Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan
Abstract
1. Introduction
2. Materials and Methods
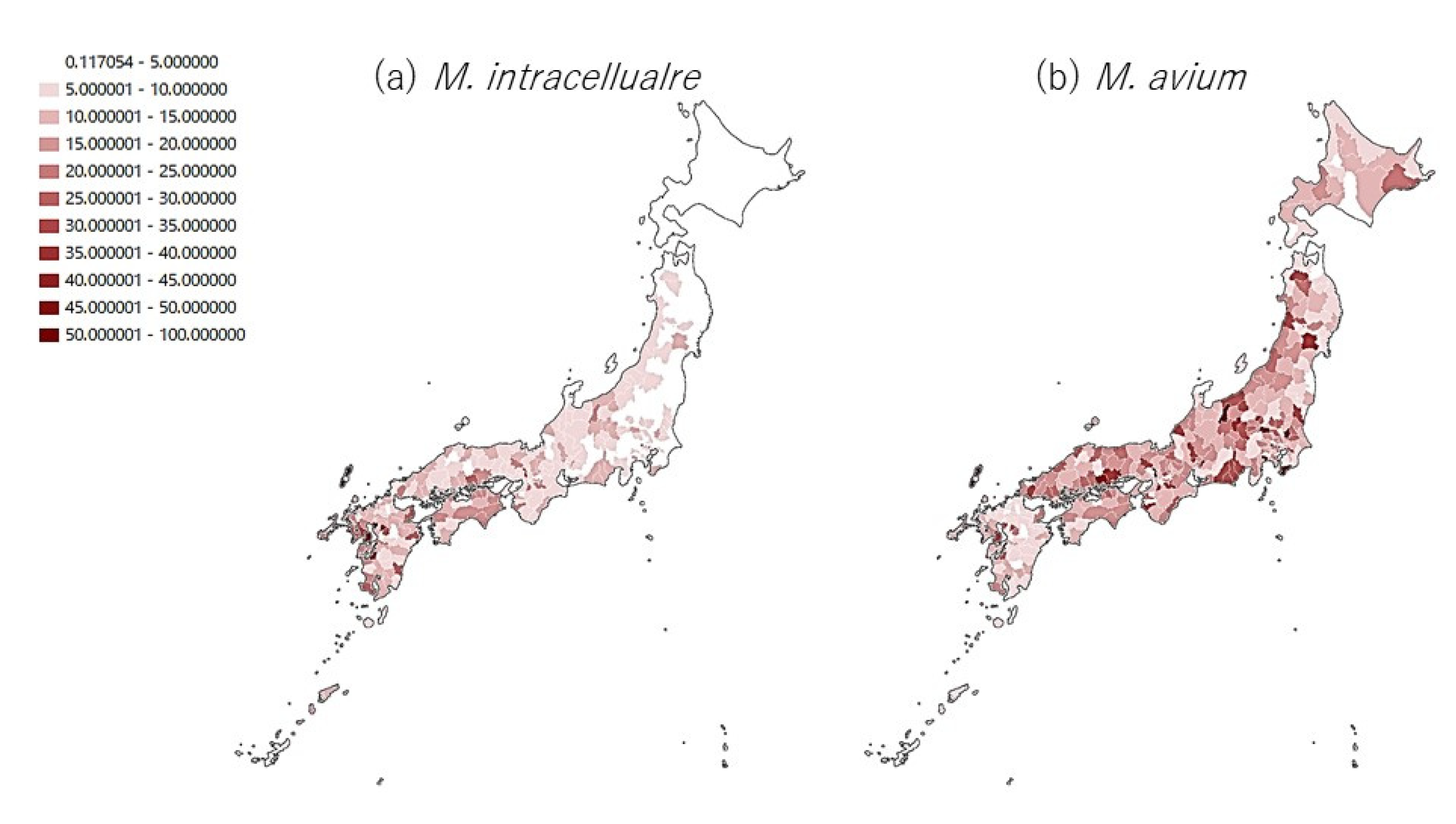
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest. Med. 2015, 36, 13–34. [Google Scholar] [CrossRef]
- Thomson, R.; Donnan, E.; Konstantinos, A. Notification of Nontuberculous Mycobacteria: An Australian Perspective. Ann. Am. Thorac. Soc. 2017, 14, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Henkle, E.; Walker, A.; Cassidy, M.; Hedberg, K.; Schafer, S. On the Reportability of Nontuberculous Mycobacterial Disease to Public Health Authorities. Ann. Am. Thorac. Soc. 2017, 14, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.M.; Raju, S.M.; Zhao, Y.; Rubin, E.J. Leveraging Advances in Tuberculosis Diagnosis and Treatment to Address Nontuberculous Mycobacterial Disease. Emerg. Infect. Dis. 2016, 22, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Bi, S.; Ji, Z.; Hu, H.; Hu, F.; Zheng, B.; Wang, B.; Ren, J.; Yang, S.; Deng, M.; et al. Distinguishing nontuberculous mycobacteria from multidrug-resistant Mycobacterium tuberculosis, China. Emerg. Infect. Dis. 2014, 20, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Iwai, K.; Uchimura, K.; Okumura, M.; Yoshiyama, T.; Yoshimori, K.; Ogata, H.; Kurashima, A.; Gemma, A.; Kudoh, S. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann. Am. Thorac. Soc. 2014, 11, 1–8. [Google Scholar] [CrossRef]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef]
- Morimoto, K.; Hasegawa, N.; Izumi, K.; Hamkoong, H.; Uchimura, K.; Yoshiyama, T.; Hoshino, Y.; Kurashima, A.; Sokunaga, J.; Shibuya, S.; et al. A laboratory-based analysis of nontuberculous mycobacterial lung disease in Japan from 2012 to 2013. Ann. Am. Thorac. Soc. 2016, 14. [Google Scholar] [CrossRef]
- Izumi, K.; Morimoto, K.; Hasegawa, N.; Uchimura, K.; Kawatsu, L.; Ato, M.; Mitarai, S. Epidemiology of Adults and Children Treated for Nontuberculous Mycobacterial Pulmonary Disease in Japan. Ann. Am. Thorac. Soc. 2019, 16, 341–347. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejek, C.; Bottger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clinical infectious diseases: An official publication of the Infectious Diseases Society of America. Clin. Inf. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef]
- Jhun, B.W.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Yoo, H.; Carriere, K.C.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: A 15-year follow-up study. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Cho, E.H.; Huh, H.J.; Song, D.J.; Moon, S.M.; Lee, S.-H.; Shin, S.Y.; Kim, C.K.; Ki, C.-S.; Koh, W.-J.; Lee, N.Y. Differences in drug susceptibility pattern between Mycobacterium avium and Mycobacterium intracellulare isolated in respiratory specimens. Journal of infection and chemotherapy. Off. J. Jpn. Soc. Chemother. 2018, 24, 315–318. [Google Scholar]
- Koh, W.J.; Jeong, B.H.; Jeon, K.; Lee, N.Y.; Lee, K.S.; Woo, S.Y.; Shin, S.J.; Kwon, O.J. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest 2012, 142, 1482–1488. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Maekura, R.; Kitada, S.; Tamaru, A.; Taguri, T.; Kira, Y.; Hiraga, T.; Hirotani, A.; Yoshimura, K.; Miki, M.; et al. The recovery of Mycobacterium avium-intracellulare complex (MAC) from the residential bathrooms of patients with pulmonary MAC. Clinical infectious diseases: An official publication of the Infectious Diseases Society of America. Clin. Inf. Dis. 2007, 45, 347–351. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Iwamoto, T.; Maruyama, F. Infection Sources of a Common Non-tuberculous Mycobacterial Pathogen, Mycobacterium avium Complex. Front. Med. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Aono, A.; Murase, Y.; Sekizuka, T.; Kurashima, A.; Takaki, A.; Sasaki, Y.; Igarashi, Y.; Chikamatsu, K.; Goto, H.; et al. Prevention of aerosol isolation of nontuberculous mycobacterium from the patient′s bathroom. ERJ Open Res. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, Y.; Tamaru, A.; Kitada, S.; Taguri, T.; Matsomoto, S.; Tateishi, Y.; Yoshimura, M.; Ozeki, Y.; Matsumoto, N.; Ogara, H.; et al. Mycobacterium avium complex organisms predominantly colonize in the bathtub inlets of patients′ bathrooms. Jpn. J. Inf. Dis. 2009, 62, 182–186. [Google Scholar]
- Arikawa, K.; Ichijo, T.; Nakajima, S.; Nishiuchi, Y.; Yano, H.; Tamaru, A.; Yoshida, S.; Maruyama, F.; Ota, A.; Nasu, M.; et al. Genetic relatedness of Mycobacterium avium subsp. hominissuis isolates from bathrooms of healthy volunteers, rivers, and soils in Japan with human clinical isolates from different geographical areas. Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Gene. Inf. Dis. 2019, 74, 103923. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Kinjo, T.; Nei, Y.; Yamashiro, S.; Fujita, J.; Kishaba, T. Causative species of nontuberculous mycobacterial lung disease and comparative investigation on clinical features of Mycobacterium abscessus complex disease: A retrospective analysis for two major hospitals in a subtropical region of Japan. PLoS ONE 2017, 12, e0186826. [Google Scholar] [CrossRef]
- Morimoto, K.; Koh, W. Asia, Global Epidemiology of NTM Disease (Except Northern America); Humana Press: Totowa, NJ, USA, 2019. [Google Scholar]
- Kim, H.S.; Lee, Y.; Lee, S.; Kim, Y.A.; Sun, Y.K. Recent trends in clinically significant nontuberculous Mycobacteria isolates at a Korean general hospital. Ann. Lab. Med. 2014, 34, 56–59. [Google Scholar] [CrossRef][Green Version]
- Koh, W.J.; Chang, B.; Jeong, B.H.; Jeon, K.; Kim, S.-Y.; Lee, N.Y.; Ki, C.-S.; Kwon, O.J. Increasing Recovery of Nontuberculous Mycobacteria from Respiratory Specimens over a 10-Year Period in a Tertiary Referral Hospital in South Korea. Tuberc. Respir. Dis. 2013, 75, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Kwon, O.J.; Jeon, K.; Kim, T.S.; Lee, K.S.; Park, Y.K.; Bai, G.H. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 2006, 129, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Nakamura, S.; Yamamoto, Y.; Kohno, Y.; Fukuda, Y.; Ikeda, H.; Sasaki, E.; Yanagihara, K.; Higashiyama, Y.; Hashiguchi, K.; et al. Epidemiology and clinical features of pulmonary nontuberculous mycobacteriosis in Nagasaki, Japan. PLoS ONE 2015, 10, e0128304. [Google Scholar]
- Yu, X.; Liu, P.; Liu, G.; Zhao, L.; Hu, Y.; Wai, G.; Luo, J.; Huang, H. The prevalence of non-tuberculous mycobacterial infections in mainland China: Systematic review and meta-analysis. J. Inf. 2016, 73, 558–567. [Google Scholar] [CrossRef]
- Yoshida, S.; Tomita, M. Pathogenic characterization of Mycobacterium intracellulare sequevar and susceptibility to clarithromycin. J. Jpn. Soc. Clin. Microbiol. 2013, 23, 29–38. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, K.; Ato, M.; Hasegawa, N.; Mitarai, S. Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan. Microbiol. Res. 2021, 12, 739-743. https://doi.org/10.3390/microbiolres12030053
Morimoto K, Ato M, Hasegawa N, Mitarai S. Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan. Microbiology Research. 2021; 12(3):739-743. https://doi.org/10.3390/microbiolres12030053
Chicago/Turabian StyleMorimoto, Kozo, Manabu Ato, Naoki Hasegawa, and Satoshi Mitarai. 2021. "Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan" Microbiology Research 12, no. 3: 739-743. https://doi.org/10.3390/microbiolres12030053
APA StyleMorimoto, K., Ato, M., Hasegawa, N., & Mitarai, S. (2021). Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan. Microbiology Research, 12(3), 739-743. https://doi.org/10.3390/microbiolres12030053





