Effect of Quercetin on Mycorrhizal Synthesis between Tuberborchii and Arbutusunedo L. In Vitro Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Inoculum Characterization
2.2. Plant Material
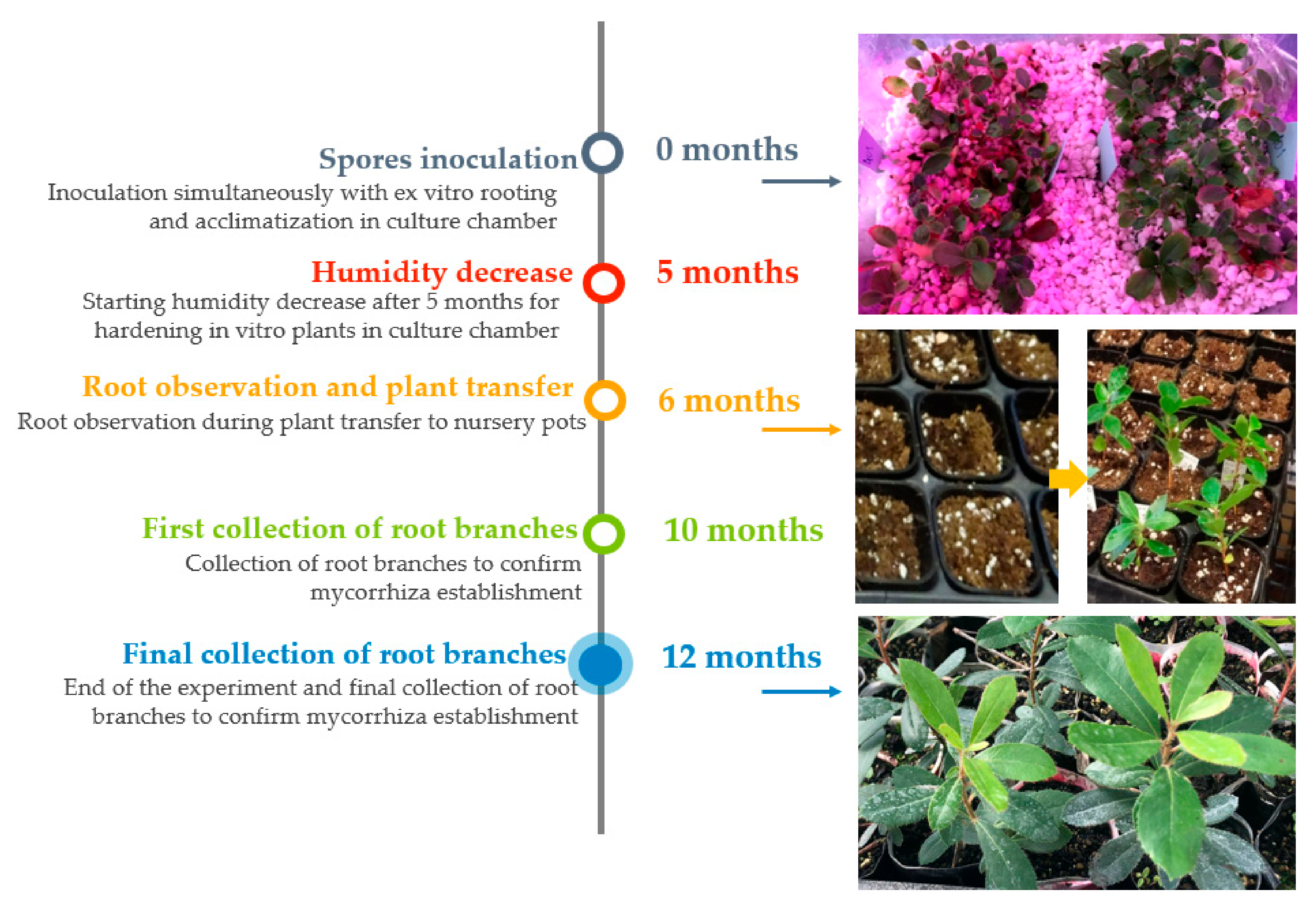
2.3. Mycorrhizal Synthesis Procedures
2.4. Morphological and Molecular Identification of Mycorrhizae
2.5. Statistical Analysis
3. Results
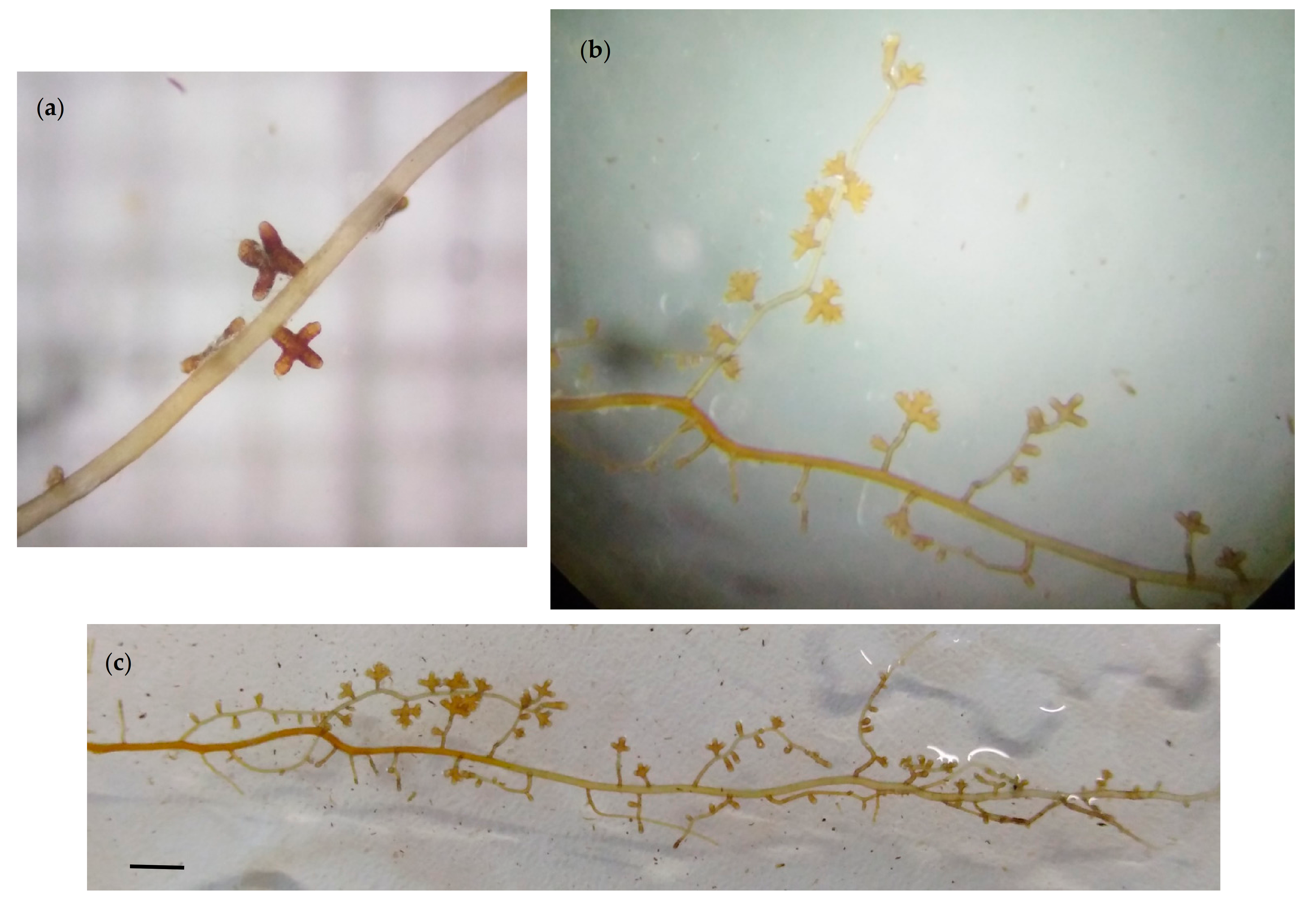
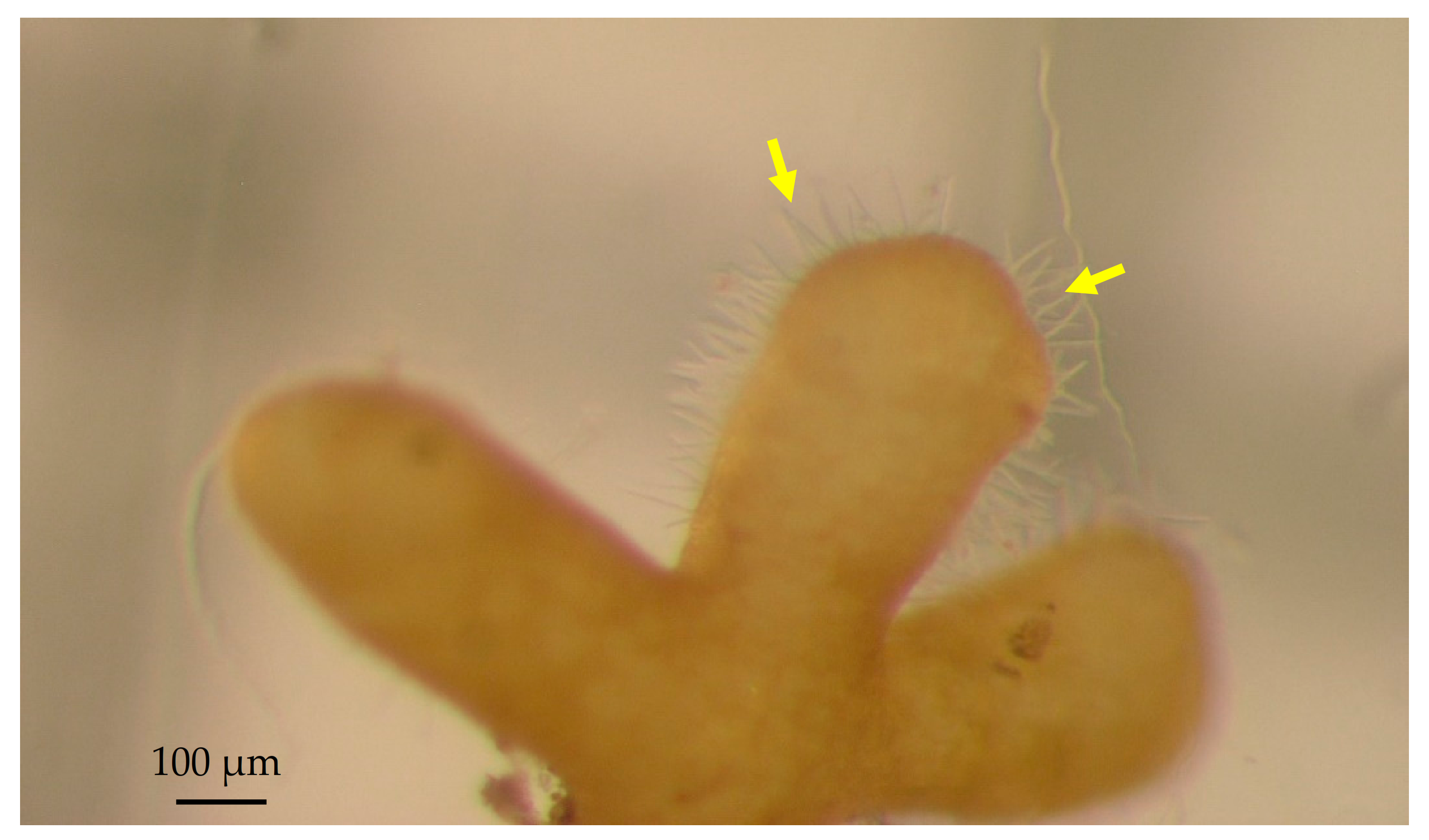
3.1. Morphological Characterization
3.2. Plant and Mycorrhizal Evaluation
3.3. Molecular Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elwes, H.J.; Henry, A. ARBUTUS. In The Trees of Great Britain and Ireland; Cambridge University Press: Cambridge, UK, 2014; pp. 558–559. [Google Scholar] [CrossRef]
- Hileman, L.C.; Vasey, M.C.; Thomas Parker, V. Phylogeny and Biogeography of the Arbutoideae (Ericaceae): Implications for the Madrean-Tethyan Hypothesis. Syst. Bot. 2001, 26, 131–143. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Maia, M.; Brigas, A.F. Physicochemical Parameters and Bioactive Compounds of Strawberry Tree (Arbutus unedo L.) Honey. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Torres, J.A.; Valle, F.; Pinto, C.; García-Fuentes, A.; Salazar, C.; Cano, E. Arbutus unedo L. Communities in Southern Iberian Peninsula Mountains. Plant Ecol. 2002, 160, 207–233. [Google Scholar] [CrossRef]
- Konstantinidis, P.; Tsiourlis, G.; Xofis, P. Effect of Fire Season, Aspect and Pre-Fire Plant Size on the Growth of Arbutus unedo L. (Strawberry tree) Resprouts. For. Ecol. Manag. 2006, 225, 359–367. [Google Scholar] [CrossRef]
- Quevedo, L.; Arnan, X.; Rodrigo, A. Selective Thinning of Arbutus Unedo Coppices Following Fire: Effects on Growth at the Individual and Plot Level. For. Ecol. Manag. 2013, 292, 56–63. [Google Scholar] [CrossRef]
- Floris, I.; Satta, A.; Ruiu, L. Honeys of Sardinia (Italy). J. Apic. Res. 2007, 46, 198–209. [Google Scholar] [CrossRef]
- Caldeira, I.; Gomes, F.; Mira, H.; Botelho, G. Distillates Composition Obtained of Fermented Arbutus unedo L. Fruits from Different Seedlings and Clonal Plants. Ann. Agric. Sci. 2019, 64, 21–28. [Google Scholar] [CrossRef]
- Plácito, F.; Ferreira, I.; Clemente, M.; Figueiredo, P.; Barrento, M.J.; Machado, H.; Gomes, F. Mycorrhizal Synthesis between Tuber Borchii and Arbutus unedo L. Seedlings and Micropropagated Plants. Acta Hortic. 2018, 1224, 91–100. [Google Scholar] [CrossRef]
- Simard, S.; Asay, A.; Beiler, K.; Bingham, M.; Deslippe, J.; He, X.; Philip, L.; Song, Y. Experimentally Testing Effects of My-corrhizal Networks on Plant-Plant Interactions and Distinguishing among Mechanisms. Mycorrhizal Netw. 2015, 224. [Google Scholar] [CrossRef]
- Machado, H.; Barrento, M.J.; Plácito, F.; Suárez, D.; Clemente, M.; Figueiredo, P.; Gomes, F. Mycorrhizal Synthesis between Lactarius Deliciosus and Arbutus unedo under Nursery Conditions. Acta Hortic. 2017, 1187, 265–272. [Google Scholar] [CrossRef]
- Richard, F.; Millot, S.; Gardes, M.; Selosse, M.A. Diversity and Specificity of Ectomycorrhizal Fungi Retrieved from an Old-Growth Mediterranean Forest Dominated by Quercus Ilex. New Phytol. 2005, 166, 1011–1023. [Google Scholar] [CrossRef]
- Massicotte, H.B.; Melville, L.H.; Molina, R.; Peterson, R.L. Structure and Histochemistry of Mycorrhizae Synthesized between Arbutus Menziesii (Ericaceae) and Two Basidiomycetes, Pisolithus Tinctorius (Pisolithaceae) and Piloderma Bicolor (Corticiaceae). Mycorrhiza 1993, 3, 1–11. [Google Scholar] [CrossRef]
- Molina, R.; Trappe, J.M. By Specificity Lack of Mycorrhizal Menziesii Hosts Arbutus the Ericaceous Uva-Ursi and Arctostaphylos. New Phytol. 1982, 90, 495–509. [Google Scholar] [CrossRef]
- Mühlmann, O.; Göbl, F. Mycorrhiza of the Host-Specific Lactarius Deterrimus on the Roots of Picea Abies and Arctostaphylos Uva-Ursi. Mycorrhiza 2006, 16, 245–250. [Google Scholar] [CrossRef]
- Gomes, F.; Suárez, D.; Santos, R.; Silva, M.; Gaspar, D.; Machado, H. Mycorrhizal Synthesis between Lactarius Deliciosus and Arbutus unedo L. Mycorrhiza 2016, 26, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.; Zampieri, E.; Zambonelli, A. Truffle Ecology: Genetic Diversity, Soil Interactions and Functioning. In Mycorrhiza—Function, Diversity, State of the Art; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 231–252. [Google Scholar] [CrossRef]
- Lancellotti, E.; Iotti, M.; Zambonelli, A.; Franceschini, A. Characterization of Tuber Borchii and Arbutus unedo Mycorrhizas. Mycorrhiza 2014, 24, 481–486. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The Role of Flavonoids in Root-Rhizosphere Signalling: Opportunities and Challenges for Improving Plant-Microbe Interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Antunes, P.M.; Rajcan, I.; Goss, M.J. Specific Flavonoids as Interconnecting Signals in the Tripartite Symbiosis Formed by Arbuscular Mycorrhizal Fungi, Bradyrhizobium Japonicum (Kirchner) Jordan and Soybean (Glycine max (L.) Merr.). Soil Biol. Biochem. 2006, 38, 533–543. [Google Scholar] [CrossRef][Green Version]
- Harrison, M.J. Isoflavonoid Accumulation and Expression of Defense Gene Transcripts during the Establishment of Vesicular-Arbuscular Mycorrhizal Associations in Roots of Medicago Truncatula. Mol. Plant-Microbe Interact. 1993, 6, 643. [Google Scholar] [CrossRef]
- Larose, G.; Chênevert, R.; Moutoglis, P.; Gagné, S.; Piché, Y.; Vierheilig, H. Flavonoid Levels in Roots of Medicago Sativa Are Modulated by the Developmental Stage of the Symbiosis and the Root Colonizing Arbuscular Mycorrhizal Fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Scervino, J.M.; Ponce, M.A.; Erra-Bassells, R.; Vierheilig, H.; Ocampo, J.A.; Godeas, A. Arbuscular Mycorrhizal Colonization of Tomato by Gigaspora and Glomus Species in the Presence of Root Flavonoids. J. Plant Physiol. 2005, 162, 625–633. [Google Scholar] [CrossRef]
- Davies, F.T.; Calderón, C.M.; Huaman, Z.; Gómez, R. Influence of a Flavonoid (Formononetin) on Mycorrhizal Activity and Potato Crop Productivity in the Highlands of Peru. Sci. Hortic. (Amst.) 2005, 106, 318–329. [Google Scholar] [CrossRef]
- Gautheret, R.J. La Culture Des Tissues Végétaux, Techniques et Réalisations; Masson & Cie: Paris, France, 1959. [Google Scholar]
- Gomes, F.; Lopes, M.L.; Santos, T.; Canhoto, J.M. Micropropagation of Selected Trees of Arbutus unedo L. Trough Axillary Shoot Proliferation and Somatic Embryogenesis. Acta Hort. 2009, 839, 111–116. [Google Scholar] [CrossRef]
- Duarte, A.; Gomes, F.; Figueiredo, P.; Santos, R.; Clemente, M. Hydric Stress Tolerance of Arbutus Unedo L. Selected Trees. In Program and Abstract, Prague, Czech Republic, 25–29 August 2014; IUFRO, Ed.; IUFRO & Faculty of Forestry and Wood Sciences: Prague, Czech Republic, 2014; p. 45. ISBN 978-80-213-2471-2478. Available online: https://www.iufro.org/fileadmin/material/publications/proceedings-archive/20402-20207-20211-prague14-abstracts.pdf (accessed on 1 January 2021).
- Gomes, F.; Botelho, G.; Franco, J.; Gama, J.; João, C.; Santos, R.; Figueiredo, P. Assessment of Arbutus Unedo L. Clonal Plants in a Field Clonal Trial. In Program and Abstract Book, Proceedings of the IUFRO Forest Tree Breeding Conference, Prague, Czech Republic, 25–29 August 2014; IUFRO, Ed.; IUFRO & Faculty of Forestry and Wood Sciences: Prague, Czech Republic, 2014; p. 29. ISBN 978-80-213-2471-2478. Available online: https://www.iufro.org/fileadmin/material/publications/proceedings-archive/20402-20207-20211-prague14-abstracts.pdf (accessed on 1 January 2021).
- Leonardi, P.; Iotti, M.; Donati Zeppa, S.; Lancellotti, E.; Amicucci, A.; Zambonelli, A. Morphological and Functional Changes in Mycelium and Mycorrhizas of Tuber Borchii Due to Heat Stress. Fungal Ecol. 2017, 29, 20–29. [Google Scholar] [CrossRef]
- Zambonelli, A.; Iotti, M.; Hall, I. Current Status of Truffle Cultivation: Recent Results and Future Perspectives. Micol. Ital. 2015, 44, 31–40. [Google Scholar] [CrossRef]
- Figueiredo, P.; Gomes, F.; Santos, R.; Pop, R.L. Rapid Propagation of Arbutus Unedo L. Adult Selected Plants Using Ex Vitro Rooting. In Program and Abstract Book, Proceedings of the 8th International Symposium on In Vitro Culture and Horticultural Breeding, Coimbra, Portugal, 2–7 June 2013; Canhoto, J., Correia, S., Eds.; UC & ISHS: Coimbra, Portugal, 2013; p. 157. Available online: https://www.uc.pt/en/congressos/IVCHB2013/ (accessed on 1 January 2021).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninski, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes: Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X Windows Interface: Flexible Strategies for Multiple Sequence Alignment Aided by Quality Analysis Tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.; Garnero, L.; Bonfante, P. Specific PCR-Primers as a Reliable Tool for the Detection of White Truffles in Mycorrhizal Roots. New Phytol. 1999, 141, 511–516. [Google Scholar] [CrossRef]
- Miller, J.C.M. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Education Limited: Edinburgh, Scotland, 2005. [Google Scholar]
- Ori, F.; Leonardi, M.; Faccio, A.; Sillo, F.; Iotti, M.; Pacioni, G.; Balestrini, R. Synthesis and Ultrastructural Observation of Arbutoid Mycorrhizae of Black Truffles (Tuber Melanosporum and T. Aestivum). Mycorrhiza 2020, 30, 715–723. [Google Scholar] [CrossRef]
- Gomes, F.; Machado, H.; San Martin, E.; Portugal, A.; Canhoto, J.M. Mycorrhizal Synthesis between Pisolithus Arhizus and Adult Clones of Arbutus Unedo in Vitro and in Nursery. J. For. Res 2013, 24, 1–12. [Google Scholar] [CrossRef][Green Version]
- García, A.N.; del Pilar Bañón Árias, S.; Morte, A.; Sánchez-Blanco, M.J. Effects of Nursery Preconditioning through Mycorrhizal Inoculation and Drought in Arbutus Unedo L. Plants. Mycorrhiza 2011, 21, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Simões, M.; Lopes, M.L.; Canhoto, J.M. Effect of Plant Growth Regulators and Genotype on the Micropropagation of Adult Trees of Arbutus Unedo L. (Strawberry Tree). N. Biotechnol. 2010, 27, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U.; Schlaman, H.R.M.; Spaink, H.P.; Sautter, C.; Rolfe, B.G.; Djordjevic, M.A. Auxin Transport Inhibition Precedes Root Nodule Formation in White Clover Roots and Is Regulated by Flavonoids and Derivatives of Chitin Oligosaccharides. Plant J. 1998, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]



| Treatment | N | Mycorrhiza 1 | SL (mm) | Leaves No. | NRP | LLR (mm) | LsR (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||||||||
| 0 µM | 153 | 1.95 ± 0.10 | b | 21.3 ± 0.63 | a | 8.03 ± 0.26 | a | 5.90 ± 0.43 | a | 58.40 ± 2.80 | a | 13.85 ± 0.84 | b |
| 0.5 µM | 130 | 1.92 ± 0.11 | b,c | 20.91 ± 0.60 | a | 8.38 ± 0.27 | a | 5.14 ± 0.37 | a | 61.18 ± 2.99 | a | 15.88 ± 1.22 | b |
| 2 µM | 111 | 2.50 ± 0.09 | a | 22.22 ± 0.60 | a | 8.52 ± 0.29 | a | 4.91 ± 0.39 | a | 51.81 ± 3.05 | a | 15.70 ± 0.94 | b |
| 4 µM | 121 | 2.07 ± 0.11 | ab | 22.47 ± 0.89 | a | 7.75 ± 0.26 | b,c | 4.79 ± 0.35 | a | 55.44 ± 3.08 | a | 15.95 ± 1.03 | b |
| 7 µM | 118 | 1.40 ± 0.12 | c | 19.04 ± 0.51 | b | 7.30 ± 0.27 | c | 5.39 ± 0.39 | a | 55.72 ± 3.01 | a | 19.55 ± 1.47 | a |
| 10 µM | 118 | 1.72 ± 0.13 | b,c | 18.58 ± 0.69 | b | 6.47 ± 0.29 | d | 5.78 ± 0.47 | a | 59.63 ± 2.60 | a | 21.47 ± 1.39 | a |
| Total | 751 | 1.92 ± 0.05 | 20.78 ± 0.28 | 7.76 ± 0.11 | 5.34 ± 0.17 | 57.20 ± 1.20 | 16.93 ± 0.48 | ||||||
| Medium | N | Mycorrhiza 1 | SL (mm) | Leaves No. | NRP | LLR (mm) | LsR (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||||||||
| H2O | 402 | 2.15 ± 0.06 | a | 19.87 ± 0.38 | b | 7.53 ± 0.16 | b | 5.17 ± 0.22 | a | 53.84 ± 1.55 | b | 15.61 ± 0.58 | b |
| KNOP | 349 | 1.66 ± 0.08 | b | 21.81 ± 0.40 | a | 8.02 ± 0.15 | a | 5.54 ± 0.25 | a | 61.07 ± 1.84 | a | 18.60 ± 0.78 | a |
| Total | 751 | 1.92 ± 0.05 | 20.78 ± 0.28 | 7.76 ± 0.11 | 5.34 ± 0.17 | 57.20 ± 1.20 | 16.93 ± 0.48 | ||||||
| Clone | N | Mycorrhiza 1 | SL (mm) | Leaves No. | NRP | LLR (mm) | LsR (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||||||||
| AL1(101) | 435 | 2.27 ± 0.05 | a | 20.84 ± 0.38 | a | 8.59 ± 0.15 | a | 6.00 ± 0.24 | a | 51.70 ± 1.23 | b | 15.28 ± 0.53 | b |
| PF1(401) | 316 | 1.45 ± 0.08 | b | 20.68 ± 0.39 | a | 6.62 ± 0.16 | b | 4.44 ± 0.20 | b | 64.78 ± 2.22 | a | 19.32 ± 0.87 | a |
| Total | 751 | 1.92 ± 0.05 | 20.78 ± 0.28 | 7.76 ± 0.11 | 5.34 ± 0.17 | 57.20 ± 1.20 | 16.93 ± 0.48 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.; Castro, F.; Santos, R.; Figueiredo, P.; Silva, M.; Vidal, M.; Ferreira, I.; Nunes, J.; Machado, H.; Gomes, F. Effect of Quercetin on Mycorrhizal Synthesis between Tuberborchii and Arbutusunedo L. In Vitro Plants. Microbiol. Res. 2021, 12, 69-81. https://doi.org/10.3390/microbiolres12010007
Gomes B, Castro F, Santos R, Figueiredo P, Silva M, Vidal M, Ferreira I, Nunes J, Machado H, Gomes F. Effect of Quercetin on Mycorrhizal Synthesis between Tuberborchii and Arbutusunedo L. In Vitro Plants. Microbiology Research. 2021; 12(1):69-81. https://doi.org/10.3390/microbiolres12010007
Chicago/Turabian StyleGomes, Bárbara, Fábio Castro, Rita Santos, Patrícia Figueiredo, Márcia Silva, Maria Vidal, Inês Ferreira, João Nunes, Helena Machado, and Filomena Gomes. 2021. "Effect of Quercetin on Mycorrhizal Synthesis between Tuberborchii and Arbutusunedo L. In Vitro Plants" Microbiology Research 12, no. 1: 69-81. https://doi.org/10.3390/microbiolres12010007
APA StyleGomes, B., Castro, F., Santos, R., Figueiredo, P., Silva, M., Vidal, M., Ferreira, I., Nunes, J., Machado, H., & Gomes, F. (2021). Effect of Quercetin on Mycorrhizal Synthesis between Tuberborchii and Arbutusunedo L. In Vitro Plants. Microbiology Research, 12(1), 69-81. https://doi.org/10.3390/microbiolres12010007






