Prevalence and Diversity of Hepatitis Virus Markers among Patients with Acute Febrile Jaundice in Chad
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Samples
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. Seroprevalence of HEV, HBV and HCV
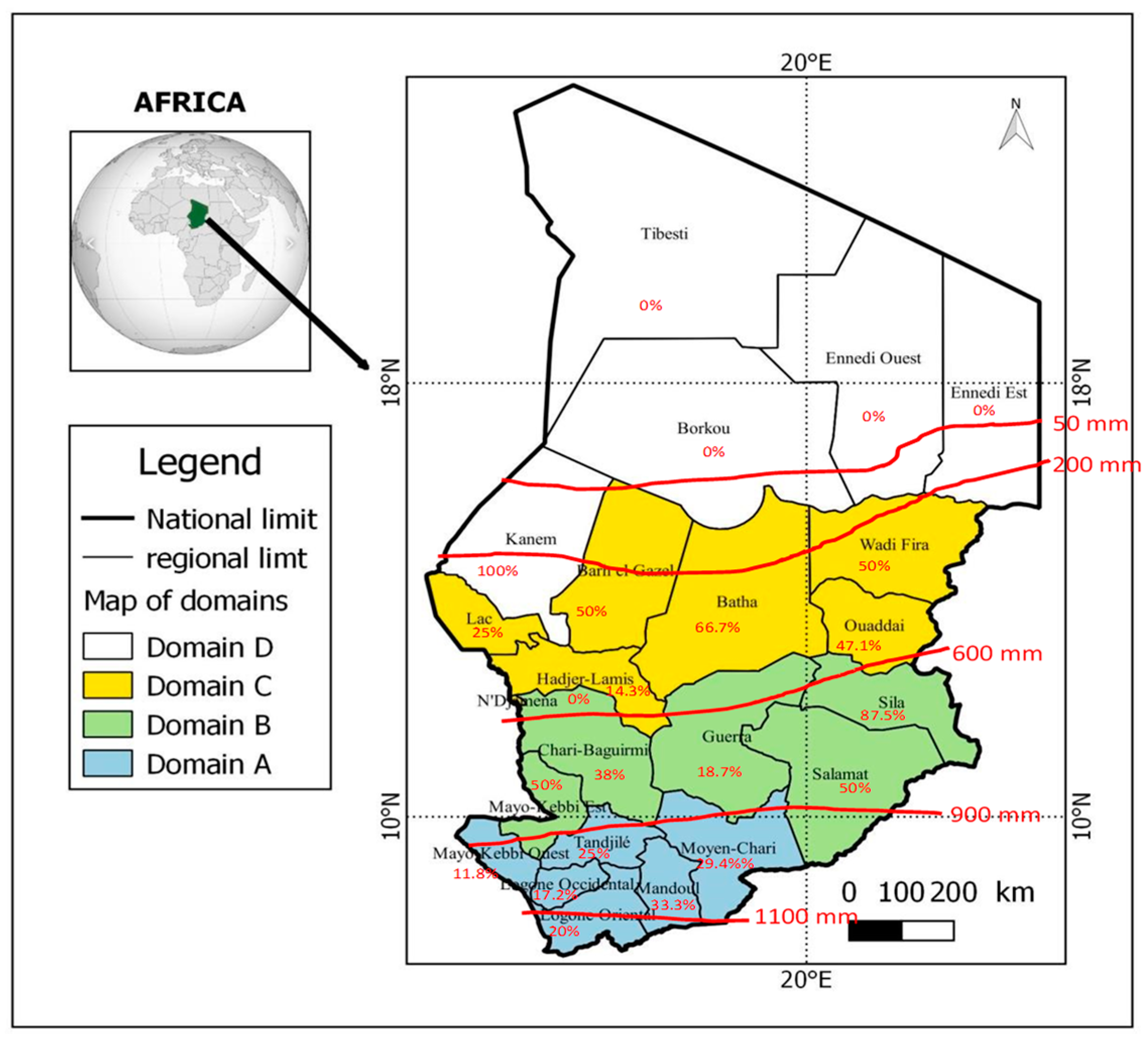
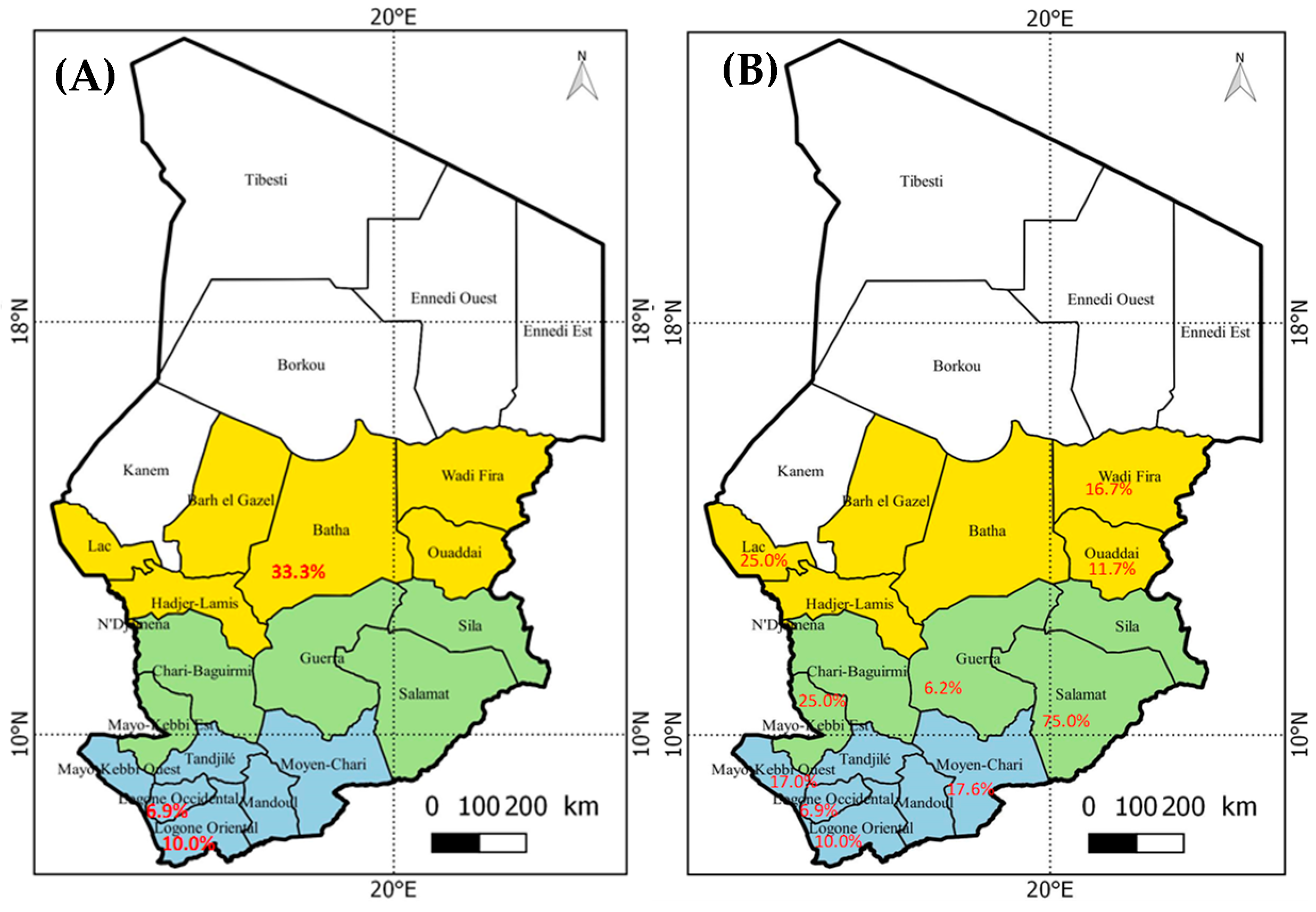
3.2. Geographical Distribution of Hepatitis Viruses
3.3. Distribution of Hepatitis Markers in Geographical Zones
3.4. Potential Risk Factors for Hepatitis Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staples, J.E. Yellow Fever: 100 Years of Discovery. JAMA 2008, 300, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Yaro, S.; Ouoba, A.R.; Zango, A.; Rouamba, J.; Drabo, A.; Ouangraoua, S.; Samandoulougou-Kirakoya, F.; Macq, J.; Robert, A.; Ouedraogo, J.B. Management Problems of Trans-Frontier Yellow Fever Cases in Burkina Faso 2010. Adv. Infect. Dis. 2013, 3, 84–88. [Google Scholar] [CrossRef]
- WHO. Vaccines and vaccination against yellow fever. WHO position paper—June 2013. Wkly. Epidemiol. Rec. 2013, 88, 269–283. [Google Scholar]
- Chen, L.H.; Wilson, M.E. Yellow fever control: Current epidemiology and vaccination strategies. Trop. Dis. Travel Med. Vaccines 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Mutebi, J.-P.; Barrett, A.D. The epidemiology of yellow fever in Africa. Microbes Infect. 2002, 4, 1459–1468. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Smart, S.J.; Zhou, L.; Yang, P.; Ou, J.; He, Y.; Ren, R.; Ma, T.; Xiang, N.; et al. Evaluating the importation of yellow fever cases into China in 2016 and strategies used to prevent and control the spread of the disease. West. Pac. Surveill. Response J. 2020, 11, 5–10. [Google Scholar] [CrossRef]
- Diagne, M.; Ndione, M.; Gaye, A.; Barry, M.; Diallo, D.; Diallo, A.; Mwakibete, L.; Diop, M.; Ndiaye, E.; Ahyong, V.; et al. Yellow Fever Outbreak in Eastern Senegal, 2020–2021. Viruses 2021, 13, 1475. [Google Scholar] [CrossRef]
- Djarma, O.M.; Elisee, D.; Bolti, M.A.; Sougoudi, D.A.; Diop, A.B.; Haggar, F.A.; Hidjab, A.; Chatté, A.; Mad-Toingue, J.; Fissou, H. Recrudescence of yellow fever in Chad: Case report of the last confirmed case in the health district of Lai-Chad. Pan. Afr. Med. J. 2021, 38, 248. [Google Scholar]
- Bissell, D.M. Formation and Elimination of Bilirubin. Gastroenterology 1975, 69, 519–538. [Google Scholar] [CrossRef]
- Billing, B.H. Twenty-five years of progress in bilirubin metabolism (1952-77). Gut 1978, 19, 481–491. [Google Scholar] [CrossRef]
- Adungo, F.; Yu, F.; Kamau, D.; Inoue, S.; Hayasaka, D.; Posadas-Herrera, G.; Sang, R.; Mwau, M.; Morita, K. Development and Characterization of Monoclonal Antibodies to Yellow Fever Virus and Application in Antigen Detection and IgM Capture Enzyme-Linked Immunosorbent Assay. Clin. Vaccine Immunol. 2016, 23, 689–697. [Google Scholar] [CrossRef]
- Makiala-Mandanda, S.; Le Gal, F.; Ngwaka-Matsung, N.; Ahuka-Mundeke, S.; Onanga, R.; Bivigou-Mboumba, B.; Pukuta-Simbu, E.; Gerber, A.; Abbate, J.L.; Mwamba, D.; et al. High Prevalence and Diversity of Hepatitis Viruses in Suspected Cases of Yellow Fever in the Democratic Republic of Congo. J. Clin. Microbiol. 2017, 55, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Vernier, L.; Lenglet, A.; Hogema, B.M.; Moussa, A.M.; Ariti, C.; Vollmer, S.; Irwin, A.; Alfani, P.; Sang, S.; Kamau, C. Sero-prevalence and risk factors of recent infection with hepatitis E virus during an acute outbreak in an urban setting in Chad, 2017. BMC Infect. Dis. 2018, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.H.; Demanou, M.; Mulders, M.; Mendez-Rico, J.; Basile, A.J. Technical viability of the YF MAC-HD ELISA kit for use in yellow fever-endemic regions. PLoS Negl. Trop. Dis. 2021, 15, e0009417. [Google Scholar] [CrossRef]
- Spina, A.; Lenglet, A.; Beversluis, D.; De Jong, M.; Vernier, L.; Spencer, C.; Andayi, F.; Kamau, C.; Vollmer, S.; Hogema, B.; et al. A large outbreak of Hepatitis E virus genotype 1 infection in an urban setting in Chad likely linked to household level transmission factors, 2016-2017. PLoS ONE 2017, 12, e0188240. [Google Scholar] [CrossRef] [PubMed]
- Coursaget, P.; Buisson, Y.; N′Gawara, M.N.; Van Cuyck-Gandre, H.; Roue, R. Role of hepatitis E virus in sporadic cases of acute and fulminant hepatitis in an endemic area (Chad). Am. J. Trop. Med. Hyg. 1998, 58, 330–334. [Google Scholar] [CrossRef]
- Bagulo, H.; Majekodunmi, A.O.; Welburn, S.C. Hepatitis E in Sub Saharan Africa—A significant emerging disease. One Health 2020, 11, 100186. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, C.; Kania, D.; Mantono, J.M.; Kagoné, T.; Zida, S.; Tassembedo, S.; Dicko, A.; Tinto, B.; Yaro, S.; Hien, H.; et al. Hepatitis E Virus Infections among Patients with Acute Febrile Jaundice in Burkina Faso. Viruses 2019, 11, 554. [Google Scholar] [CrossRef]
- Lagare, A.; Testa, J.; Kadadé, G.; Zaneidou, M.; Ibrahim, A.; Issaka, B.; Ousmane, S.; Ibrahim, A. Outbreak of Hepatitis E Virus Infection in Displaced Persons Camps in Diffa Region, Niger, 2017. Am. J. Trop. Med. Hyg. 2018, 99, 1055–1057. [Google Scholar] [CrossRef]
- Modiyinji, A.F.; Amougou-Atsama, M.; Monamele, C.G.; Nola, M.; Njouom, R. Seroprevalence of hepatitis E virus antibodies in different human populations of Cameroon. J. Med. Virol. 2019, 91, 1989–1994. [Google Scholar] [CrossRef]
- Behloul, N.; Zhang, M.; Meng, J. Binding Preference of Anti-HEV Antibodies in Sera Collected in Algeria for Antigens Derived From HEV Genotype 1. Zahedan J. Res. Med. Sci. 2016, 16, e35312. [Google Scholar] [CrossRef]
- Azman, A.S.; Bouhenia, M.; Iyer, A.S.; Rumunu, J.; Laku, R.L.; Wamala, J.F.; Rodriguez-Barraquer, I.; Lessler, J.; Gignoux, E.; Luquero, F.J.; et al. High Hepatitis E Seroprevalence Among Displaced Persons in South Sudan. Am. J. Trop. Med. Hyg. 2017, 96, 1296–1301. [Google Scholar] [CrossRef]
- Junaid, S.A.; Agina, S.E.; Abubakar, K.A. Epidemiology and Associated Risk Factors of Hepatitis E Virus Infection in Plateau State, Nigeria. Virol. Res. Treat. 2014, 5, VRT.S15422–26. [Google Scholar] [CrossRef]
- Howard, C.M.; Handzel, T.; Hill, V.R.; Grytdal, S.P.; Blanton, C.; Kamili, S.; Drobeniuc, J.; Hu, D.; Teshale, E. Novel risk factors associated with hepatitis E virus infection in a large outbreak in northern Uganda: Results from a case-control study and en-vironmental analysis. Am. J. Trop. Med. Hyg. 2010, 83, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Amanya, G.; Kizito, S.; Nabukenya, I.; Kalyango, J.; Atuheire, C.; Nansumba, H.; Abwoye, S.A.; Opio, D.N.; Kibuuka, E.; Karamagi, C. Risk factors, person, place and time characteristics associated with Hepatitis E Virus outbreak in Napak District, Uganda. BMC Infect. Dis. 2017, 17, 451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Traoré, K.A.; Roques, P.; Huot, N.; Ouoba, J.B.; Barro, N.; Pavio, N.; Traoré, A.S.; Dumarest, M.; Rogée, S. Hepatitis E Virus Exposure is Increased in Pork Butchers from Burkina Faso. Am. J. Trop. Med. Hyg. 2015, 93, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Sachs, K.; Lierz, M.; Appel, B.; Jansen, A.; Johne, R. Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virol. J. 2009, 6, 58. [Google Scholar] [CrossRef]
- Ministère de l’Élevage et des Productions Animales (MEPA). Plan National de Développement de L’Élevage, PNDE 2: 2017–2021. 2017. pp. 1–103. Available online: http://www.plateforme-pastorale-tchad.org/classified/PNDE_2__version_finale.pdf (accessed on 7 November 2021).
- Guthmann, J.-P.; Klovstad, H.; Boccia, D.; Hamid, N.; Pinoges, L.; Nizou, J.-Y.; Tatay, M.; Diaz, F.; Moren, A.; Grais, R.F.; et al. A Large Outbreak of Hepatitis E among a Displaced Population in Darfur, Sudan, 2004: The Role of Water Treatment Methods. Clin. Infect. Dis. 2006, 42, 1685–1691. [Google Scholar] [CrossRef]
- Tucker, T.J.; Kirsch, R.E.; Louw, S.J.; Isaacs, S.; Kannemeyer, J.; Robson, S.C. Hepatitis E in South Africa: Evidence for sporadic spread and increased seroprevalence in rural areas. J. Med. Virol. 1996, 50, 117–119. [Google Scholar] [CrossRef]
- Teshale, E.H.; Grytdal, S.P.; Howard, C.; Barry, V.; Kamili, S.; Drobeniuc, J.; Hill, V.; Okware, S.; Hu, D.J.; Holmberg, S.D. Evidence of Person-to-Person Transmission of Hepatitis E Virus during a Large Outbreak in Northern Uganda. Clin. Infect. Dis. 2010, 50, 1006–1010. [Google Scholar] [CrossRef]
- Muchiri, I.; Okoth, F.A.; Ngaira, J.; Tuei, S. Seroprevalence of HAV, HBV, HCV, and HEV among acute hepatitis patients at kenyatta national hospital in Nairobi, Kenya. East Afr. Med. J. 2012, 89, 199–205. [Google Scholar]
- Shimakawa, Y.; Njai, H.F.; Takahashi, K.; Berg, L.; Ndow, G.; Jeng-Barry, A.; Ceesay, A.; Tamba, S.; Opoku, E.; Taal, M.; et al. Hepatitis E virus infection and acute-on-chronic liver failure in West Africa: A case-control study from The Gambia. Aliment. Pharmacol. Ther. 2015, 43, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.A.; Aviyase, J.T.; Tettey, Y.; Adu-Gyamfi, C.; Mingle, J.A.; Ayeh-Kumi, P.F.; Adiku, T.K.; Gyasi, R.K. Hepatitis E virus infection among pig handlers in Accra, Ghana. East Afr. Med. J. 2009, 86, 359–363. [Google Scholar]
- Rao, V.B.; Johari, N.; du Cros, P.; Messina, J.; Ford, N.; Cooke, G.S. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15, 819–824. [Google Scholar] [CrossRef]
- Bessimbaye, N.; Moussa, A.M.; Mbanga, D.; Tidjani, A.; Mahamat, S.O.; Ngawara, M.N.; Ngarnayal, G.; Fissou, H.Y.; Sangare, L.; Ndoutamia, G. Séroprévalence de l’Ag HBs et de l’anticorps Anti VHC chez les personnes infectées par le VIH1 à N’Djamena, Tchad. Bull. De La Société De Pathol. Exot. 2014, 107, 327–331. [Google Scholar] [CrossRef]
- Suesstrunk, J.; Djongali, F.B. Hepatitis B virus prevalence in rural areas in south-west Chad. Trop. Dr. 2017, 47, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Florence, K.; Djeneba, O.; Charlemagne, G.; Djigma, F.; Obiri-Yeboah, D.; Compaore, T.R.; Théodora, Z.; Marius, B.; Paul, O.; Simpore, J. Hepatitis e in pregnant women at the saint camille hospital of ouagadougou in burkina faso: Prevalence and infection risk factors. Int. J. Recent Adv. Multidiscip. Res. 2016, 3, 15–79. [Google Scholar]
- Patterson, J.; Abdullahi, L.; Hussey, G.D.; Muloiwa, R.; Kagina, B.M. A systematic review of the epidemiology of hepatitis A in Africa. BMC Infect. Dis. 2019, 19, 651. [Google Scholar] [CrossRef]
| Markers of HEV | Total HEV Tested % n = 255 | AgHBs | HCV IgG |
|---|---|---|---|
| Positive for anti-HEV IgM | 11 (4.3%) | 1 | 0 |
| Positive for anti-HEV IgM and IgG | 32 (12.5%) | 4 | 1 |
| Positive for anti-HEV IgG | 44 (17.3%) | 9 | 1 |
| HEV positive total | 87 (34.1%) | 14 | 2 |
| Negative for HEV | 168 (65.9%) | 13 | 3 |
| Domains | Total | HBsAg (%) | Ab HCV (%) | HEV (%) | Neg of All (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM+ and IgG− | IgM+, IgG+ | IgM− and IgG+ | |||||||||||
| Domain A | 135 | 15 | (11.1) | 4 | (3.0) | 8 | (5.9) | 9 | (6.7) | 20 | (14.8) | 79 | (58.5) |
| Domain B | 61 | 7 | (11.5) | 0 | (-) | 3 | (4.9) | 13 | (21.3) | 13 | (21.3) | 25 | (41.0) |
| Domain C | 55 | 5 | (9.1) | 1 | (1.8) | 0 | (-) | 9 | (16.4) | 9 | (16.4) | 31 | (56.4) |
| Domain D | 4 | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (25.0) | 2 | (50.0) | 1 | (25.0) |
| Total | 255 | 27 | (10.6) | 5 | (2.0) | 11 | (4.3) | 32 | (12.5) | 44 | (17.3) | 136 | (53.3) |
| Variables | Anti-HEV IgG+ IgM− | Anti-HEV IgM+ IgG+ | Anti-HEV IgM+ IgG− | HBsAg + | Antibodies HCV | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | N (Rate%) | OR [CI 95%] | p-Value | N (Rate%) | OR [CI 95%] | p-Value | N (Rate%) | OR [CI 95%] | p-Value | N (Rate%) | OR [CI 95%] | p-Value | N (Rate%) | OR [CI 95%] | p-Value | |
| Sex | 0.90 | 0.004 | 0.83 | 0.41 | 0.91 | |||||||||||
| Male | 147 | 36 (24.49) | 11 (7.48) | 6 (4.08) | 14 (9.52) | 3 (2.04) | ||||||||||
| Female | 108 | 40 (37.04) | 2.0 [1.10–3.64] | 21 (19.44) | 2.98 [1.39–6.7] | 5 (4.63) | 1.14 [0.32–3.87] | 13 (12.03) | 1.39 [0.61–3.18] | 2 (1.85) | ||||||
| Age | 0.12 | 0.03 | 0.29 | 0.01 | ||||||||||||
| ≤9 years | 113 | 13 (11,50) | 10 (8.85) | 10 (8.85) | 0.02 | 7 (6.19) | 0 (0.0) | |||||||||
| [10–20] | 59 | 11 (18.64) | 1.50 [0.68–3.32] | 5 (8.47) | 3.85 [1.24–13.41] | 1 (1.69) | 9 (15.52) | 3.14 [1.06–9.35] | 1 (1.72) | |||||||
| [20–30] | 38 | 10 (26.31) | 4.04 [1.77–9.23] | 10 (26.31) | 1.04 [0.35–3.51] | (0) | 5 (12.82) | 2.36 [0.69–8.09] | 1 (2.56) | 1.65 [0.06–40.4] | ||||||
| ≥30 years | 45 | 10 (22.22) | 2.69 [1.21–6.02] | 7 (15.56) | 1.99 [0.59–7.17] | (0) | 6 (13.33) | 2.56 [0.79–8.22] | 3 (6.66) | 1.75 [0.06–45.43] | ||||||
| Ggeograpical zone | 0.09 | 0.003 | 0.22 | 0.81 | 0.59 | |||||||||||
| Soudanese | 212 | 33 (15.56) | 20 (9.43) | 3.24 [1.39–7.29] | 11 (5.16) | 23 (10.95) | 4 (1.9) | |||||||||
| Sahelian | 43 | 12 (27.90) | 1.93 [0.86–4.15] | 12 (27.90) | (0) | 4 (9.52) | 0.61 [0.15–2.60] | 1 (2.3) | ||||||||
| Domains | 0.21 | 0.01 | 0.31 | 0,95 | 0,46 | |||||||||||
| A | 135 | 20 (14.8) | 9 (6. 7) | 8 (5.9) | 15 (11.1) | 4 (3.0) | ||||||||||
| B | 61 | 13 (21.3) | 13 (21.3) | 3.79 [1.53–9.74] | 3 (4.9) | 7 (11.5) | 0 (0) | |||||||||
| C | 55 | 9 (16.4) | 9 (16.4) | 2.73 [1.01–7.43] | 0 (0) | 5 (9.1) | 1 (1.8) | |||||||||
| D | 4 | 2 (50) | 1 (25) | 4.67 [0.22–41] | 0 (0) | 0 (0) | 0 (0) | |||||||||
| Number Per Zone | Total Tested | HBsAg (%) | Ab HCV (%) | Positive for Anti-HEV (%) | Neg of All (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM+ and IgG− | IgM+, IgG+ | IgM− and IgG+ | |||||||||||
| Soudanian | 212 | 23 | (10.8) | 4 | (1.9) | 11 | (5.2) | 20 | (9.4) | 33 | (15.6) | 121 | (57.1) |
| Sahelian | 43 | 4 | (9.3) | 1 | (2.3) | 0 | (-) | 12 | (27.9) | 12 | (27.9) | 14 | (32.6) |
| Both | 255 | 27 | (10.6) | 5 | (2.0) | 11 | (4.3) | 32 | (12.5) | 45 | (17.6) | 135 | (52.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yandai, F.H.; Traore, K.A.; Moussa, A.M.; Ouoba, B.L.; Ouoba, J.B.; Bolti, M.A.; Abakar, M.F.; Hota, M.; Gamougam, K.; Nadlao, B.; et al. Prevalence and Diversity of Hepatitis Virus Markers among Patients with Acute Febrile Jaundice in Chad. Microbiol. Res. 2021, 12, 878-887. https://doi.org/10.3390/microbiolres12040064
Yandai FH, Traore KA, Moussa AM, Ouoba BL, Ouoba JB, Bolti MA, Abakar MF, Hota M, Gamougam K, Nadlao B, et al. Prevalence and Diversity of Hepatitis Virus Markers among Patients with Acute Febrile Jaundice in Chad. Microbiology Research. 2021; 12(4):878-887. https://doi.org/10.3390/microbiolres12040064
Chicago/Turabian StyleYandai, Fissou Henry, Kuan Abdoulaye Traore, Ali Mahamat Moussa, Bruno Lalidia Ouoba, Jean Bienvenue Ouoba, Mahamat Ali Bolti, Mahamat Fayiz Abakar, Mathieu Hota, Kadidja Gamougam, Bessimbaye Nadlao, and et al. 2021. "Prevalence and Diversity of Hepatitis Virus Markers among Patients with Acute Febrile Jaundice in Chad" Microbiology Research 12, no. 4: 878-887. https://doi.org/10.3390/microbiolres12040064
APA StyleYandai, F. H., Traore, K. A., Moussa, A. M., Ouoba, B. L., Ouoba, J. B., Bolti, M. A., Abakar, M. F., Hota, M., Gamougam, K., Nadlao, B., Uwimbabazi, J.-C., Tao, N. E., Ngandolo, B. N., Roques, P., & Barro, N. (2021). Prevalence and Diversity of Hepatitis Virus Markers among Patients with Acute Febrile Jaundice in Chad. Microbiology Research, 12(4), 878-887. https://doi.org/10.3390/microbiolres12040064






