Negativibacillus massiliensis gen. nov., sp. nov., a New Bacterial Genus Isolated from a Human Left Colon Sample
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. Bacterial Strain and Identification
2.3. Phenotypic Characteristics
2.3.1. Optimal Growth Conditions
2.3.2. Morphologic and Biochemical Characteristics, and Antibiotic Susceptibility
2.3.3. Fatty Acid Methyl Ester (FAME) Analysis by GC/MS
2.4. Genomic Characteristics
2.4.1. Genome Sequencing
2.4.2. Genome Assemblage, Annotation and Comparison
3. Results
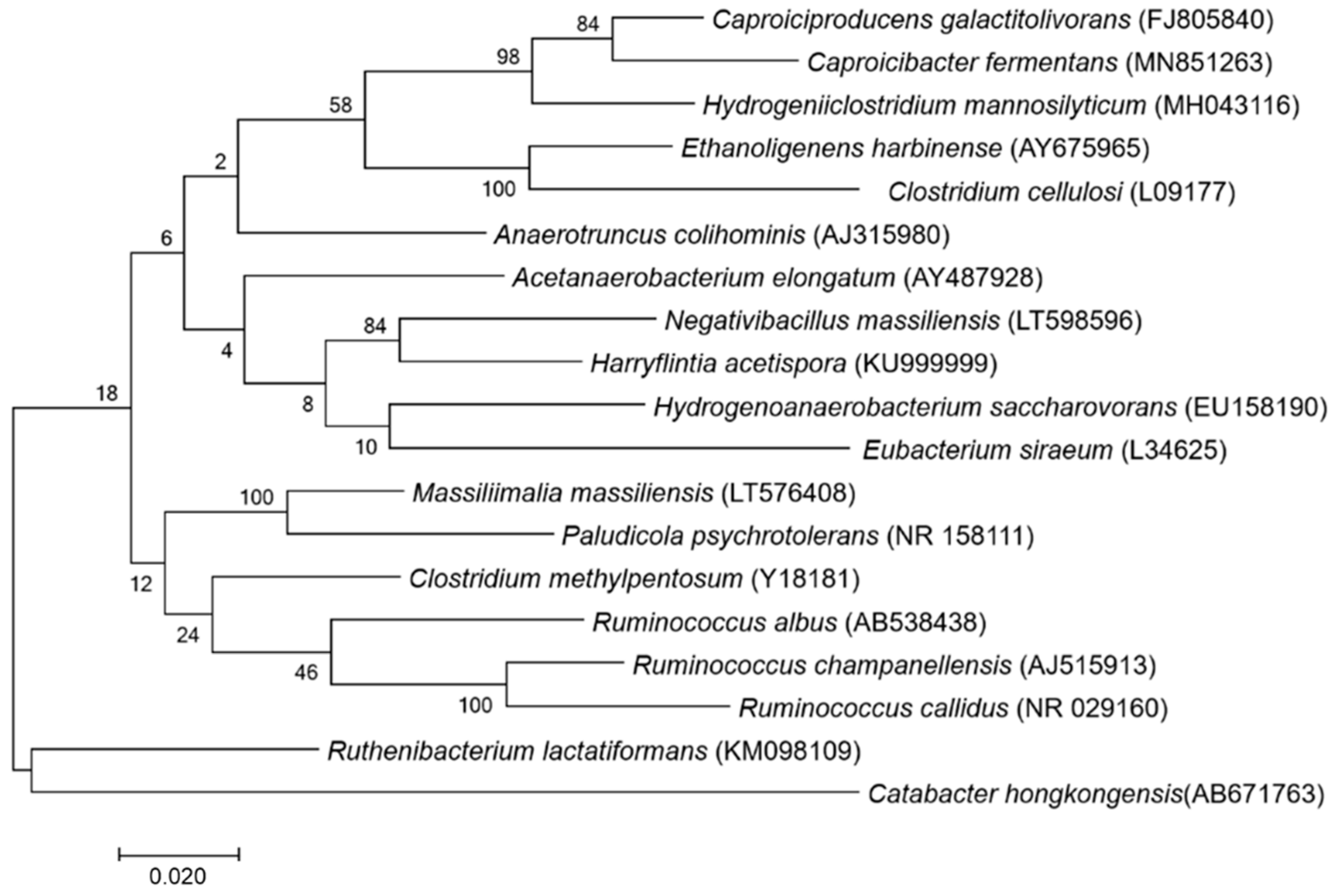
3.1. Strain Identification
3.2. Phenotypic Description
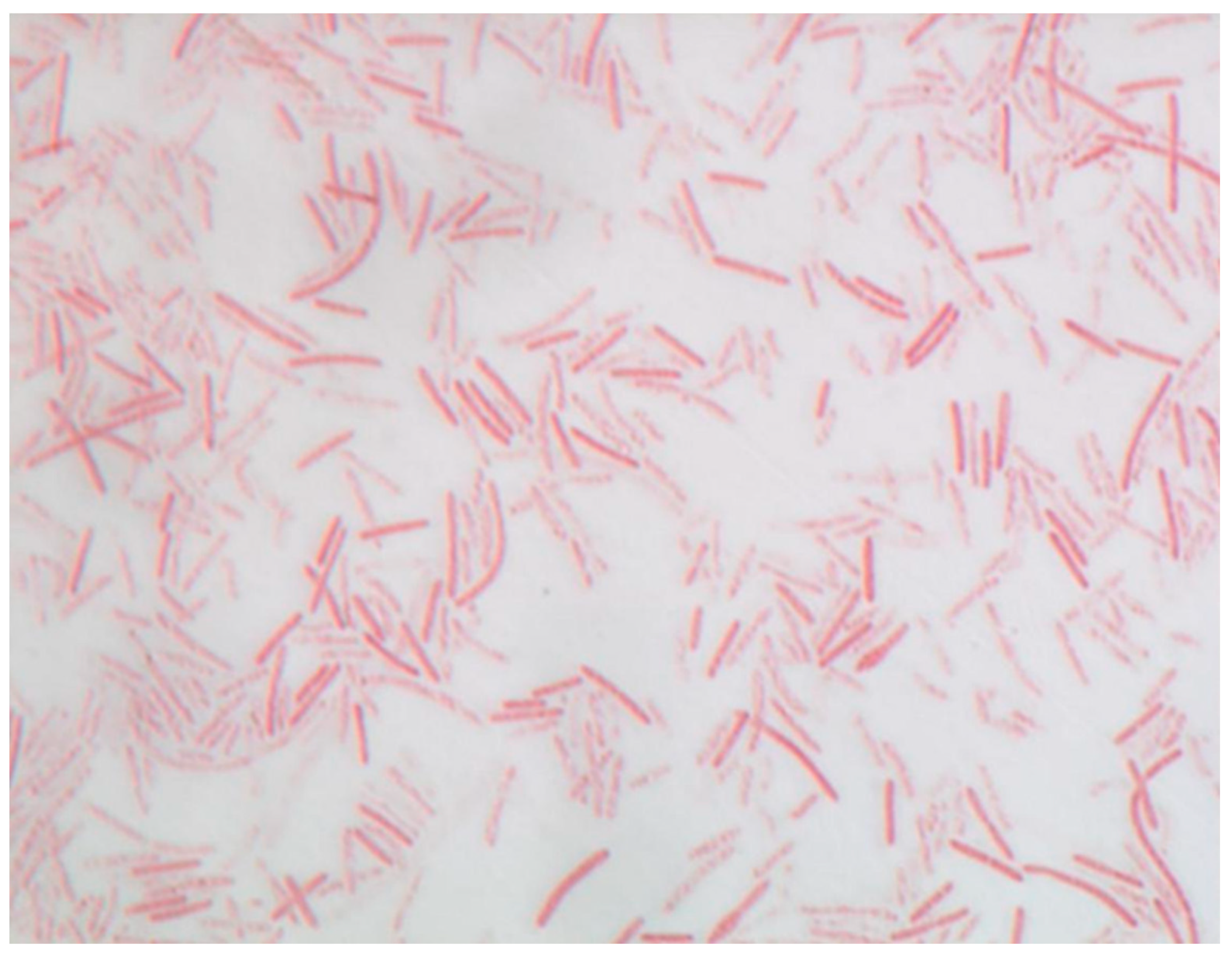
3.2.1. Morphologic and Biochemical Characteristics
3.2.2. Optimal Growth Conditions
3.2.3. Antibiotic Susceptivity
3.3. Genomic Characteristics
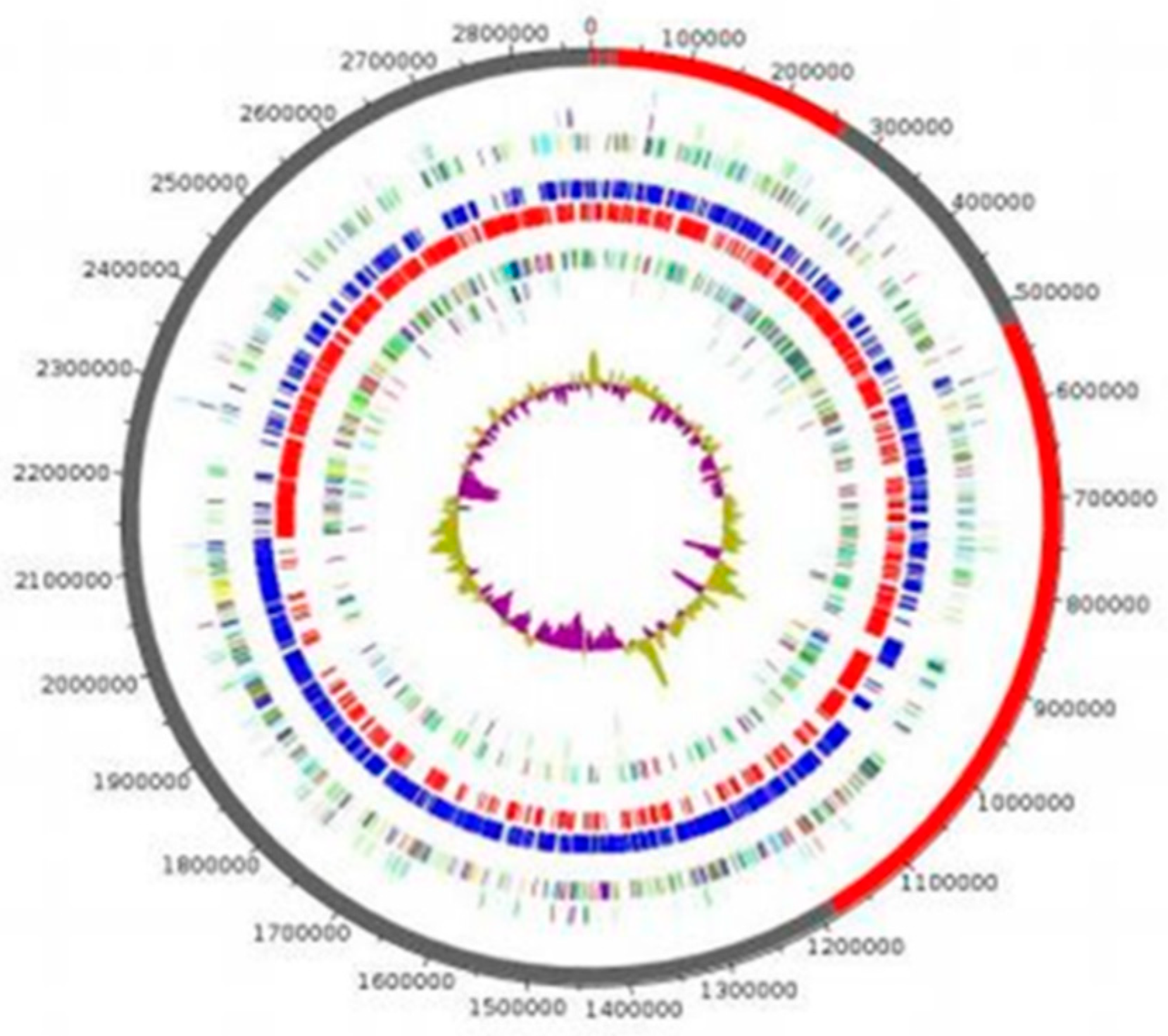
3.3.1. Genome Properties
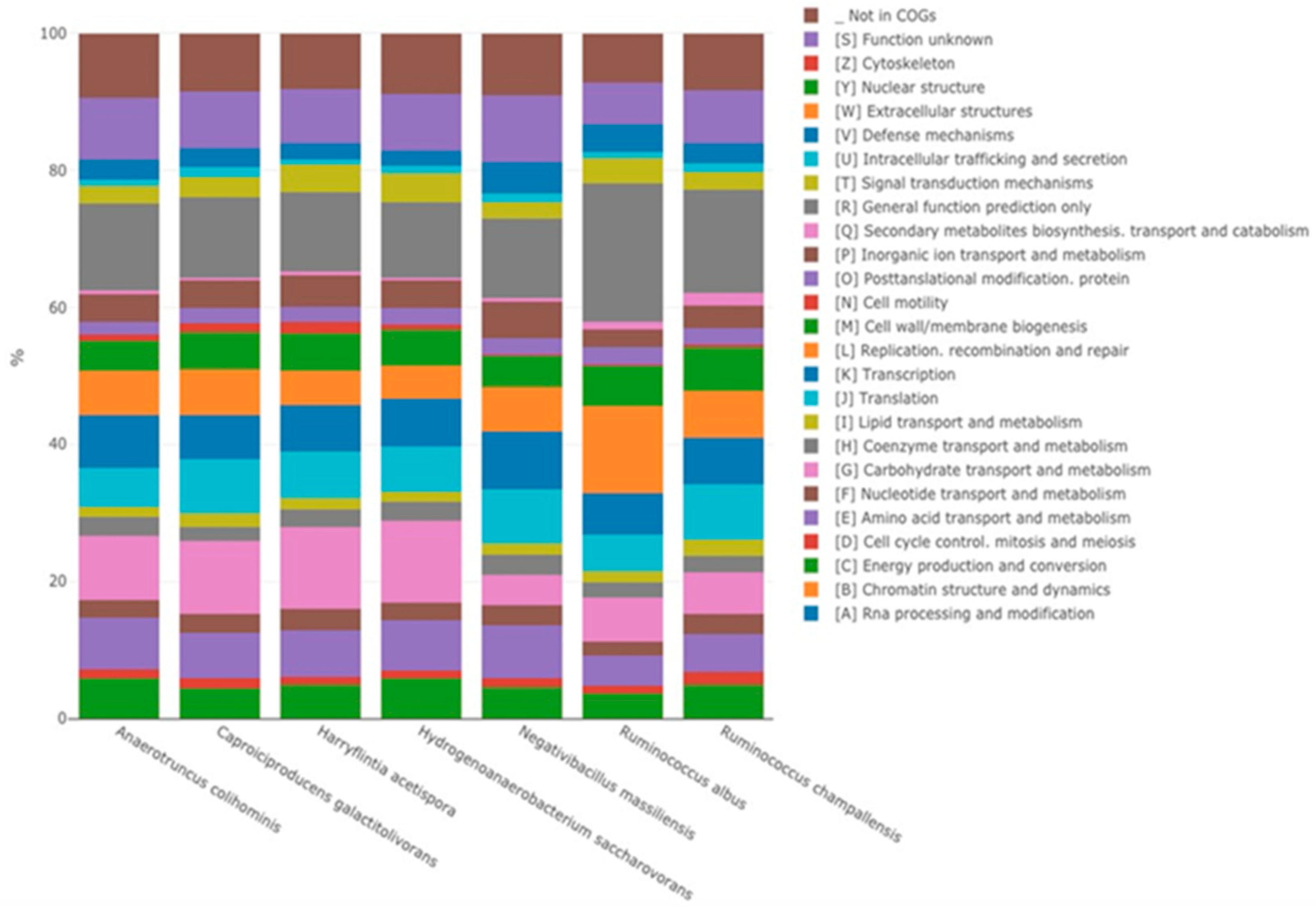
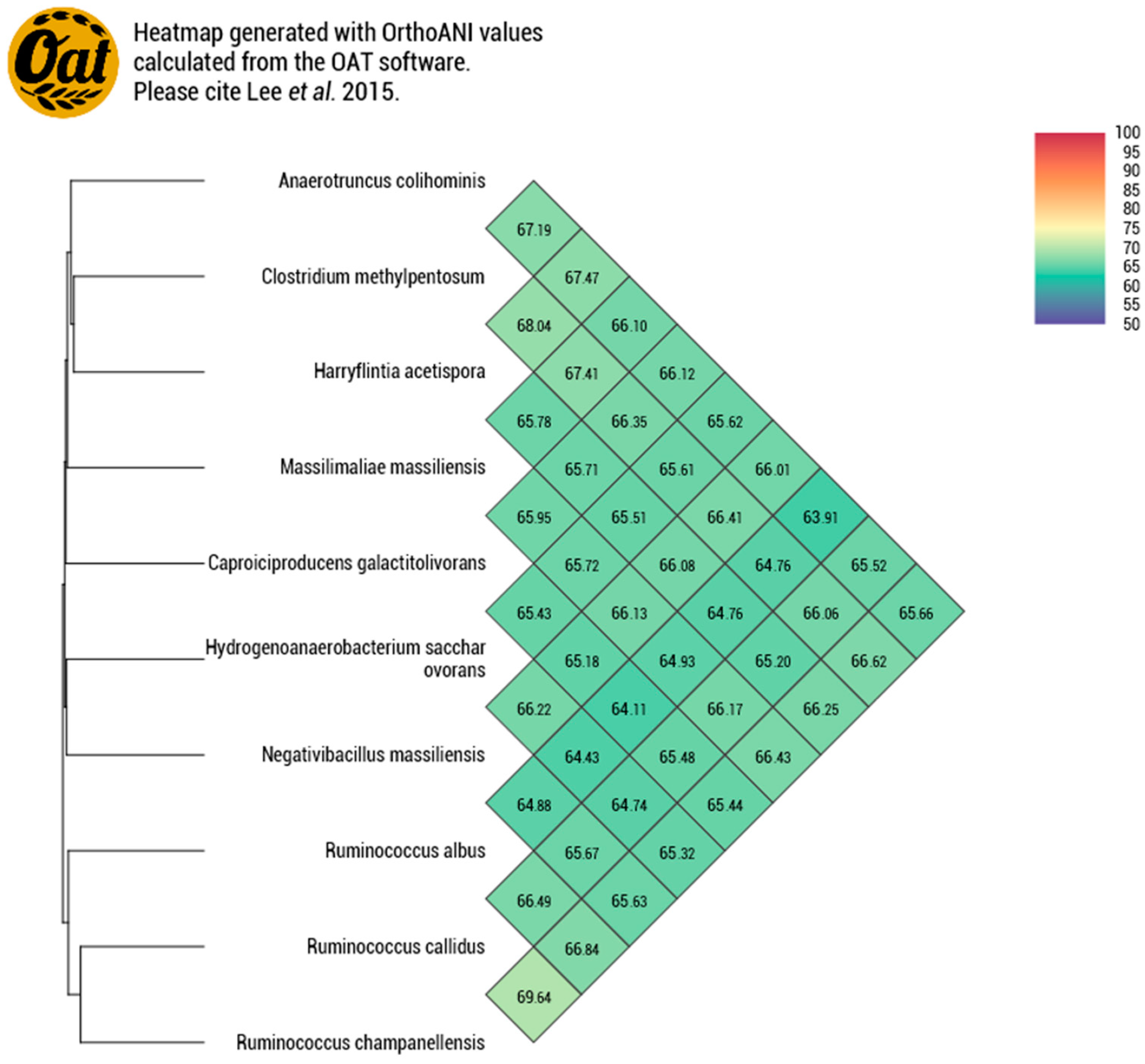
3.3.2. Genomic Comparison
4. Conclusions
4.1. Nucleotide Sequence Accession Number
4.2. Deposit in Culture Collections
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajilić-Stojanović, M.; de Vos, W.M. The First 1000 Cultured Species of the Human Gastrointestinal Microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Hugon, P.; Khelaifia, S.; Fournier, P.-E.; La Scola, B.; Raoult, D. The Rebirth of Culture in Microbiology through the Example of Culturomics to Study Human Gut Microbiota. Clin. Microbiol. Rev. 2015, 28, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Million, M.; Hugon, P.; Armougom, F.; Raoult, D. Human Gut Microbiota: Repertoire and Variations. Front. Cell. Infect. Microbiol. 2012, 2. [Google Scholar] [CrossRef]
- Tidjani Alou, M.; Naud, S.; Khelaifia, S.; Bonnet, M.; Lagier, J.-C.; Raoult, D. State of the Art in the Culture of the Human Microbiota: New Interests and Strategies. Clin. Microbiol. Rev. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Armougom, F.; Million, M.; Hugon, P.; Pagnier, I.; Robert, C.; Bittar, F.; Fournous, G.; Gimenez, G.; Maraninchi, M.; et al. Microbial Culturomics: Paradigm Shift in the Human Gut Microbiome Study. Clin. Microbiol. Infect. 2012, 18, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.; Dufour, J.-C.; Lagier, J.-C.; Cadoret, F.; Daoud, Z.; Dubourg, G.; Raoult, D. The Contribution of Culturomics to the Repertoire of Isolated Human Bacterial and Archaeal Species. Microbiome 2018, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Mailhe, M.; Ricaboni, D.; Vitton, V.; Gonzalez, J.-M.; Bachar, D.; Dubourg, G.; Cadoret, F.; Robert, C.; Delerce, J.; Levasseur, A.; et al. Repertoire of the Gut Microbiota from Stomach to Colon Using Culturomics and Next-Generation Sequencing. BMC Microbiol. 2018, 18, 157. [Google Scholar] [CrossRef]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Firmicutes. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-95041-9. [Google Scholar]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Robert, C.; Bernalier-Donadille, A. The Cellulolytic Microflora of the Human Colon: Evidence of Microcrystalline Cellulose-Degrading Bacteria in Methane-Excreting Subjects. FEMS Microbiol. Ecol. 2003, 46, 81–89. [Google Scholar] [CrossRef]
- Okeke, F.; Roland, B.C.; Mullin, G.E. The Role of the Gut Microbiome in the Pathogenesis and Treatment of Obesity. Glob. Adv. Health Med. 2014, 3, 44–57. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.-S.; Park, S.-C.; Chun, J. Towards a Taxonomic Coherence between Average Nucleotide Identity and 16S RRNA Gene Sequence Similarity for Species Demarcation of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Lagier, J.-C.; Dubourg, G.; Raoult, D. From Culturomics to Taxonomogenomics: A Need to Change the Taxonomy of Prokaryotes in Clinical Microbiology. Anaerobe 2015, 36, 73–78. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Khelaifia, S.; Alou, M.T.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Traore, S.I.; Seck, E.H.; et al. Culture of Previously Uncultured Members of the Human Gut Microbiota by Culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Abat, C.; Rolain, J.M.; Colson, P.; Lagier, J.-C.; Gouriet, F.; Fournier, P.E.; Drancourt, M.; La Scola, B.; Raoult, D. Identification of Rare Pathogenic Bacteria in a Clinical Microbiology Laboratory: Impact of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2013, 51, 2182–2194. [Google Scholar] [CrossRef] [PubMed]
- Drancourt, M.; Bollet, C.; Carlioz, A.; Martelin, R.; Gayral, J.P.; Raoult, D. 16S Ribosomal DNA Sequence Analysis of a Large Collection of Environmental and Clinical Unidentifiable Bacterial Isolates. J. Clin. Microbiol. 2000, 38, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Ebers, J. Taxonomic Parameters Revisited: Tarnished Gold Standards. Microbiol. Today 2006, 33, 152. [Google Scholar]
- Brown, D.F.J.; Brown, L. Evaluation of the E Test, a Novel Method of Quantifying Antimicrobial Activity. J. Antimicrob. Chemother. 1991, 27, 185–190. [Google Scholar] [CrossRef]
- Sasser, M. Bacterial Identification by Gas Chromatographic Analysis of Fatty Acid Methyl Esters (GC-FAME); MIDI, Inc.: Newark, DE, USA, 1990; p. 6. [Google Scholar]
- Dione, N.; Sankar, S.A.; Lagier, J.-C.; Khelaifia, S.; Michele, C.; Armstrong, N.; Richez, M.; Abrahão, J.; Raoult, D.; Fournier, P.-E. Genome Sequence and Description of Anaerosalibacter massiliensis sp. Nov. New Microbes New Infect. 2016, 10, 66–76. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic Gene Recognition and Translation Initiation Site Identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. TRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Genomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and Rapid Annotation of Ribosomal RNA Genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L.L. A Combined Transmembrane Topology and Signal Peptide Prediction Method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Gouret, P.; Thompson, J.D.; Pontarotti, P. PhyloPattern: Regular Expressions to Identify Complex Patterns in Phylogenetic Trees. BMC Bioinform. 2009, 10, 298. [Google Scholar] [CrossRef]
- Lechner, M.; Findeiß, S.; Steiner, L.; Marz, M.; Stadler, P.F.; Prohaska, S.J. Proteinortho: Detection of (Co-)Orthologs in Large-Scale Analysis. BMC Bioinform. 2011, 12, 124. [Google Scholar] [CrossRef]
- Auch, A.F.; von Jan, M.; Klenk, H.-P.; Göker, M. Digital DNA-DNA Hybridization for Microbial Species Delineation by Means of Genome-to-Genome Sequence Comparison. Stand Genom. Sci. 2010, 2, 117–134. [Google Scholar] [CrossRef]
- Ramasamy, D.; Mishra, A.K.; Lagier, J.-C.; Padhmanabhan, R.; Rossi, M.; Sentausa, E.; Raoult, D.; Fournier, P.-E. A Polyphasic Strategy Incorporating Genomic Data for the Taxonomic Description of Novel Bacterial Species. Int. J. Syst. Evol. Microbiol. 2014, 64, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef]
- Integration of Evolutionary Biology Concepts for Functional Annotation and Automation of Complex Research in Evolution: The Multi-Agent Software System DAGOBAH|SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-3-642-20763-1_5 (accessed on 9 December 2020).
- Gouret, P.; Vitiello, V.; Balandraud, N.; Gilles, A.; Pontarotti, P.; Danchin, E.G. FIGENIX: Intelligent automation of genomic annotation: Expertise integration in a new software platform. BMC Bioinform. 2005, 6, 198. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An Improved Algorithm and Software for Calculating Average Nucleotide Identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acids | Name | Mean Relative % (a) |
|---|---|---|
| 16:0 | Hexadecanoic acid | 49.8 ± 0.5 |
| 14:0 | Tetradecanoic acid | 21.0 ± 0.8 |
| 18:1n9 | 9-Octadecenoic acid | 9.0 ± 0.3 |
| 18:2n6 | 9,12-Octadecadienoic acid | 8.4 ± 0.1 |
| 18:0 | Octadecanoic acid | 7.4 ± 0.1 |
| 12:0 | Dodecanoic acid | 1.6 ± 0.2 |
| 18:1n7 | 11-Octadecenoic acid | 1.5 ± 0.1 |
| 15:0 | Pentadecanoic acid | TR |
| 17:0 | Heptadecanoic acid | TR |
| 16:1n7 | 9-Hexadecenoic acid | TR |
| 15:0 iso | 13-methyl-tetradecanoic acid | TR |
| Attribute | Genome (Total) | |
|---|---|---|
| Value | % of Total | |
| Size | 2,876,881 | 100 |
| G + C content (%) | 1,306,499 | 45.41 |
| Coding region (bp) | 2,512,042 | 87.31 |
| Total genes | 2779 | 100 |
| RNA genes | 63 | 2.27 |
| Protein-coding genes | 2716 | 97.73 |
| Genes with function prediction | 2453 | 90.32 |
| Genes assigned to COGs | 1827 | 65.74 |
| Genes with peptide signals | 345 | 12.70 |
| Genes with transmembrane helices | 631 | 23.23 |
| CRISPR repeats | ||
| ORFans genes | 21 | 0.77 |
| Genes associated with PKS or NRPS | 4 | 0.15 |
| N° of antibiotic resistance genes | 2 | 0.074 |
| Name of Organisms | RefSeq | Size (Mb) | G + C (%) | Protein-Coding Genes | Total Genes (ORFs) |
|---|---|---|---|---|---|
| Anaerotruncus colihominis | NZ_DS544194 | 3.72 | 54.2 | 3525 | 3704 |
| Ruminococcus champanellensis | NC_021039 | 2.57 | 53.3 | 2276 | 2383 |
| Harryflintia acetispora | NZ_SLUK01000001 | 2.93 | 60.0 | 2704 | 2786 |
| Negativibacillus massiliensis | NZ_FTRU01000008 | 2.88 | 45.4 | 268 | 2781 |
| Hydrogenoanaerobcaterium saccharovorans | NZ_RKRD01000001 | 3.18 | 42.7 | 2906 | 2994 |
| Caprociproducens galactitovorans | NZ_SRMQ01000001 | 2.58 | 48.1 | 2413 | 2535 |
| Ruminococcus albus | NZ_FOAT01000022 | 3.85 | 45.2 | 3334 | 3484 |
| Clostridium methylpentosum | PRJNA30029 | 3.48 | 50.7 | 3907 | 3964 |
| Massiliimalia massiliensis | NZ_FUHT00000000.1 | 2.84 | 47.2 | 2681 | 2769 |
| Ruminococcus callidus | PRJNA18185 | 3.1 | 49.0 | 2719 | 2866 |
| Negativibacillus massiliensis | |||
|---|---|---|---|
| Code | Description | Value | % of Total |
| [A] | Rna processing and modification | 0 | 0.00 |
| [B] | Chromatin structure and dynamics | 0 | 0.00 |
| [C] | Energy production and conversion | 91 | 4.53 |
| [D] | Cell cycle control. mitosis and meiosis | 26 | 1.29 |
| [E] | Amino acid transport and metabolism | 156 | 7.77 |
| [F] | Nucleotide transport and metabolism | 58 | 2.89 |
| [G] | Carbohydrate transport and metabolism | 89 | 4.43 |
| [H] | Coenzyme transport and metabolism | 59 | 2.94 |
| [I] | Lipid transport and metabolism | 33 | 1.64 |
| [J] | Translation | 159 | 7.91 |
| [K] | Transcription | 172 | 8.56 |
| [L] | Replication. recombination and repair | 126 | 6.27 |
| [M] | Cell wall/membrane biogenesis | 94 | 4.68 |
| [N] | Cell motility | 2 | 0.10 |
| [O] | Posttanslational modification. protein turnover.chaperones | 51 | 2.54 |
| [P] | Inorganic ion transport and metabolism | 107 | 5.33 |
| [Q] | Secondary metabolites biosynthesis. transport and catabolism | 10 | 0.50 |
| [R] | General function prediction only | 232 | 11.55 |
| [T] | Signal transduction mechanisms | 49 | 2.44 |
| [U] | Intracellular trafficking and secretion | 24 | 1.19 |
| [V] | Defense mechanisms | 95 | 4.73 |
| [W] | Extracellular structures | 0 | 0.00 |
| [Y] | Nuclear structure | 0 | 0.00 |
| [Z] | Cytoskeleton | 0 | 0.00 |
| [S] | Function unknown | 194 | 9.66 |
| _ | Not in COGs | 182 | 9.06 |
| Acetanaerobacterium elongatum | Anaerotruncus colihominis | Caproiciproducens galactitolivorans | Clostridium methylpentosum | Ethanoligenens harbinense | Harryflintia acetispora | Hydrogenoanaerobacterium saccharovorans | Massilimaliae massiliensis | Negativibacillus massiliensis | Ruminococcus albus | Ruminococcus callidus | Ruminococcus champanellensis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetanaerobacterium elongatum | 20.60% | 23% | 17.50% | 20% | 18.70% | 19.40% | 37.50% | 24% | 26.60% | 29.40% | 33.80% | |
| Anaerotruncus colihominis | 35% | 21.10% | 19.60% | 22.60% | 27.20% | 33.90% | 32.30% | 28.80% | 26.80% | 35.90% | ||
| Caproiciproducens galactitolivorans | 25.10% | 23.60% | 30.20% | 27.80% | 32.90% | 28.60% | 26% | 31.30% | 27.70% | |||
| Clostridium methylpentosum | 18.20% | 20.30% | 21.10% | 22.90% | 27.80% | 25.80% | 24% | 28.10% | ||||
| Ethanoligenens harbinense | 18.80% | 25.50% | 31.40% | 27.90% | 27.90% | 29.70% | 30.40% | |||||
| Harryflintia acetispora | 24.20% | 24.50% | 35.60% | 29.30% | 23.40% | 20.2% | ||||||
| Hydrogenoanaerobacterium saccharovorans | 23.70% | 26.20% | 27.50% | 21.90% | 35.50% | |||||||
| Massilimaliae massiliensis | 25.20% | 34.90% | 20.20% | 29.70% | ||||||||
| Negativibacillus massiliensis | 24.30% | 25.50% | 24.40% | |||||||||
| Ruminococcus albus | 22.70% | 24.50% | ||||||||||
| Ruminococcus callidus | 20.90% | |||||||||||
| Ruminococcus champanellensis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valles, C.; Mailhe, M.; Ricaboni, D.; Armstrong, N.; Alibar, S.; Vitton, V.; Lagier, J.-C.; Raoult, D.; Tidjani Alou, M. Negativibacillus massiliensis gen. nov., sp. nov., a New Bacterial Genus Isolated from a Human Left Colon Sample. Microbiol. Res. 2021, 12, 29-42. https://doi.org/10.3390/microbiolres12010004
Valles C, Mailhe M, Ricaboni D, Armstrong N, Alibar S, Vitton V, Lagier J-C, Raoult D, Tidjani Alou M. Negativibacillus massiliensis gen. nov., sp. nov., a New Bacterial Genus Isolated from a Human Left Colon Sample. Microbiology Research. 2021; 12(1):29-42. https://doi.org/10.3390/microbiolres12010004
Chicago/Turabian StyleValles, Camille, Morgane Mailhe, Davide Ricaboni, Nicholas Armstrong, Stéphane Alibar, Véronique Vitton, Jean-Christophe Lagier, Didier Raoult, and Maryam Tidjani Alou. 2021. "Negativibacillus massiliensis gen. nov., sp. nov., a New Bacterial Genus Isolated from a Human Left Colon Sample" Microbiology Research 12, no. 1: 29-42. https://doi.org/10.3390/microbiolres12010004
APA StyleValles, C., Mailhe, M., Ricaboni, D., Armstrong, N., Alibar, S., Vitton, V., Lagier, J.-C., Raoult, D., & Tidjani Alou, M. (2021). Negativibacillus massiliensis gen. nov., sp. nov., a New Bacterial Genus Isolated from a Human Left Colon Sample. Microbiology Research, 12(1), 29-42. https://doi.org/10.3390/microbiolres12010004







