Secondary Metabolite Production and Terpenoid Biosynthesis in Endophytic Fungi Cladosporium cladosporioides Isolated from Wild Cymbopogon martinii (Roxb.) Wats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Authentication of Plant
2.2. Plant Extraction Method
2.3. Isolation and Characterization of Endophytic Fungi
2.4. Characterization of Endophytic Fungi
2.5. Extraction of Secondary Metabolites
2.6. Phytochemical Analysis
2.7. Gas Chromatography and Mass Spectroscopy-(GC-MS)
3. Results
3.1. Isolation and Identification of Endophytic Fungi
3.2. Phytochemical Screening of Endophytic Fungi
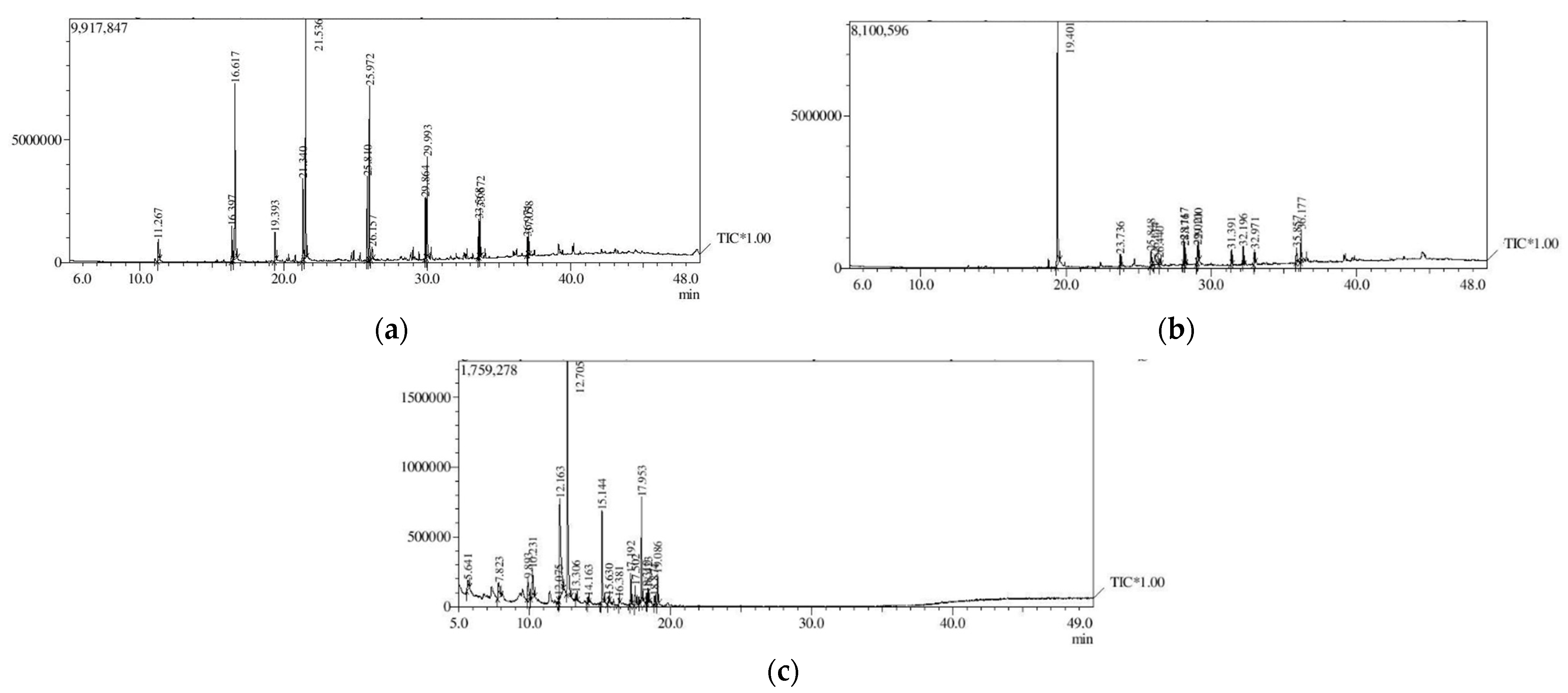
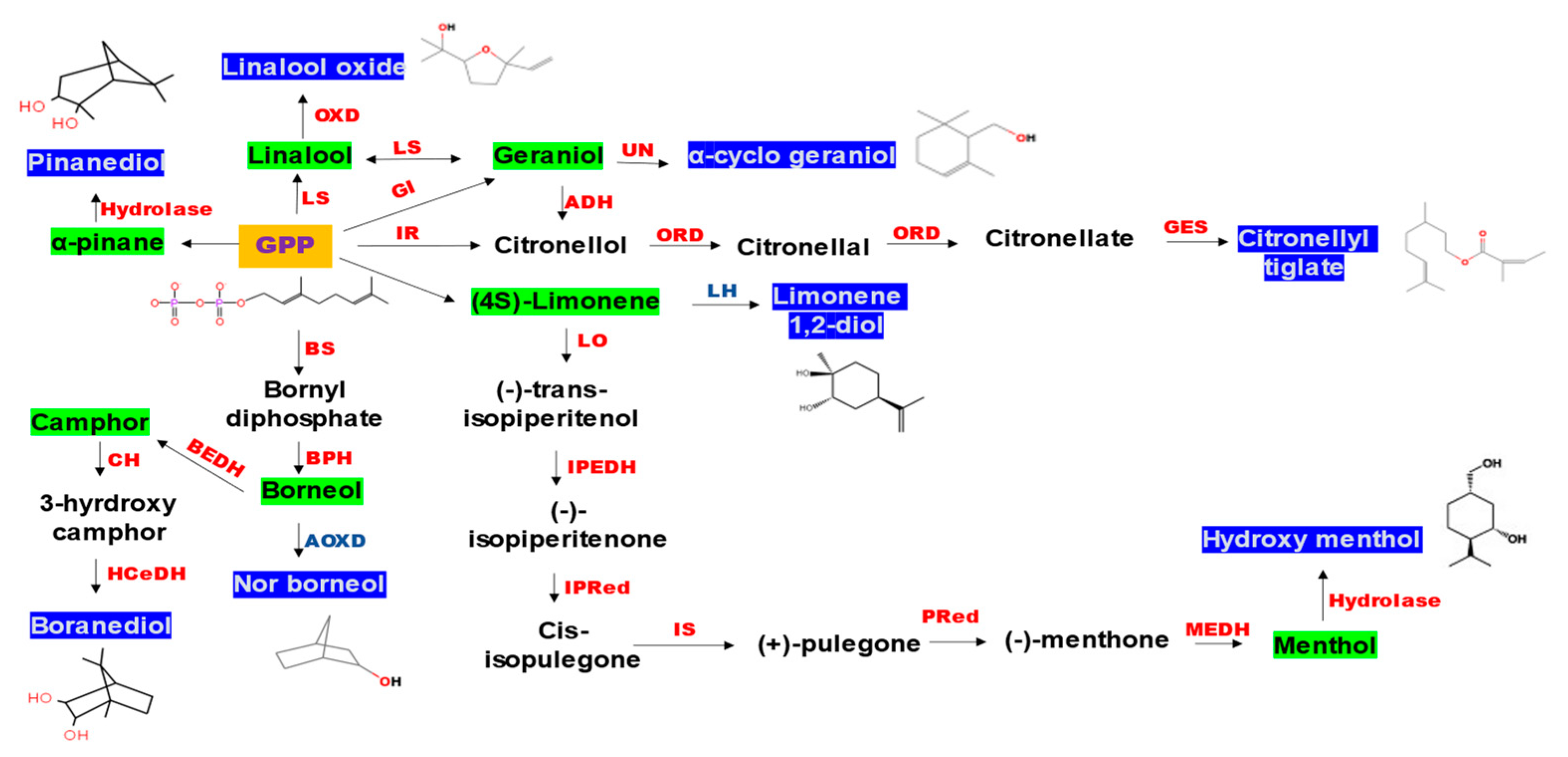
3.3. Analysis of Secondary Metabolites (GC-MS)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bor, N.L. The genus Cymbopogon Spreng. India, Burma and Ceylon, Part. I. J. Bombay Nat. Hist. Soc. 1953, 51, 890–916. [Google Scholar]
- Promila, P. A review on the medicinal and aromatic plant Cymbopogon martini (Roxb.) Watson (Palmarosa). Int. J. Chem. Stud. 2018, 6, 1311–1315. [Google Scholar]
- Ashwini Murthy, A.; Thara Saraswathi, K.T. Chemical profiling of leaf extract and essential oil in wild Cymbopogon martinii (Roxb.) collected from Devarayanadurga hills (Tumkur, Karnataka). Int. J. Sci. Res. Rev. 2019, 8, 1–9. [Google Scholar]
- Smitha, G.R.; Dhaduk, H.L. A new chemotype of palmarosa [Cymbopogon martini (Roxb.) W. Watson] identified from ‘The Aravali Range’ of Rajasthan, India. Med. Plants 2018, 10, 203–209. [Google Scholar] [CrossRef]
- Grenera. Nutrients: Lemon Grass Oil. Retrieved from Essential Oil. 2008. [Google Scholar]
- Farh, M.E.; Jeon, J. Roles of fungal volatiles from perspective of distinct lifestyles in filamentous fungi. Plant Pathol. J. 2020, 36, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, B.; Xing, J.; Li, C. Endophytes: The novel sources for plant terpenoid biosynthesis. Appl. Microbiol. Biotechnol. 2021, 105, 4501–4513. [Google Scholar] [CrossRef] [PubMed]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Roy, S.; Banerjee, D. Volatile Organic Compounds from Endophytic fungi. In Recent Advance in White Biotechnology through Fungi, Fungal Biology; Springer: Cham, Germany, 2019; pp. 149–176. [Google Scholar]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues and challenges. Microorganisms 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, Staf (lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharad, S.; Kapur, S. Free radicals and oxidative stress in neurodegenerative diseases: Relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 2004, 5, 218–225. [Google Scholar]
- Balakrishnan, B.; Paramasivam, S.; Arulkumar, A. Evaluation of the lemongrass plant (Cymbopogon citratus) extracted in different solvents for antioxidant and antibacterial activity against human pathogens. Asian Pac. J. Trop. Dis. 2014, 4, 134–139. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Duan, J.; Charles, T.C.; Glick, B.R. A bioinformatics approach to the determination of genes involved in endophytic behavior in Burkholderia spp. J. Theor. Biol. 2014, 343, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth-promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Udayashankar and Sudisha Jogaiah. Detection and Characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Ganjewala, D.; Gupta, A.K. Lemongrass (Cymbopogon flexuosus. Steud.) Wats essential oil: Overview and biological activities. Recent Prog. Med. Plants 2013, 37, 235–271. [Google Scholar]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef] [PubMed]
- Potshang Bam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal volatile organic compounds: A review with emphasison their biotechnological potential. Fungal Biol. Rev. 2012, 30, 73–83. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutfia, A.; Munir, E.; Yurnaliza, Y.; Basyuni, M. Chemical analysis and anticancer activity of sesterterpenoid from an endophytic fungus Hypomontagnella monticulosa Zg15SU and its host Zingiber griffithii Baker. Heliyon 2021, 7, e06292. [Google Scholar] [CrossRef]
- Kusari, S.; Zühlke, S.; Spiteller, M. An endophytic fungus from Camptotheca acuminata that produces camptothecin and analogues. J. Nat. Prod. 2009, 72, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, L.; Li, Q.; Shen, Y.; Shao, A.; Lin, S.; Huang, L. Volatile metabolites analysis and molecular identification of endophytic fungi bn12 from Cinnamomum camphora var. borneol. China J. Chin. Mater. Med. 2011, 36, 3217–3221. [Google Scholar] [CrossRef]
- Stierle, A.; Stierle, D.; Stierle, S. Bioactive compounds from four endophytic Penicillium sp. of a northwest pacific yew tree. Nat. Prod. Chem. 2000, 24, 933–977. [Google Scholar]
- Zhu, D.; Wang, J.; Zeng, Q.; Zhang, Z.; Yan, R. A novel endophytic Huperzine A-producing fungus Shiraia sp. Slf14, isolated from Huperzia serrata. J. Appl. Microbiol. 2010, 109, 1469–1478. [Google Scholar] [CrossRef]
- Kasaei, A.; Mobini-Dehkordi, M.; Mahjoubi, F.; Saffar, B. Isolation of Taxol-producing endophytic fungi from Iranian yew through novel molecular approach and their effects on human breast cancer cell line. Curr. Microbiol. 2017, 74, 702–709. [Google Scholar] [CrossRef]
- Pimentel, I.C.; Glienke-Blanco, C.; Gabardo, J.; Stuart, R.M.; Azevedo, J.L. Identification and colonization of endophytic fungi from soyabean (Glycine max (L.) Merril) under different environmental conditions. Braz. Arch. Biol. Technol. 1998, 49, 705–711. [Google Scholar] [CrossRef]
- Landum, M.C.; do Rosário Félix, M.; Alho, J.; Garcia, R.; Cabrita, M.J.; Rei, F. Antagonistic activity of fungi of Olea europaea L. against Colletotrichum acutatum. Microbiol. Res. 2016, 183, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa, 2nd ed.; Scientific Research; Spectrum Books Limited: Ibadan, Nigeria, 1993; pp. 1–153. [Google Scholar]
- Harborne, A.J. Phytochemical Methods—A Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Dordrecht, The Netherlands, 1973. [Google Scholar] [CrossRef]
- Danmalam, U.H.; Hanwa, U.A.; Abdurrazak, A.; Hassan Maidoki, A.L.; Ambi, A.A. Phytochemical analysis of some crotalaria species growing in Samaru-zaria, Nigeria for the presence of pyrrolizidine alkaloids. Trends Sci. Technol. J. 2017, 2, 1035–1037. [Google Scholar]
- Desire, M.H.; Bernard, F.; Forsah, M.R.; Assang, C.T.; Denis, O.N. Enzymes and qualitative phytochemical screening of endophytic fungi isolated from Lantana camara Linn. Leaves. J. Appl. Biol. Biotechnol. 2014, 2, 1–6. [Google Scholar]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Malik, S.K.; Ahmed, M.; Khan, F. Identification of novel anticancer terpenoids from Prosopis juliflora (Sw) DC (Leguminosae) pods. Trop. J. Pharm. Res. 2018, 17, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Eghbaliferiz, S.; Emami, S.A.; Tayarani-Najaran, Z.; Iranshahi, M.; Shakeri, A.; Hohmann, J.; Asili, J. Cytotoxic diterpene quinones from Salvia tebesana Bunge. Fitoterapia 2018, 128, 97–101. [Google Scholar] [CrossRef]
- Unuigbe, C.; Enahoro, J.; Erharuyi, O.; Okeri, H.A. Phytochemical analysis and antioxidant evaluation of lemon grass (Cymbopogon citratus DC.) Stapf leaves. J. Appl. Sci. Environ. Manag. 2019, 23, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M. Investigation of In-Vitro Antioxidant Potential, Brine Shrimp Lethality and Thrombolytic Activity in Ficus Mollis Vahl Leaves along with Phytochemical Screening. Bachelor’s Thesis, BRAC University, Dhaka, Bangladesh, 2018. [Google Scholar]
- Deshmukh, S.K.; Kolet, M.J.; Verekar, S.A. Distribution of endophytic fungi in lemon grass (Cymbopogon citratus (DC). Stapf.). J. Cell Tissue Res. 2010, 10, 2263–2267. [Google Scholar]
- Krishnamurthy, Y.L.; Hemalatha, T.V. Isolation of endophytic fungi from some grasses. J. Mycol. Plant Pathol. 2003, 33, 305–306. [Google Scholar]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The genus Cladosporium: A rich source of diverse and bioactive natural compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: A review. J. Fungi 2021, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, L.; Wang, J.; Shan, T.; Zhong, L.; Liu, X.; Gao, X. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. Technol. Educ. Trop. Appl. Microbiol. Microbial. Biotechnol. 2010, 1, 567–576. [Google Scholar]
- Sharma, D.; Paramanik, A.; Agarwal, P.K. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don. 3 Biotech 2016, 6, 210. [Google Scholar] [CrossRef] [Green Version]
- Kanjana, M.; Kanimozhi, G.; Udayakumar, R.; Panneerselvam, A. GC-MS analysis of bioactive compounds of endophytic fungi Chaetomium globosum, Cladosporium tenuissimum and Penicillium janthinellum. J. Biomed. Pharm. Sci. 2019, 2, 123. [Google Scholar]
- Kaur, N.; Arora, D.S.; Kalia, N.; Kaur, M. Bioactive potential of endophytic fungus Chaetomium globosum and GC-MS analysis of its responsible components. Sci. Rep. 2020, 10, 18792. [Google Scholar] [CrossRef]
- Li, J.Y.; Sidhu, R.S.; Ford, E.; Long, D.; Hess, W.; Strobel, G. The induction of taxol production in the endophytic fungus–Periconia sp. From Torreya grandifolia. J. Ind. Microbiol. Biotechnol. 1998, 20, 259–264. [Google Scholar] [CrossRef]
- Miao, L.Y.; Mo, X.C.; Xi, X.Y.; Zhou, L.; De, G.; Ke, Y.S.; Liu, P.; Song, F.J.; Jin, W.W.; Zhang, P. Transcriptome analysis of a taxol-producing endhphytic fungus Cladosporium cladosporioides MD2. AMB Express 2018, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chen, X.; Xu, C.; Zhao, H.; Zhang, X.; Zeng, G.; Qian, Y.; Liu, R.; Guo, N.; Mi, W.; et al. Horizontal gene transfer allowed the emergence of broad host range entomopathogens. Proc. Natl. Acad. Sci. USA 2019, 116, 7982–7989. [Google Scholar] [CrossRef] [Green Version]
- Paul, D.; Park, K.S. Identification of Volatile produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth. Sensors 2013, 13, 13969–13977. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xu, A.; Tang, X. Isolation, identification and volatile compound analysis of an aroma-producing endophytic yeast from romaine lettuce. Food Sci. 2011, 23, 33. [Google Scholar]
- Nithya, K.; Muthumary, J. Secondary metabolite from Phomopsis sp. isolated from Plumeria acutifolia Poiret. Recent Res. Sci. Technol. 2010, 2, 99–103. [Google Scholar]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Nuangmek, W.; Matsui, K.; Lumyong, S. Biofumigation with the endophytic fungus Nodulisporium spp. CMU-UPE34 to control postharvest decay of citrus fruit. Crop Prot. 2013, 45, 63–70. [Google Scholar] [CrossRef]
- De Souza, J.J.; Vieira, I.J.C.; Rodrigues-Filho, E.; Braz-Filho, R. Terpenoids form endophytic fungi. Molecules 2011, 16, 10604–10618. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Cogoon, P.; Mcphail, A.T. Plant as antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Pandey, S.P.; Spiteller, M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 2013, 91, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Are microbial endophytes the ‘actual’ producers of bioactive antitumor agents? Trends Cancer 2018, 4, 662–670. [Google Scholar] [CrossRef]
- Germaine, K.; Liu, X.; Cabellos, G.; Hogan, J.; Ryan, D.; Dowling, D.N. Bacterial endophyte-enhanced phyto-remediation of the organochlorine herbicide 2,4 dichlorophenoxyacetic acid. FEMS Microbiol. Ecol. 2006, 57, 302–310. [Google Scholar] [CrossRef]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef]
- Tiwari, P.; Bae, H. Horizontal gene transfer and endophytes: An implication for the acquisition of novel traits. Plants 2020, 9, 305. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Inamadar, A.A. Are some fungal volatile organic compounds (VOCs) mycotoxins? Toxins 2015, 7, 3785–3804. [Google Scholar] [CrossRef] [Green Version]
- Bensh, K.; Groenewald, J.Z.; Starink-Willemse, M.; Andersen, B.; Sumerell, B.A.; Shin, H.D. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellacear, Capnodiales). Stud. Mycol. 2010, 67, 1–94. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [Green Version]

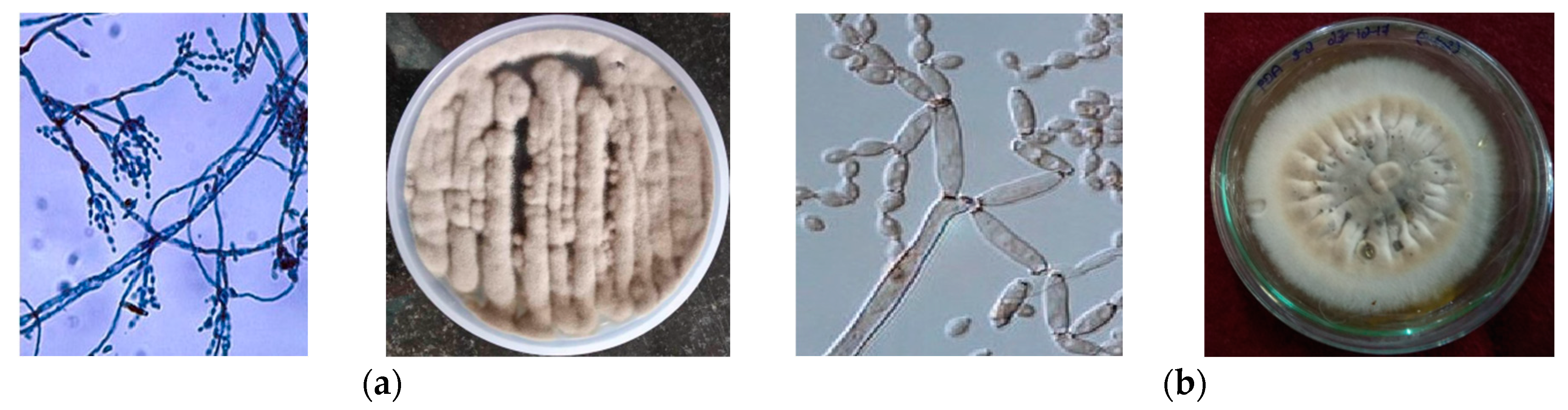

| Sl No. | Test | Host Plant Extract (C. martinii) | PDB (Control) (without Supplementation of Plant Extract) | PDB (Test) (with Supplementation of Plant Extract) | |
|---|---|---|---|---|---|
| Cladosporium cladosporioides (F1) | Cladosporium tenuissimum (F2) | Cladosporium cladosporioides (F1) | |||
| 1 | Alkaloid | +ve | +ve | +ve | +ve |
| 2 | Flavonoid | +ve | +ve | +ve | +ve |
| 3 | Phenol | +ve | +ve | +ve | +ve |
| 4 | Tannin | +ve | +ve | −ve | +ve |
| 5 | Terpenoid | +ve | +ve | +ve | +ve |
| 6 | Quinine | +ve | −ve | −ve | −ve |
| 7 | Essential oil | +ve | +ve | −ve | +ve |
| Sl No. | Compound | Area% | ||
|---|---|---|---|---|
| Extract of Plant C. martinii | Extract of Endophytes | |||
| F1a | F2 | |||
| 1 | Tetradecane | 2.08 | 0.97 | 2.12 |
| 2 | Dodecane | 2.88 | - | - |
| 3 | 1S-Endo-bornyl acetate | 3.73 | - | - |
| 4 | Tridecane | 4.39 | - | - |
| 5 | Geraniol | 5.45 | 1.2 | - |
| 6 | Farnesane | 0.34 | - | - |
| 7 | Geranyl acetate | 21.44 | 0.91 | - |
| 8 | Heptadecane | 30.39 | 3.17 | - |
| 9 | (-)-Beta-caryophyllene | 0.63 | - | - |
| 10 | Decane, 2,3,5,8-tetramethyl- | 0.44 | - | - |
| 11 | Hexadecane | 10.95 | 19.4 | 4.59 |
| 12 | Gamma-cadinene | 0.63 | - | |
| 13 | 2-(3-Isopropenyl-4-methyl-4-vinylcyclohexyl)-2-propanol | 0.50 | - | - |
| 14 | (-)-Beta-caryophyllene epoxide | 3.09 | - | - |
| 15 | Tridecane | 2.07 | - | - |
| 16 | Cubenol | 9.81 | 0.39 | - |
| 17 | (-)-Guaiol | 0.83 | - | - |
| 18 | Agarospirol | 1.79 | - | - |
| 19 | Beta-eudesmol | 0.94 | - | - |
| 20 | Globulol | 3.07 | - | - |
| 21 | 2,4-Ditert-butylphenol | - | 9.34 | - |
| 22 | E-14-Hexadecanal | - | 9.01 | - |
| 23 | Octadecane | - | 20.99 | - |
| 24 | 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione | - | 2.10 | 1.40 |
| 25 | Heneicosane, 4-cyclohexyl- | - | 2.20 | - |
| 26 | E-15-heptadecenal | - | 6.78 | - |
| 27 | Eicosane | - | 10.69 | - |
| 28 | Decane 4-cycloheyl-, 4-cyclohexyl- | - | 1.25 | - |
| 29 | Tricosane | - | 5.23 | - |
| 30 | 1-Heneicosanol | - | 1.96 | - |
| 31 | Tetracosane | - | 2.28 | - |
| 32 | 1,2-Benzenedicarboxylic acid | - | 3.62 | 6.24 |
| 33 | Cyclooctanone | - | - | 7.01 |
| 34 | Cycloheptanone | - | - | 2.66 |
| 35 | 3-Methyl-2-butenoic acid, pentadecyl ester | - | - | 2.61 |
| 36 | Valeric acid, 2-pentadecyl ester | - | - | 1.21 |
| 37 | 3-(tert-Butyl dimethylsilyl) oxyiminobutan-2-one | - | - | 12.48 |
| 38 | Pentadecane | - | - | 3.12 |
| 39 | 4,5,7-Trihydroxy-2-octenoic acid | - | - | 42.26 |
| 40 | Erythro-cis (1,4), trans (1,4)-4,4-dihyrdoxybicyclooctyl | - | - | 9.04 |
| 41 | Nonadecane | - | - | 1.37 |
| Sl No. | Compound | Area % | |||
|---|---|---|---|---|---|
| 5 mL | 10 mL | 15 mL | 20 mL | ||
| 1. | 2-Hydroxy menthol | 0.11 | 0.14 | 0.15 | - |
| 2. | Nor-borneol | 0.15 | 0.28 | - | 0.38 |
| 3. | Cedrane | 0.14 | - | 1.06 | - |
| 4. | Cedral acetate | 2.92 | - | - | - |
| 5. | Alpha cyclo geraniol | - | 0.4 | - | - |
| 6. | Campesterol | - | 0.41 | - | - |
| 7. | 1,4-Epoxynaphthalene-menthol | - | 0.55 | - | - |
| 8. | Elemicin | - | - | 1.26 | - |
| 9. | Cetonal | - | - | 0.35 | - |
| 10. | Limonene 1,2-diol | - | - | - | 0.4 |
| 11. | Beta-cyclo homo geraniol | - | - | - | 0.13 |
| 12. | Linalool oxide | - | - | - | 0.24 |
| 13. | 2,3-Bornanediol | - | - | - | 1.4 |
| 14. | 2,3-pinanediol | - | - | - | 0.11 |
| 15. | Epiglobulol | - | - | - | 0.84 |
| 16. | Calamenene | - | - | - | 0.66 |
| 17. | Citronellyl tiglate | - | - | - | 1.11 |
| Sl No. | Compound | Area % | RT |
|---|---|---|---|
| 1. | Nonanal | 0.04 | 6.074 |
| 2. | 1,3-Butanediol, diacetate | 0.03 | 6.361 |
| 3. | 3-tert-Butyl-2-pyrazolin-5-one | 0.06 | 8.167 |
| 4. | Nonanoic acid | 0.05 | 8.292 |
| 5. | 1-Tetradecene | 0.08 | 9.964 |
| 6. | 2-hydroxy menthol | 0.11 | 10.054 |
| 7. | Cyclohexane butanal, | 0.6 | 10.14 |
| 8. | exo-nor borneol | 0.15 | 10.283 |
| 9. | Phenol, | 0.23 | 11.542 |
| 10. | Benzoic acid, | 0.05 | 11.75 |
| 11. | 1,7-Dioxaspiro [5.5] undec-2-ene | 0.06 | 11.918 |
| 12. | Dodecanoic acid | 0.18 | 12.076 |
| 13. | 1-Heptadecene | 0.85 | 12.431 |
| 14. | Octadecane | 0.63 | 12.512 |
| 15. | Oxirane | 1.44 | 12.816 |
| 16. | Cedrane | 0.14 | 13.547 |
| 17. | Tridecanal | 0.08 | 13.859 |
| 18. | E-8-Methyl-9-tetradecen-1-ol acetate | 0.92 | 14.334 |
| 19. | 1-Nonadecene | 12.61 | 14.662 |
| 20. | Spiro [4.5] decan-7-one | 0.29 | 15.334 |
| 21. | Cedrol acetate | 2.92 | 16.019 |
| 22. | Widdrol hydroxyether | 1.43 | 16.295 |
| 23. | n-hexa decanoic acid | 20.98 | 16.447 |
| 24. | Oleic Acid | 3.32 | 17.276 |
| 25. | 1-Octadecanol | 0.27 | 17.568 |
| 26. | Octadecanoic acid | 7.9 | 18.307 |
| 27. | Decanoic acid, | 0.37 | 19.100 |
| 28. | 1-Tetracosanol | 1.38 | 20.234 |
| 29. | Octadecanal | 0.3 | 20.553 |
| 30. | 2-palmitoyl glycerol | 1.72 | 21.228 |
| 31. | 1,2-Benzenedicarboxylic acid, mono (2-ethylhexyl | 1.32 | 21.553 |
| 32. | 1-Triacontanol | 1.86 | 21.805 |
| 33. | 10,12,14-Nonacosatriynoic acid | 0.72 | 22.263 |
| 34. | Abietic acid | 1.3 | 22.385 |
| 35. | Retinoic acid | 0.43 | 22.544 |
| 36. | Glyceryl mono acetate | 3.99 | 22.629 |
| 37. | Stearin | 0.97 | 22.787 |
| 38. | Squalene | 0.93 | 23.568 |
| 39. | Corticosterone 21-acetate | 0.63 | 23.796 |
| 40. | Trans-beta-lonone | 0.58 | 23.934 |
| 41. | Dehydroergosterol 3,5-dinitrobenzoate | 0.92 | 24.848 |
| 42. | Cholest-5-en-3-ol (3. beta) | 0.24 | 26.134 |
| 43. | Cycloeucalenol | 0.36 | 26.73 |
| 44. | Stigmasta-4,7,22-trien-3. alpha-ol | 0.88 | 30.15 |
| 45. | Hexa decanoic acid | 1.42 | 30.562 |
| 46. | Rhodopin | 0.83 | 31.201 |
| 47. | 1,4-Epoxynaphthalene-1(2H)-methanol | 1.63 | 32.554 |
| 48. | Ergosta-8,24(28)-dien-3-ol, 14-methyl-, (3. beta,5. alpha) | 1.79 | 33.576 |
| Sl No. | Compound | Area % | R Time |
|---|---|---|---|
| 1. | 1-Tetradecene | 0.1 | 9.951 |
| 2. | 2-hydroxymenthol | 0.14 | 10.041 |
| 3. | 2-hydroxybutyl acrylate | 0.45 | 10.129 |
| 4. | Oxo-borneol | 0.28 | 10.271 |
| 5. | 1-Heptadecene | 0.93 | 12.418 |
| 6. | Alpha-cyclo geraniol | 0.4 | 14.319 |
| 7. | 1-Nonadecene | 2.12 | 14.649 |
| 8. | 2,11-Dodecadiene | 0.4 | 14.906 |
| 9. | Cetonal | 0.36 | 15.318 |
| 10. | diisobutyl phthalate | 19.28 | 15.547 |
| 11. | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 1.12 | 16.297 |
| 12. | n-Hexadecanoic acid | 7.45 | 16.389 |
| 13. | Dibutyl phthalate | 12.3 | 16.494 |
| 14. | Phthalic acid, | 14.64 | 16.69 |
| 15. | Oleic Acid | 6.87 | 18.074 |
| 16. | Benzenedicarboxylic acid | 2.51 | 17.017 |
| 17. | 9-Tricosene | 2.52 | 18.522 |
| 18. | 1-Tetracosanol | 1.22 | 20.219 |
| 19. | 1-Triacontanol | 0.61 | 21.793 |
| 20. | Oleoyl chloride | 0.86 | 22.617 |
| 21. | Tetrapentacontane | 0.49 | 23.255 |
| 22. | Tetracosamethyl-cyclododecasiloxane | 1.92 | 24.362 |
| 23. | Dehydroergosterol 3,5-dinitrobenzoate | 0.36 | 24.838 |
| 24. | Dithianone | 0.37 | 25.048 |
| 25. | Cyclononasiloxane | 0.88 | 25.359 |
| 26. | Stigmast-5-en-3-ol | 1.64 | 25.909 |
| 27. | Isoindole-1,3(1H,3H)-dione | 0.78 | 27.085 |
| 28. | Campesterol | 0.41 | 27.241 |
| 29. | Chondrillasterol | 0.57 | 27.581 |
| 30. | gamma-Sitosterol | 2.22 | 28.297 |
| 31. | 1,4-Epoxynaphthalene-1(2H)-methanol | 0.55 | 32.525 |
| Sl No. | Compound | Area % | R Time |
|---|---|---|---|
| 1. | Propanoic acid | 0.35 | 5.478 |
| 2. | Malic acid | 0.18 | 5.68 |
| 3. | Butanedioic acid | 0.13 | 5.856 |
| 4. | 2-Octenoic acid | 0.4 | 7.513 |
| 5. | 2-Decenoic acid | 11.95 | 9.538 |
| 6. | 1-Tetradecene | 0.12 | 9.958 |
| 7. | 2-Hydroxymenthol | 0.15 | 10.05 |
| 8. | Elemicin | 1.26 | 12.069 |
| 9. | 1-Hexadecanol | 1.46 | 12.438 |
| 10. | Cyclodecanamine | 3.46 | 12.629 |
| 11. | D-Galactose | 0.54 | 12.945 |
| 12. | Cedrane | 0.1 | 13.547 |
| 13. | 9,10-Dimethyltricyclo [4.2.1.1(2,5)] decane-9,10-diol | 0.87 | 14.333 |
| 14. | 1-Nonadecene | 1.54 | 14.658 |
| 15. | 2-Cyclohexen-1-one | 0.18 | 14.83 |
| 16. | 3.alpha,7 beta-Dihydroxy-5 beta,6 beta-epoxycholestane | 0.1 | 15.127 |
| 17. | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | 0.04 | 15.24 |
| 18. | Cetonal | 0.35 | 15.327 |
| 19. | Di isobutyl phthalate | 7.35 | 15.556 |
| 20. | Phthalic acid | 21.69 | 16.236 |
| 21. | n-Hexadecanoic acid | 9.16 | 16.417 |
| 22. | Dibutyl phthalate | 11.78 | 16.505 |
| 23. | Oleic acid | 7.73 | 17.261 |
| 24. | Acetophenone | 0.49 | 18.384 |
| 25. | Pentaerythrityl tetrachloride | 0.36 | 18.815 |
| 26. | 4-Bromobutanoic acid | 0.29 | 19.057 |
| 27. | Pentaerythrityl tetrachloride | 0.91 | 19.826 |
| 28. | 1-Tetracosanol | 1.02 | 20.226 |
| 29. | Carbamazepine | 0.33 | 20.651 |
| 30. | 1-Triacontanol | 0.55 | 21.795 |
| 31. | glyceryl mono oleate | 2.36 | 22.621 |
| 32. | 1-Hexacosanol | 0.35 | 23.255 |
| 33. | 1-Nonadecene | 2.01 | 18.53 |
| Sl No. | Compound | Area % | R Time |
|---|---|---|---|
| 1. | Glycerine | 0.11 | 4.264 |
| 2. | 2-Nonadecanone | 0.13 | 7.756 |
| 3. | Limonene glycol or P-menth-8-ene | 0.4 | 9.191 |
| 4. | Beta-cyclohomo geraniol | 0.13 | 9.487 |
| 5. | Linalool oxide | 0.24 | 9.56 |
| 6. | 2,3-Bornanediol | 1.4 | 10.046 |
| 7. | Cyclohexanebutanal | 1.15 | 10.146 |
| 8. | 2-Norborneol | 0.38 | 10.278 |
| 9. | Alpha-campholene aldehyde | 0.18 | 10.418 |
| 10. | Lomustine | 0.13 | 10.637 |
| 11. | Cyclohexanol | 0.14 | 10.754 |
| 12. | 10-Methyl-8-tetradecen-1-ol acetate | 0.18 | 11.021 |
| 13. | 4-Nonanone | 0.22 | 11.245 |
| 14. | Benzoic acid, 4-ethoxy-, ethyl ester | 0.11 | 11.742 |
| 15. | 1,7-Dioxaspiro [5.5] undec-2-ene | 0.58 | 11.908 |
| 16. | 1-Heptadecene | 1.12 | 12.427 |
| 17. | 6-Acetyl-4,4,7-trimethylbicyclo [4.1.0] heptan-2-one | 0.42 | 12.735 |
| 18. | 2,3-pinanediol | 0.1 | 13.03 |
| 19. | Bicyclo(3.1.1)heptane-2,3-diol, 2,6,6-trimethyl- | 0.67 | 13.544 |
| 20. | 1H-Benzocyclohepten-7-ol, 2,3,4,4a,5,6,7,8-octahydro-1,1,4a,7-tetramethyl- | 0.23 | 14.18 |
| 21. | 1-Cyclohexanone, 2-methyl-2-(3-methyl-2-oxobutyl) | 7.3 | 14.337 |
| 22. | 1-Nonadecene | 3.41 | 14.662 |
| 23. | 2,5,9-Tetradecatriene, 3,12-diethyl- | 0.23 | 14.839 |
| 24. | 2-Dodecen-1-yl (-) succinic anhydride | 1.57 | 14.998 |
| 25. | Epiglobulol | 0.84 | 15.131 |
| 26. | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | 1.28 | 15.246 |
| 27. | Spiro [4.5] decan-7-one, 1,8-dimethyl-8,9-epoxy-4-isopropyl- | 0.42 | 15.326 |
| 28. | Diisobutyl phthalate | 7.91 | 15.556 |
| 29. | 3-Methyl-5-(1,4,4-trimethylcyclohex-2-enyl) pentan-1-ol | 1.72 | 15.666 |
| 30. | Calamenene | 0.66 | 15.780 |
| 31. | 2,6-Bis-(acetamido)-pyridine | 0.93 | 16.115 |
| 32. | Phthalic acid, isobutyl 2-pentyl ester | 1.21 | 16.231 |
| 33. | Widdrol hydroxyether | 3.35 | 16.302 |
| 34. | n-Hexadecanoic acid | 8.03 | 16.429 |
| 35. | Dibutyl phthalate | 4.65 | 16.504 |
| 36. | 1,2-Benzenedicarboxylic acid, butyl decyl ester | 1.53 | 16.579 |
| 37. | Phthalic acid, isobutyl octadecyl ester | 9.23 | 16.709 |
| 38. | 2,4,7,14-Tetramethyl-4-vinyl-tricyclo [5.4.3.0(1,8)]t | 1.38 | 16.945 |
| 39. | 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester | 0.79 | 17.024 |
| 40. | 2-naphthalene butanoic acid | 5.94 | 17.161 |
| 41. | 2-Methyl-5-(2,6,6-trimethyl-cyclohex-1-enyl)-pentane-2,3-diol | 0.35 | 17.336 |
| 42. | Phthalic acid, isobutyl 2-pentyl ester | 0.56 | 17.416 |
| 43. | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl) hexa-1,3,5-trienyl]cy | 1.11 | 17.516 |
| 44. | Citronellyl tiglate | 1.11 | 17.761 |
| 45. | Oleic acid | 5.41 | 18.110 |
| 46. | Octadecanoic acid | 1.68 | 18.288 |
| 47. | Benzo[b]dihydropyran, 6-hydroxy-4,4,5,7,8-pentamethyl- | 1.6 | 18.390 |
| 48. | 1-Tricosanol | 2.78 | 18.529 |
| 49. | Beta-sitosterol | 0.17 | 18.867 |
| 50. | 1-Tetracosanol | 1.67 | 20.226 |
| 51. | Oxirane, hexadecyl | 0.15 | 20.546 |
| 52. | Hexadecanoic acid, | 0.41 | 21.223 |
| 53. | 1,2-Benzenedicarboxylic acid, | 3.68 | 21.548 |
| 54. | 1-Triacontanol | 1.06 | 21.795 |
| 55. | 10,12,14-Nonacosatriynoic acid | 0.24 | 22.259 |
| 56. | Glyceryl monooleate | 1.73 | 22.620 |
| 57. | 1-Hexacosanol | 0.77 | 23.252 |
| 58. | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl- | 0.21 | 23.559 |
| 59. | Dehydroergosterol 3,5-dinitrobenzoate | 0.44 | 24.836 |
| 60. | 9,19-Cycloergost-24(28)-en-3-ol, 4,14-dimethyl-, | 0.18 | 26.716 |
| 61. | Ergosta-5,8,22-trien-3-ol, (3 beta,22E) | 0.54 | 27.008 |
| 62. | Anthraergostatetraenol | 0.13 | 27.239 |
| 63. | Stigmasta-7,25-dien-3-ol, (3 beta, 5 alpha) | 0.11 | 27.831 |
| 64. | 17-Pentatriacontene | 0.14 | 28.588 |
| 65. | Rhodopin | 0.96 | 31.174 |
| 66. | 1,4-Epoxynaphthalene-1(2H)-methanol, 4,5,7-tris(1,1-dimethylethyl)-3 | 0.72 | 32.520 |
| 67. | Cholesta-8,14-dien-3-ol, 4,4-dimethyl- | 0.43 | 33.532 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaram, H.; Marigowda, V.; Thara Saraswathi, K.J. Secondary Metabolite Production and Terpenoid Biosynthesis in Endophytic Fungi Cladosporium cladosporioides Isolated from Wild Cymbopogon martinii (Roxb.) Wats. Microbiol. Res. 2021, 12, 812-828. https://doi.org/10.3390/microbiolres12040059
Jayaram H, Marigowda V, Thara Saraswathi KJ. Secondary Metabolite Production and Terpenoid Biosynthesis in Endophytic Fungi Cladosporium cladosporioides Isolated from Wild Cymbopogon martinii (Roxb.) Wats. Microbiology Research. 2021; 12(4):812-828. https://doi.org/10.3390/microbiolres12040059
Chicago/Turabian StyleJayaram, Hemalatha, Vinutha Marigowda, and Kunigal Jagadishchandra Thara Saraswathi. 2021. "Secondary Metabolite Production and Terpenoid Biosynthesis in Endophytic Fungi Cladosporium cladosporioides Isolated from Wild Cymbopogon martinii (Roxb.) Wats" Microbiology Research 12, no. 4: 812-828. https://doi.org/10.3390/microbiolres12040059
APA StyleJayaram, H., Marigowda, V., & Thara Saraswathi, K. J. (2021). Secondary Metabolite Production and Terpenoid Biosynthesis in Endophytic Fungi Cladosporium cladosporioides Isolated from Wild Cymbopogon martinii (Roxb.) Wats. Microbiology Research, 12(4), 812-828. https://doi.org/10.3390/microbiolres12040059






