda, Y.; Hayashi, F.; Doi, T.; Suzuki, J.; Miura, K.; Kozai, K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J. Med. Microbiol. 2005, 54, 661–665. [Google Scholar] [CrossRef]
- Hata, S.; Hata, H.; Miyasawa-Hori, H.; Kudo, A.; Mayanagi, H. Quantitative detection of Streptococcus mutans in the dental plaque of Japanese preschool children by real-time PCR. Lett. Appl. Microbiol. 2006, 42, 127–131. [Google Scholar] [CrossRef]
- Saraithong, P.; Pattanaporn, K.; Chen, Z.; Khongkhunthian, S.; Laohapensang, P.; Chhun, N.; Gaw, H.Y.; Li, Y. Streptococcus mutans and Streptococcus sobrinus colonization and caries experience in 3- and 5-year-old Thai children. Clin. Oral Investig. 2015, 19, 1955–1964. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).