Hurdle Effects of Ethanolic Plant Extracts with Antimicrobials Commonly Used in Food against Foodborne Pathogenic Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation
2.2. Plant-Extract Preparation
2.3. Gas Chromatography-Mass Spectroscopy (GC–MS) Analysis
2.4. Screening of Plant Extracts for Antimicrobial Activity
2.5. MIC and MBC Determination
2.6. Combined Effect of Plant Extracts with Glycine and NaOAc
2.7. Effect of Individual and Combined Clove Extract with Antimicrobials on Survival of E. coli O157:H7 in Phosphate-Buffered Saline (PBS) under Different pH and Incubation-Temperature Levels
2.8. Statistical Analysis
3. Results and Discussion
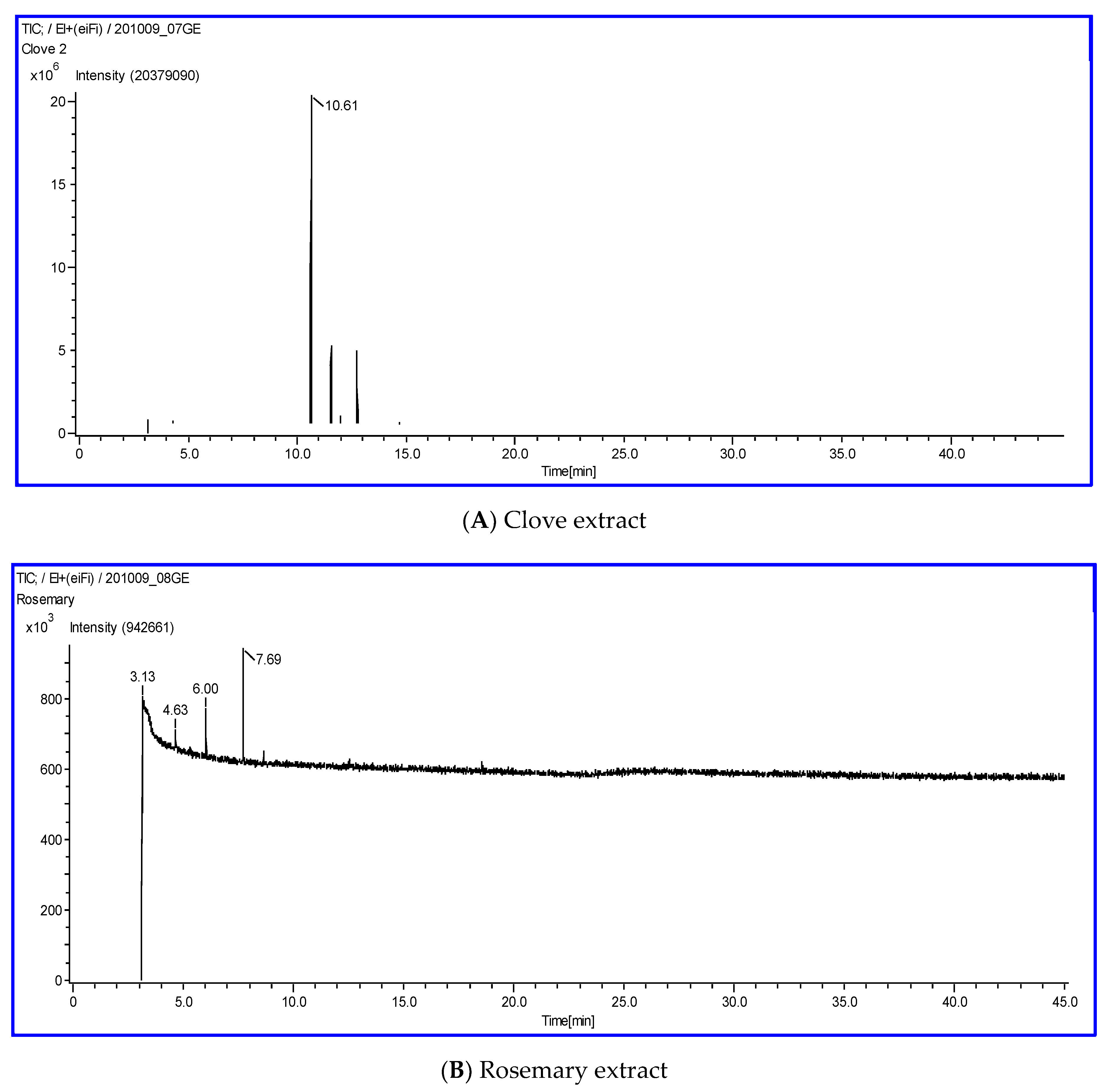
3.1. Chemical Composition of Plant Extracts
3.2. Antimicrobial Activities of Plant Extracts against Tested Bacteria
3.3. MIC and MBC of Plant Extracts against E. coli
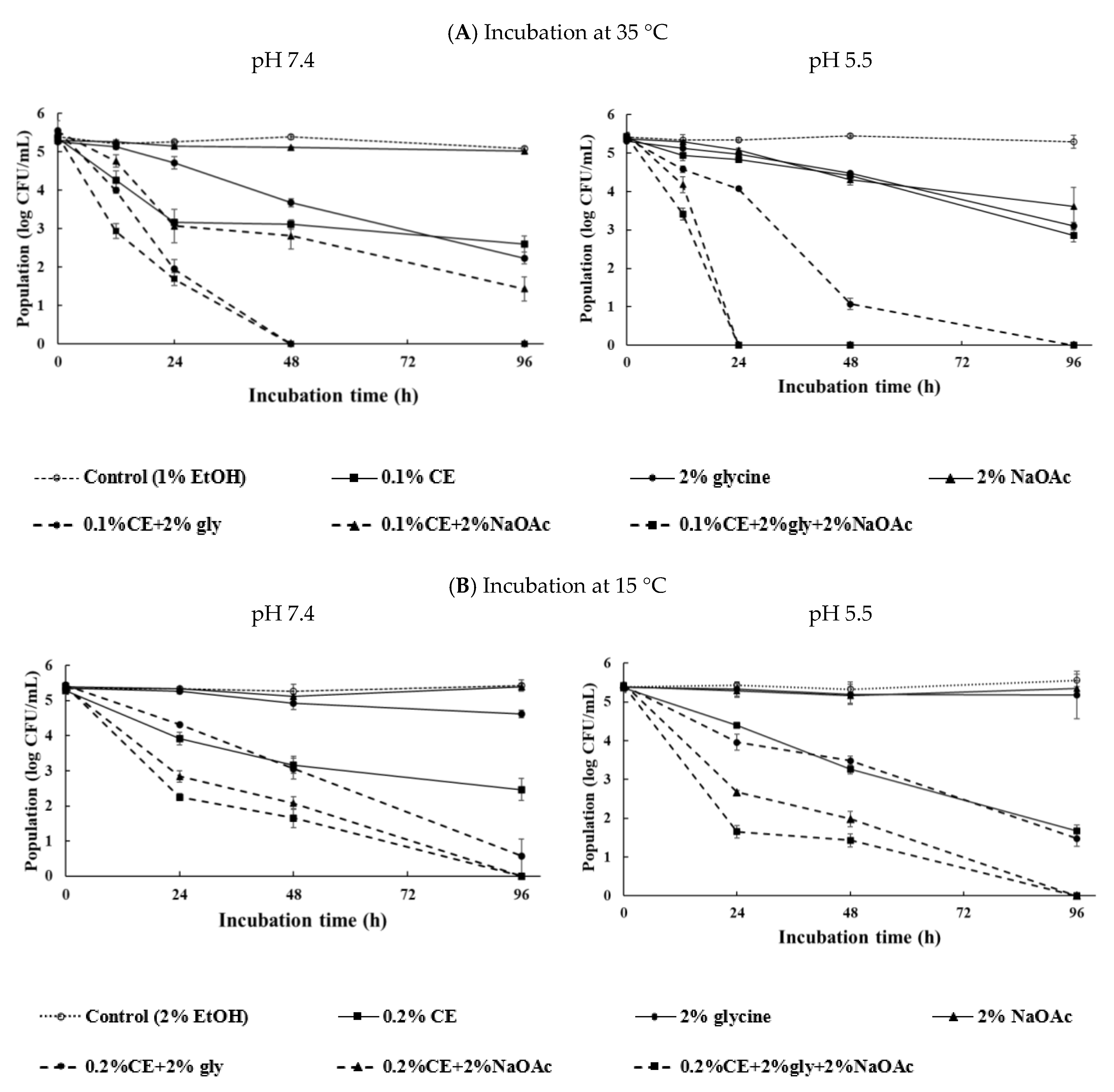
3.4. Effect of Glycine or NaOAc on Antibacterial Activity of Plant Extracts against E. coli in Neutral and Mildly Acidic Media
3.5. Combined Effects of Clove Extract and Antimicrobials on E. coli O157:H7 in PBS under Different pH and Incubation-Temperature Levels
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Center for Desease Control and Prevention (CDC). Available online: https://www.cdc.gov/ecoli/outbreaks.html (accessed on 3 December 2020).
- Dabija, A.; Codină, G.G.; Ropciuc, S.; Gâtlan, A.-M.; Rusu, L. Assessment of the Antioxidant Activity and Quality Attributes of Yogurt Enhanced with Wild Herbs Extracts. J. Food Qual. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, B.; Xiong, Y.L.; Sun, X. Antimicrobial Activities of Spice Extracts against Pathogenic and Spoilage Bacteria in Modified Atmosphere Packaged Fresh Pork and Vacuum Packaged Ham Slices Stored at 4 °C. Meat Sci. 2009, 81, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. The in vitro Antibacterial Activity of Dietary Spice and Medicinal Herb Extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Leistner, L.; Gorris, L.G.M. Food Preservation by Hurdle Technology. Trends Food Sci. Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Fan, X.; Jin, T.; Niemira, B.A. Influence of Antimicrobial Agents on the Thermal Sensitivity of Foodborne Pathogens: A Review. J. Food Prot. 2019, 82, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.A.; Brandelli, A.; Malheiros, P.D.S. Combining Natural Antimicrobials and Nanotechnology for Disinfecting Food Surfaces and Control Microbial Biofilm Formation. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Ando, T.; Hashikawa, S.; Torii, K.; Hasegawa, T.; Israel, D.A.; Ina, K.; Kusugami, K.; Goto, H.; Ohta, M. Effect of Glycine on Helicobacter pylori in vitro. Antimicrob. Agents Chemother. 2004, 48, 3782–3788. [Google Scholar] [CrossRef]
- Sallam, K.H.I.; Samejima, K. Microbiological and Chemical Quality of Ground Beef Treated with Sodium Lactate and Sodium Chloride during Refrigerated Storage. LWT Food Sci. Technol. 2004, 37, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Daifas, D.P.; El-Khoury, W.; Koukoutsis, J.; El-Khoury, A. Shelf Life and Safety Concerns of Bakery Products—a Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 19–55. [Google Scholar] [CrossRef]
- Mohammadzadeh-Aghdash, H.; Sohrabi, Y.; Mohammadi, A.; Shanehbandi, D.; Dehghan, P.; Dolatabadi, J.E.N. Safety Assessment of Sodium Acetate, Sodium Diacetate and Potassium Sorbate Food Additives. Food Chem. 2018, 257, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pundir, K.R.; Jain, P.; Sharma, C. Antimicrobial Activity of Ethanolic Extracts of Syzygium aromaticum and Allium sativum against Food Associated Bacteria and Fungi. Ethnobot. Leafl. 2010, 14, 334–360. [Google Scholar]
- Weerakkody, N.S.; Caffin, N.; Turner, M.S.; Dykes, G.A. In Vitro Antimicrobial Activity of Less-Utilized Spice and Herb Extracts against Selected Food-Borne Bacteria. Food Control 2010, 21, 1408–1414. [Google Scholar] [CrossRef]
- Cui, H.; Gabriel, A.A.; Nakano, H. Antimicrobial Efficacies of Plant Extracts and Sodium Nitrite against Clostridium botulinum. Food Control 2010, 21, 1030–1036. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C. Antibacterial Activity of Some Essential Oil Components against Five Foodborne Pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Zurenko, G.E. In Vitro Activities of Linezolid Combined with Other Antimicrobial Agents against Staphylococci, Enterococci, Pneumococci, and Selected Gram-Negative Organisms. Antimicrob. Agents Chemother. 2003, 47, 1902–1906. [Google Scholar] [CrossRef]
- Alshaikh, N.; Perveen, K. Anti-Candidal Activity and Chemical Composition of Essential Oil of Clove (Syzygium aromaticum). J. Essent. Oil Bear. Plants 2017, 20, 951–958. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Kayira, T.M.; Nakano, H. Antibacterial Effects of Plant Extracts with Hurdle Technology against Vibrio cholerae. FEMS Microbiol. Lett. 2020, 367, 1–6. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant Nutraceuticals as Antimicrobial Agents in Food Preservation: Terpenoids, Polyphenols and Thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-N.; Wang, C.-Y.; Wang, C.-L.; Chou, C.-H.; Leu, Y.-L.; Chen, B.-Y. Antimicrobial Effects and Mechanisms of Ethanol Extracts of Psoralea corylifolia Seeds against Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2019, 16, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Meira, N.V.B.; Holley, R.A.; Bordin, K.; Macedo, R.E.F.; de Luciano, F.B. Combination of Essential Oil Compounds and Phenolic Acids against Escherichia coli O157:H7 in Vitro and in Dry-Fermented Sausage Production. Int. J. Food Microbiol. 2017, 260, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The Potential Application of Plant Essential Oils as Natural Food Preservatives in Soft Cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Sepahi, M.; Jalal, R.; Mashreghi, M. Antibacterial Activity of Poly-l-Arginine under Different Conditions. Iran. J. Microbiol. 2017, 9, 103–111. [Google Scholar]
- Ehsani, A.; Jasour, M.S.; Hashemi, M.; Mehryar, L.; Khodayari, M. Zataria multiflora Boiss Essential Oil and Sodium Acetate: How They Affect Shelf Life of Vacuum-Packaged Trout Burgers. Int. J. Food Sci. Technol. 2014, 49, 1055–1062. [Google Scholar] [CrossRef]
- Takahashi, H.; Takahashi, T.; Miya, S.; Yokoyama, H.; Kuda, T.; Kimura, B. Growth Inhibition Effects of Ferulic Acid and Glycine/Sodium Acetate on Listeria monocytogenes in Coleslaw and Egg Salad. Food Control. 2015, 57, 105–109. [Google Scholar] [CrossRef]
- Govaris, A.; Solomakos, N.; Pexara, A.; Chatzopoulou, P.S. The Antimicrobial Effect of Oregano Essential Oil, Nisin and Their Combination against Salmonella Enteritidis in Minced Sheep Meat during Refrigerated Storage. Int. J. Food Microbiol. 2010, 137, 175–180. [Google Scholar] [CrossRef]
- Kotzekidou, P.; Giannakidis, P.; Boulamatsis, A. Antimicrobial Activity of Some Plant Extracts and Essential Oils against Foodborne Pathogens in Vitro and on the Fate of Inoculated Pathogens in Chocolate. LWT Food Sci. Technol. 2008, 41, 119–127. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The Antimicrobial Effect of Thyme Essential Oil, Nisin, and Their Combination against Listeria monocytogenes in Minced Beef during Refrigerated Storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Kim, S.-S.; Kang, D.-H. Susceptibility of Escherichia coli O157:H7 Grown at Low Temperatures to the Krypton-Chlorine Excilamp. Sci. Rep. 2019, 9, 563. [Google Scholar] [CrossRef] [PubMed]
| Scientific Name | Common Name | Part |
|---|---|---|
| Cinnamomum verum | Cinnamon | Bark |
| Syzygium aromaticum | Clove | Flower bud |
| Helichrysum italicum | Curry plant | Leaves |
| Corymbia citriodora | Lemon eucalyptus | Leaves |
| Glycyrrhiza glabra | Liquorice | Root |
| Myristica fragrans | Mace | Seed coat |
| Myristica fragrans | Nutmeg | Seed |
| Rosmarinus officinalis | Rosemary | Leaves |
| Salvia officinalis | Sage | Leaves |
| Mentha spicaaand | Spearmint | Leaves |
| Thymus vulgaris | Thyme | Leaves |
| Monodora myristica | Calabash nutmeg | Seed |
| Piper guineense | West African black pepper | Seed |
| Tetrepleura tetraptera | Aidan | Fruit |
| Afaramomun melegueta | Grains of paradise | Seed |
| Xylopia aethiopica | Negro pepper | Fruit |
| Pimpenella anisum | Aniseed | Seed |
| Rauvolfia vomitoria | Rauvolfia | Root |
| Parkia biglobosa | African locust bean | Seed |
| Piper nigrum | Black pepper | Seed |
| Capsicum annuum | Cayenne | Fruit |
| Ocimum basilicum | Sweet basil | Leaves |
| Plant Extract | RT | Identified Compound | Molecular Formula | Peak Area (%) |
|---|---|---|---|---|
| Clove | 10.61 | Eugenol | C10H12O2 | 63.73 |
| 11.52 | Caryophyllene | C15H24 | 13.97 | |
| 12.72 | Phenol, 2-methoxy-4-(2-propenyl)-, acetate | C12H14O3 | 12.72 | |
| Rosemary | 3.13 | Glycidol | C3H6O2 | 67.98 |
| 4.63 | 1-Isopropyl-4-methylbicyclo[3.1.0]hex-2-ene | C10H16 | 4.66 | |
| 6.00 | Eucalyptol | C10H18O | 6.14 | |
| 7.69 | Camphor | C10H16O | 17.15 |
| Plant Extract | Diameter of Inhibition Zone 1 (mm) | ||||
|---|---|---|---|---|---|
| E. coli | E. coli O157:H7 | S. aureus | B. cereus | ||
| IFO 3301 | HCIPH 92655 | HCIPH 92656 | 209P | IFO 3457 | |
| Clove | 13.0 ± 0.8 a | 12.2 ± 0.5 a | 17.2 ± 1.9 a | 19.0 ± 1.4 b | 11.0 ± 0 d |
| Curry plant | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 33.5 ± 2.1 a | 22.0 ± 1.4 a |
| Lemon eucalyptus | 8.2 ± 0.5 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 14.5 ± 0.7 d,e,f | 12.0 ± 0.0 c |
| Liquorice | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 15.0 ± 0.0 d,e | 9.5 ± 0.7 e |
| Mace | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 18.5 ± 2.1 bc | 11.0 ± 0.0 d |
| Nutmeg | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 12.5 ± 0.7 f,g | 8.0 ± 0.0 f |
| Rosemary | 11.2 ± 1.0 b | 11.8 ± 1.0 a | 12.0 ± 0.0 b | 13.5 ± 0.7 e,f,g | 8.0 ± 0.0 f |
| Sage | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 16.5 ± 0.0 c,d | 11.5 ± 0.7 c,d |
| Thyme | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 19.5 ± 0.7 b | 8.0 ± 0.0 f |
| Calabash nutmeg | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 17.5 ± 0.7 b,c | 12.0 ± 0.0 c |
| Grains of paradise | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 12.0 ± 0.0 g | 8.0 ± 0.0 f |
| Negro pepper | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 13.5 ± 0.7 e,f,g | 16.5 ± 0.7 b |
| Aniseed | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 11.5 ± 0.7 g | 8.0 ± 0.0 f |
| African locust bean | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 18.5 ± 2.1 b,c | 11.5 ± 0.7 c,d |
| Sweet basil | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 12.5 ± 0.7 f,g | 8.0 ± 0.0 f |
| Control (ethanol) | 8.0 ± 0.0 c | 8.0 ± 0.0 b | 8.0 ± 0.0 c | 8.0 ± 0.0 h | 8.0 ± 0.0 f |
| Compounds | Concentration (%) | |||
|---|---|---|---|---|
| E. coli | E. coli O157:H7 | |||
| IFO 3310 | HCIPH 92655 | HCIPH 92656 | ||
| Clove Extract | MIC | 0.4 | 0.4 | 0.4 |
| MBC | 0.4 | 0.4 | 0.4 | |
| Rosemary Extract | MIC | 0.6 | 0.6 | 0.6 |
| MBC | 0.8 | 0.8 | 0.8 | |
| Glycine | MIC | 4.0 | 4.0 | 4.0 |
| MBC | 4.0 | 4.0 | 4.0 | |
| Sodium Acetate | MIC | 6.0 | 4.0 | 4.0 |
| MBC | 8.0 | 8.0 | 8.0 | |
| Plant Extract | Individual MIC (%) | MIC When Combined with Antimicrobials (%) | FIC Index 1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 7.0 | pH 5.5 | pH 7.0 | pH 5.5 | pH 7.0 | pH 5.5 | |||||||||||||
| NaOAc | Glycine | NaOAc | Glycine | NaOAc | Glycine | NaOAc | Glycine | |||||||||||
| 0.1 | 0.2 | 1.0 | 2.0 | 0.1 | 0.2 | 1.0 | 2.0 | 0.1 | 0.2 | 1.0 | 2.0 | 0.1 | 0.2 | 1.0 | 2.0 | |||
| Clove | 0.4 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.53 | 0.55 | 0.75 | 1.00 | 0.70 | 0.90 | 1.25 | 1.50 |
| Rosemary | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.4 | 0.2 | 0.2 | 0.6 | 0.6 | 1.03 | 1.05 | 1.25 | 1.17 | 0.53 | 0.73 | 1.25 | 1.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusalaruk, W.; Nakano, H. Hurdle Effects of Ethanolic Plant Extracts with Antimicrobials Commonly Used in Food against Foodborne Pathogenic Escherichia coli. Microbiol. Res. 2021, 12, 288-298. https://doi.org/10.3390/microbiolres12020020
Kusalaruk W, Nakano H. Hurdle Effects of Ethanolic Plant Extracts with Antimicrobials Commonly Used in Food against Foodborne Pathogenic Escherichia coli. Microbiology Research. 2021; 12(2):288-298. https://doi.org/10.3390/microbiolres12020020
Chicago/Turabian StyleKusalaruk, Waraporn, and Hiroyuki Nakano. 2021. "Hurdle Effects of Ethanolic Plant Extracts with Antimicrobials Commonly Used in Food against Foodborne Pathogenic Escherichia coli" Microbiology Research 12, no. 2: 288-298. https://doi.org/10.3390/microbiolres12020020
APA StyleKusalaruk, W., & Nakano, H. (2021). Hurdle Effects of Ethanolic Plant Extracts with Antimicrobials Commonly Used in Food against Foodborne Pathogenic Escherichia coli. Microbiology Research, 12(2), 288-298. https://doi.org/10.3390/microbiolres12020020