Long COVID-19: A Concept Analysis
Abstract
1. Introduction
Purpose of the Analysis
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Analysis
3. Results
3.1. Dictionary Definitions of Long-COVID-19
3.2. Defining Attributes
3.3. Model, Borderline and Contrary Cases
3.3.1. Model Case
3.3.2. Borderline Case
3.3.3. Contrary Case
3.4. Antecedents
3.5. Consequences
3.6. Empirical Referents
3.7. Definition of the Concept
4. Impact of the Findings
5. Conclusions/Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Res. 2020, 4, 536–544. [Google Scholar] [CrossRef]
- World Health Organization. WHO COVID-19 Dashboard. 2023. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 1 October 2023).
- Hindsley, G. The Lost Month: How a Failure to Test Blinded the U.S. to Covid-19 By Grant Hindsley for The New York Time. The New York Times, 28 March 2020. Available online: https://www.nytimes.com/2020/03/28/us/testing-coronavirus-pandemic.html#selection-321.0-321.66 (accessed on 8 July 2025).
- International Monetary Fund. International Monetary Fund Policy Responses to COVID19; International Monetary Fund. Available online: https://www.imf.org/en/Topics/imf-and-covid19/Policy-Responses-to-COVID-19 (accessed on 1 October 2023).
- Haleem, A.; Javaid, M.; Vaishya, R. Effects of COVID-19 pandemic in daily life. Curr. Med. Res. Pract. 2020, 10, 78–79. [Google Scholar] [CrossRef]
- Larsen, J.R.; Martin, M.R.; Martin, J.D.; Kuhn, P.; Hicks, J.B. Modeling the Onset of Symptoms of COVID-19. Front. Public Health 2020, 8, 551338. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, F.; Pizzol, D.; Marotta, C.; Antunes, M.; Racalbuto, V.; Veronese, N.; Smith, L. Coronavirus diseases (COVID-19) current status and future perspectives: A narrative review. Int. J. Environ. Res. Public Health 2020, 17, 2690. [Google Scholar] [CrossRef]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Tian, S.; Chang, Z.; Wang, Y.; Wu, M.; Zhang, W.; Zhou, G.; Zou, X.; Tian, H.; Xiao, T.; Xing, J.; et al. Clinical Characteristics and Reasons for Differences in Duration From Symptom Onset to Release From Quarantine Among Patients with COVID-19 in Liaocheng, China. Front. Med. 2020, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Carfi, B.R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Res. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Res. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Jackson, J.L.; George, S.; Hinchey, S. Medically unexplained physical symptoms. Intern. Med. 2009, 24, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-acute sequelae of COVID-19: A metabolic perspective. Elife 2022, 11, e78200. [Google Scholar] [CrossRef] [PubMed]
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining post-covid symptoms (Post-acute covid, long covid, persistent post-covid): An integrative classification. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef]
- Daitch, V.; Yelin, D.; Awwad, M.; Guaraldi, G.; Milić, J.; Mussini, C.; Falcone, M.; Tiseo, G.; Carrozzi, L.; Pistelli, F.; et al. Characteristics of long-COVID among older adults: A cross-sectional study. Int. J. Infect. Dis. 2022, 125, 287–293. [Google Scholar] [CrossRef]
- Cegolon, L.; Mauro, M.; Sansone, D.; Tassinari, A.; Gobba, F.M.; Modenese, A.; Casolari, L.; Liviero, F.; Pavanello, S.; Scapellato, M.L.; et al. A Multi-Center Study Investigating Long COVID-19 in Healthcare Workers from North-Eastern Italy: Prevalence, Risk Factors and the Impact of Pre-Existing Humoral Immunity—ORCHESTRA Project. Vaccines 2023, 11, 1769. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J. Long COVID: A clinical update. Lancet 2024, 404, 10453. [Google Scholar] [CrossRef]
- Nishimi, K.; Neylan, T.C.; Bertenthal, D.; Seal, K.H.; O’Donovan, A. Association of psychiatric disorders with clinical diagnosis of long COVID in US veterans. Psychol. Med. 2024, 54, 2024–2032. [Google Scholar] [CrossRef]
- Mateu, L.; Tebe, C.; Loste, C.; Santos, J.R.; Lladós, G.; López, C.; España-Cueto, S.; Toledo, R.; Font, M.; Chamorro, A.; et al. Determinants of the onset and prognosis of the Post-COVID-19 Condition:A 2-year prospective observational cohort study. Lancet Reg. Health–Europe. 2024, 54, 2024–2032. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Sabir, S.; Jan, A. Physiology, Immune Response; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539801/ (accessed on 10 July 2025).
- Su, S.; Zhao, Y.; Zeng, N.; Liu, X.; Zheng, Y.; Sun, J.; Zhong, Y.; Wu, S.; Ni, S.; Gong, Y.; et al. Epidemiology, clinical presentation, pathophysiology, and management of long COVID: An update. Mol. Psychiatry 2023, 28, 4056–4069. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Torres, J.F.; Romero-Ibarguengoitia, M.E.; Garza-Silva, A.; Rivera-Cavazos, A.; Hurtado-Cabrera, M.; Kalife-Assad, R.; Villarreal-Parra, D.L.; Loose-Esparza, A.; Gutierrez-Arias, J.J.; Mata-Porras, Y.G.; et al. Association between Mexican vaccination schemes and the duration of long COVID syndrome symptoms. Sci Rep. 2025, 15, 5301. [Google Scholar] [CrossRef]
- Kuodi, P.; Gorelik, Y.; Zayyad, H.; Wertheim, O.; Wiegler, K.B.; Abu Jabal, K.; Dror, A.A.; Nazzal, S.; Glikman, D.; Edelstein, M. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: Cross-sectional study 2020-21, Israel. npj Vaccines 2022, 7, 101. [Google Scholar] [CrossRef]
- Öztürk, F.; Emiroğlu, C.; Aypak, C. The Relationship Between Long Covid Symptoms and Vaccination Status in COVID-19 Survivors. J. Eval. Clin. Pract. 2025, 31, e70004. [Google Scholar] [CrossRef]
- Centers for Disease Control. Long COVID Basics. Available online: https://www.cdc.gov/long-covid/about/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcovid%2Flong-term-effects%2Findex.html (accessed on 22 July 2025).
- Bartsch, S.M.; Chin, K.L.; Strych, U.; John, D.C.; Shah, T.D.; Bottazzi, M.E.; O’Shea, K.J.; Robertson, M.; Weatherwax, C.; Heneghan, J.; et al. The Current and Future Burden of Long COVID in the United States. J. Infect. Dis. 2025, 231, 1581–1590. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deeks, S.G. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. 2022, 43, 268–270. [Google Scholar] [CrossRef]
- Coombs, J.R.; Daniels, L.B. Philosophical Inquiry: Conceptual Analysis. In Forms of Curriculum Inquiry; Short, E.C., Ed.; SUNY Press: Albany, NY, USA, 1991; pp. 27–41. [Google Scholar]
- Srikanth, S.; Boulos, J.R.; Dover, T.; Boccuto, L.; Dean, D. Identification and diagnosis of long COVID-19: A scoping review. Prog. Biophys. Mol. Biol. 2023, 182, 1–7. [Google Scholar] [CrossRef]
- Aiyegbusi, O.L.; Hughes, S.E.; Turner, G.; Rivera, S.C.; McMullan, C.; Chandan, J.S.; Haroon, S.; Price, G.; Davies, E.H.; Nirantharakumar, K.; et al. Symptoms, complications and management of long COVID: A review. J. R. Soc. Med. 2021, 114, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Berger, Z.; Altiery De Jesus, V.; Assoumou, S.A.; Greenhalgh, T. Long COVID and Health Inequities: The Role of Primary Care. Milbank Q. 2021, 99, 519–541. [Google Scholar] [CrossRef]
- Walker, L.; Avant, K. Strategies for Theory Construction in Nursing, 5th ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Oxford English Dictionary. “Long”. Available online: https://www.oed.com/search/dictionary/?scope=Entries&q=long (accessed on 25 November 2024).
- Oxford English Dictionary. “COVID-19”. Available online: https://www.oed.com/search/dictionary/?scope=Entries&q=Covid-19 (accessed on 25 November 2024).
- Datta, S.D.; Talwar, A.; Lee, J.T. A Proposed Framework and Timeline of the Spectrum of Disease Due to SARS-CoV-2 Infection: Illness beyond Acute Infection and Public Health Implications. JAMA 2020, 324, 2251–2252. [Google Scholar] [CrossRef]
- Caliman-Sturdza, O.A.; Gheorghita, R.; Lobiuc, A. Neuropsychiatric Manifestations of Long COVID-19: A Narrative Review of Clinical Aspects and Therapeutic Approaches. Life 2025, 15, 439. [Google Scholar] [CrossRef]
- Huang, L.W.; Li, H.M.; He, B.; Wang, X.B.; Zhang, Q.Z.; Peng, W.X. Prevalence of cardiovascular symptoms in post-acute COVID-19 syndrome: A meta-analysis. BMC Med. 2025, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Patton, M.J.; Benson, D.; Robison, S.W.; Raval, D.; Locy, M.L.; Patel, K.; Grumley, S.; Levitan, E.B.; Morris, P.; Might, M.; et al. Characteristics and determinants of pulmonary long COVID. J. Clin. Investig. 2024, 9, e177518. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Montes-Ibarra, M.; Oliveira, C.L.P.; Orsso, C.E.; Landi, F.; Marzetti, E.; Prado, C.M. The Impact of Long COVID-19 on Muscle Health. Clin. Geriatr. Med. 2022, 38, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Swank, Z.; Borberg, E.; Chen, Y.; Senussi, Y.; Chalise, S.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Henrich, T.J.; et al. Measurement of circulating viral antigens post-SARS-CoV-2 infection in a multicohort study. Clin. Microbiol. Infect. 2024, 30, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Bayarri-Olmos, R.; Brinda, R.; Alec Rath Constable, R.; Colom Diaz, P.A.; Kwon, D.; Rodrigues, G.; Wenxue, L.; Baker, C.; Bhattacharjee, B.; et al. A causal link between autoantibodies and neurological symptoms in long COVID. medRxiv 2024. [Google Scholar] [CrossRef]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood–brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E.; et al. Mitochondrial dysfunction in long COVID: Mechanisms, consequences, and potential therapeutic approaches. GeroScience 2024, 46, 5267–5286. [Google Scholar] [CrossRef]
- Ancona, G.; Alagna, L.; Alteri, C.; Palomba, E.; Tonizzo, A.; Pastena, A.; Muscatello, A.; Gori, A.; Bandera, A. Gut and airway microbiota dysbiosis and their role in COVID-19 and long-COVID. Front. Immunol. 2023, 14, 1080043. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, V.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef]
- Krishna, B.A.; Lim, E.Y.; Metaxaki, M.; Jackson, S.; Mactavous, L.; NIHR BioResource; Lyons, P.A.; Doffinger, R.; Bradley, J.R.; Smith, K.G.C.; et al. Spontaneous, persistent, T cell-dependent IFN-γ release in patients who progress to Long Covid. Sci. Adv. 2024, 10, eadi9379. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Berends, M.S.; Homburg, M.; Kupers, T.; Meijer, E.N.; Bos, I.; Verheij, R.; Kuiper, J.; Berger, M.Y.; Peters, L.L. Impact of pre-existing comorbidities and multimorbidities, demography and viral variants on post-acute sequelae of COVID-19 (‘Long COVID’) in Dutch primary care: A retrospective cohort study. Int. J. Infect. Dis. 2025, 156, 107912. [Google Scholar] [CrossRef]
- Luo, Y.S.; Zhang, K.; Cheng, Z.S. Absence of Association between a Long COVID and Severe COVID-19 Risk Variant of FOXP4 and Lung Cancer. Front. Genet. 2023, 14, 1258829. [Google Scholar] [CrossRef] [PubMed]
- Lammi, V.; Nakanishi, T.; Jones, S.E.; Andrews, S.J.; Karjalainen, J.; Cortés, B.; O’Brien, H.E.; Fulton-Howard, B.E.; Haapaniemi, H.H.; Schmidt, A.; et al. Genome-wide Association Study of Long COVID. Nat. Genet 2025, 1–16. [Google Scholar] [CrossRef]
- Berentschot, J.C.; Drexhage, H.A.; Aynekulu Mersha, D.G.; Wijkhuijs, A.J.M.; GeurtsvanKessel, C.H.; Koopmans, M.P.G.; Voermans, J.; Heijenbrok-Kal, M.H.; Bek, L.M.; Ribbers, G.M.; et al. Severe fatigue as symptom of long COVID is characterized by increased expression of inflammatory genes in monocytes, increased serum pro-inflammatory cytokines, and increased CD+ T-lymphocytes: T-lymphocytes: A putative dysregulation of the immune-brain axis, the coagulation process, and auto-inflammation to explain the diversity of long COVID symptoms. medRxiv 2022. [Google Scholar] [CrossRef]
- Constantinescu-Bercu, A.; Lobiuc, A.; Căliman-Sturdza, O.A.; Oiţă, R.C.; Iavorschi, M.; Pavăl, N.E.; Șoldănescu, I.; Dimian, M.; Covasa, M. Long COVID: Molecular Mechanisms and Detection Techniques. Int. J. Mol. Sci. 2024, 25, 408. [Google Scholar] [CrossRef]
- Qi, P.; Huang, M.; Zhu, H. Exploring potential biomarkers and therapeutic targets of long COVID-associated inflammatory cardiomyopathy. Front. Med. 2023, 10, 1191354. [Google Scholar] [CrossRef]
- da Silva, R.; de Sarges, K.M.L.; Cantanhede, M.H.D.; da Costa, F.P.; dos Santos, E.F.; Rodrigues, F.B.B.; de Nazaré do Socorro de Almeida Viana, M.; de Meira Leite, M.; da Silva, A.L.S.; de Brito, M.T.M.; et al. Thrombophilia and Immune-Related Genetic Markers in Long COVID. Viruses 2023, 15, 885. [Google Scholar] [CrossRef]
- Gutman, E.G.; Salvio, A.L.; Fernandes, R.A.; Duarte, L.A.; Raposo-Vedovi, J.V.; Alcaraz, H.F.; Teixeira, M.A.; Passos, G.F.; de Medeiros, K.Q.M.; Hammerle, M.B.; et al. Long COVID: Plasma levels of neurofilament light chain in mild COVID-19 patients with neurocognitive symptoms. Mol. Psychiatry 2024, 29, 3106–3116. [Google Scholar] [CrossRef]
- Ercegovac, M.; Asanin, M.; Savic-Radojevic, A.; Ranin, J.; Matic, M.; Djukic, T.; Coric, V.; Jerotic, D.; Todorovic, N.; Milosevic, I.; et al. Antioxidant Genetic Profile Modifies Probability of Developing Neurological Sequelae in Long-COVID. Antioxidants 2022, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; Baldassarri, M.; d’Alessandro, M.; Brunelli, G.; Fabbri, G.; Zguro, K.; Degl’Innocenti, A.; Mari, F.; Daga, S.; Meloni, I.; et al. Ultra-rare RTEL1 gene variants associate with acute severity of COVID-19 and evolution to pulmonary fibrosis as a specific long COVID disorder. Respir. Res. 2023, 24, 158. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hadrawi, D.S.; Maes, M. Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection. J. Clin. Med. 2023, 12, 511. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 408. [Google Scholar] [CrossRef]
- Aghaei, A.; Zhang, R.; Taylor, S.; Tam, C.-C.; Yang, C.-H.; Li, X.; Qiao, S. Impact of COVID-19 symptoms on social aspects of life among female long haulers: A. qualitative study. Res. Sq. [Preprint] 2022, 2, 511. [Google Scholar] [CrossRef]
- Padilla, S.; Ledesma, C.; García-Abellán, J.; García, J.A.; Fernández-González, M.; de la Rica, A.; Galiana, A.; Gutiérrez, F.; Masiá, M. Long COVID across SARS-CoV-2 variants, lineages, and sublineages. iScience 2024, 27, 109536. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Ren, S.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Guo, Y.; Lipsitch, M.; Daugherty, S.E. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2022, 376, e068414. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Khunti, K.; Alwan, N.; Steves, C.; Greenhalgh, T.; Macdermott, N.; Sagan, A.; Mckee, M. In the Wake of the Pandemic Preparing for Long Covid Health Systems and Policy Analysis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK569598/?report=printable (accessed on 27 June 2025).
- Di Maio, M.; Basch, E.; Denis, F.; Fallowfield, L.J.; Ganz, P.A.; Howell, D.; Kowalski, C.; Perrone, F.; Stover, A.M.; Sundaresan, P.; et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann. Oncol. 2022, 33, 878–892. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Yan, D.; Liu, Y.; Chen, R.; Zhou, L.; Wang, C.; Ma, A.H.Y.; Chen, X.; Song, Q.; Qian, G. Follow-up of long COVID based on the definition of WHO: A multi-centre cross-sectional questionnaire-based study. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Carlile, O.; Briggs, A.; Henderson, A.D.; Butler-Cole, B.F.C.; Tazare, J.; Tomlinson, L.A.; Marks, M.; Jit, M.; Lin, L.-Y.; Bates, C.; et al. Impact of long COVID on health-related quality-of-life: An OpenSAFELY population cohort study using patient-reported outcome measures (OpenPROMPT). Available online: https://opensafely.org/ (accessed on 11 June 2024).
- Saltzman, L.Y.; Longo, M.; Hansel, T.C. Long-COVID stress symptoms: Mental health, anxiety, depression, or posttraumatic stress. Psychol. Trauma Theory Res. Prac. Policy 2023, 16, 1169–1178. [Google Scholar] [CrossRef]
- Taribagil, P.; Creer, D.; Tahir, H. Long COVID syndrome. BMJ Case Rep. 2021, 14, e241485. [Google Scholar] [CrossRef]
- Straub, R.K.; Powers, C.M. Chronic fatigue syndrome: A case report highlighting diagnosing and treatment challenges and the possibility of Jarisch–Herxheimer reactions if high infectious loads are present. Healthcare 2021, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Yu, X.; Zhang, Y.; Chen, X. A Case of Adult Epstein-Barr Virus-Associated Pneumonia with Multiple Cavitary Pulmonary Lesions Confirmed by Lung Biopsy mNGS: A Case Report. Infect. Drug Resist. 2023, 16, 7507–7513. [Google Scholar] [CrossRef]
- Justiz Vaillant, A.A.; Goyal, A.; Varacallo, M.A. Systemic Lupus Erythematosus [Updated 2023 Aug 4]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535405/ (accessed on 14 July 2025).
- Chauhan, K.; Jandu, J.S.; Brent, L.H.; Al-Dhahir, M.A. “Rheumatoid Arthritis” [Updated 2023 May 25]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441999/ (accessed on 14 July 2025).
- Carruthers, B.M.; Van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Hatam, E.; Cameron, A.; Petsikas, D.; Messenger, D.; Ball, I. A Case of Severe Accidental Hypothermia Successfully Treated with Cardiopulmonary Bypass. Lin. Prac. Cases Emerg. Med. 2017, 1, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Collins, L.F.; Malani, P. Long-term Health Consequences of COVID-19. JAMA 2020, 324, 1723–1724. [Google Scholar] [CrossRef] [PubMed]
- Kompaniyets, L.; Bull-Otterson, L.; Boehmer, T.K.; Baca, S.; Alvarez, P.; Hong, K.; Hsu, J.; Harris, A.M.; Gundlapalli, A.V.; Saydah, S. Post–COVID-19 Symptoms and Conditions Among Children and Adolescents—United States, 1 March 2020–31 January 2022. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9368731/ (accessed on 5 June 2024).
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Engelmann, I.; Moreau, A.S.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein–Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now 2021, 51, 296–299. [Google Scholar] [CrossRef]
- Sansone, D.; Tassinari, A.; Valentinotti, R.; Kontogiannis, D.; Ronchese, F.; Centonze, S.; Maggiore, A.; Cegolon, L.; Filon, F.L. Persistence of Symptoms 15 Months since COVID-19 Diagnosis: Prevalence, Risk Factors and Residual Work Ability. Life 2022, 13, 97. [Google Scholar] [CrossRef]
- Voruz, P.; Assal, F.; Péron, J.A. The economic burden of the post-COVID-19 condition: Underestimated long-term consequences of neuropsychological deficits. Glob. Health 2023, 13, 03019. [Google Scholar] [CrossRef]
- Wang, S.; Quan, L.; Chavarro, J.E.; Slopen, N.; Kubzansky, L.D.; Koenen, K.C.; Kang, J.H.; Weisskopf, M.G.; Branch-Elliman, W.; Roberts, A.L. Associations of Depression, Anxiety, Worry, Perceived Stress, and Loneliness Prior to Infection With Risk of Post-COVID-19 Conditions. JAMA Psychiatry 2022, 79, 1081–1091. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Ahrnsbrak, R.; Rekito, A. Evidence Mounts That About 7% of US Adults Have Had Long COVID. JAMA 2024, 331, 5–6. [Google Scholar] [CrossRef]
- Cutler, D.M. The Costs of Long COVID. JAMA Health Forum 2022, 3, e221809. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The Impact of Long COVID-19 on Mental Health: Observational 6-Month Follow-Up Study. JMIR Ment. Health 2022, 9, e33704. [Google Scholar] [CrossRef]
- Living with Long COVID Key Points. Available online: https://www.cdc.gov/long-covid/living-with/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcovid%2Flong-term-effects%2Fliving-with-long-covid.html (accessed on 23 October 2024).
- Venkatesh, A.K.; Yu, H.; Malicki, C.; Gottlieb, M.; Elmore, J.G.; Hill, M.J.; Idris, A.H.; Montoy, J.C.C.; Laughlin, K.N.O.; Rising, K.L.; et al. The association between prolonged SARS-CoV-2 symptoms and work outcomes. PLoS ONE 2024, 19, e0300947. [Google Scholar] [CrossRef]
- Mayo Clinic Staff, Long COVID: Lasting Effects of COVID-19. Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-long-term-effects/art-20490351 (accessed on 10 July 2025).
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef]
- Ndugga, N.; Hill, L.; Artiga, S. COVID-19 Cases and Deaths, Vaccinations, and Treatments by Race Ethnicity as of Fall 2022. KFF Health News Nov 17, 2022. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-cases-and-deaths-vaccinations-and-treatments-by-race-ethnicity-as-of-fall-2022/ (accessed on 11 June 2025).
- Tanne, J.H. COVID-19: US studies show racial and ethnic disparities in long covid. BMJ 2023, 380, 535. [Google Scholar] [CrossRef] [PubMed]
- US-HHS, ADA, Section 504, and Section 1557: Long COVID as a Disability. Available online: https://archive.ada.gov/long_covid_joint_guidance.pdf (accessed on 9 July 2024).
- Jennifer, C.F. Clues to Long COVID SCIENCE. Available online: https://www.science.org/content/article/what-causes-long-covid-three-leading-theories (accessed on 21 September 2024).
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- NICE 2020. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. (NG188) Evidence Review 5: Interventions, 2020. 18 December 2020. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 21 September 2024).
- Merckelbach, H.; Dandachi-FitzGerald, B.; van Helvoort, D.; Jelicic, M.; Otgaar, H. When Patients Overreport Symptoms: More Than Just Malingering. Urr. Dir. Psychol. Sci. 2019, 28, 321–326. [Google Scholar] [CrossRef]
- Prusinski, C. Multidisciplinary Management Strategies for Long COVID: A Narrative Review. Cureus 2024, 16, e59478. [Google Scholar] [CrossRef]
- Goldenberg, D.L. How to understand the overlap of long COVID, chronic fatigue syndrome/myalgic encephalomyelitis, fibromyalgia and irritable bowel syndromes. Semin. Arthritis Rheum. 2024, 67, 152455. [Google Scholar] [CrossRef]
- Vivaldi, G.; Pfeffer, P.E.; Talaei, M.; Basera, T.J.; Shaheen, S.O.; Martineau, A.R. Long-term symptom profiles after COVID-19 vs other acute respiratory infections: An analysis of data from the COVIDENCE UK study. eClinicalMedicine 2023, 65, 102251. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-A systemic review and comparison of clinical presentation and symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study. Ann Intern Med. 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Gurvich, C.; Huang, K.; Gooley, P.R.; Armstrong, C.W. The underlying sex differences in neuroendocrine adaptations relevant to Myalgic Encephalomyelitis Chronic Fatigue Syndrome. Front. Neuroendocr. 2022, 66, 100995. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Lipkin, W.I. ME/CFS and Long COVID share similar symptoms and biological abnormalities: Road map to the literature. Front. Med. 2023, 10, 1187163. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, D.; Ramasawmy, M.; Pantelic, M.; Hayer, J.; Begum, F.; Faghy, M.; Nasir, N.; Causer, B.; Heightman, M.; Allsopp, G.; et al. Barriers to healthcare access and experiences of stigma: Findings from a coproduced Long Covid case-finding study. Health Expect. 2024, 27, e14037. [Google Scholar] [CrossRef] [PubMed]
- McNabb, K.C.; Bergman, A.J.; Smith-Wright, R.; Seltzer, J.; Slone, S.E.; Tomiwa, T.; Alharthi, A.; Davidson, P.M.; Commodore-Mensah, Y.; Ogungbe, O. It was almost like it’s set up for people to fail’ A qualitative analysis of experiences and unmet supportive needs of people with Long COVID. BMC Public Health 2023, 23, 2131. [Google Scholar] [CrossRef]
- Missailidis, D.; Ebrahimie, E.; Dehcheshmeh, M.M.; Allan, C.; Sanislav, O.; Fisher, P.; Gras, S.; Annesley, S.J. A blood-based mRNA signature distinguishes people with Long COVID from recovered individuals. Front. Immunol. 2024, 15, 1450853. [Google Scholar] [CrossRef]
- Babicki, M.; Lejawa, M.; Osadnik, T.; Kapusta, J.; Banach, M.; Jankowski, P.; Mastalerz-Migas, A.; Kałuzińska-Kołat, Ż.; Kołat, D.; Chudzik, M.; et al. LC risk score—Development and evaluation of a scale for assessing the risk of developing long COVID. Arch. Med. Sci. 2024, 21, 121–130. [Google Scholar] [CrossRef]
- Ye, G.; Zhu, Y.; Bao, W.; Zhou, H.; Lai, J.; Zhang, Y.; Xie, J.; Ma, Q.; Luo, Z.; Ma, S.; et al. The Long COVID Symptoms and Severity Score: Development, Validation, and Application. Value Health 2024, 27, 1085–1091. [Google Scholar] [CrossRef]
- Nair, P.; Nair, C.V.; Kulirankal, K.G.; Corley, E.M.; Edathadathil, F.; Gutjahr, G.; Moni, M.; Sathyapalan, D.T. Characterization and predictive risk scoring of long COVID in a south indian cohort after breakthrough COVID infection; a prospective single centre study. BMC Infect. Dis. 2023, 23, 670. [Google Scholar] [CrossRef] [PubMed]
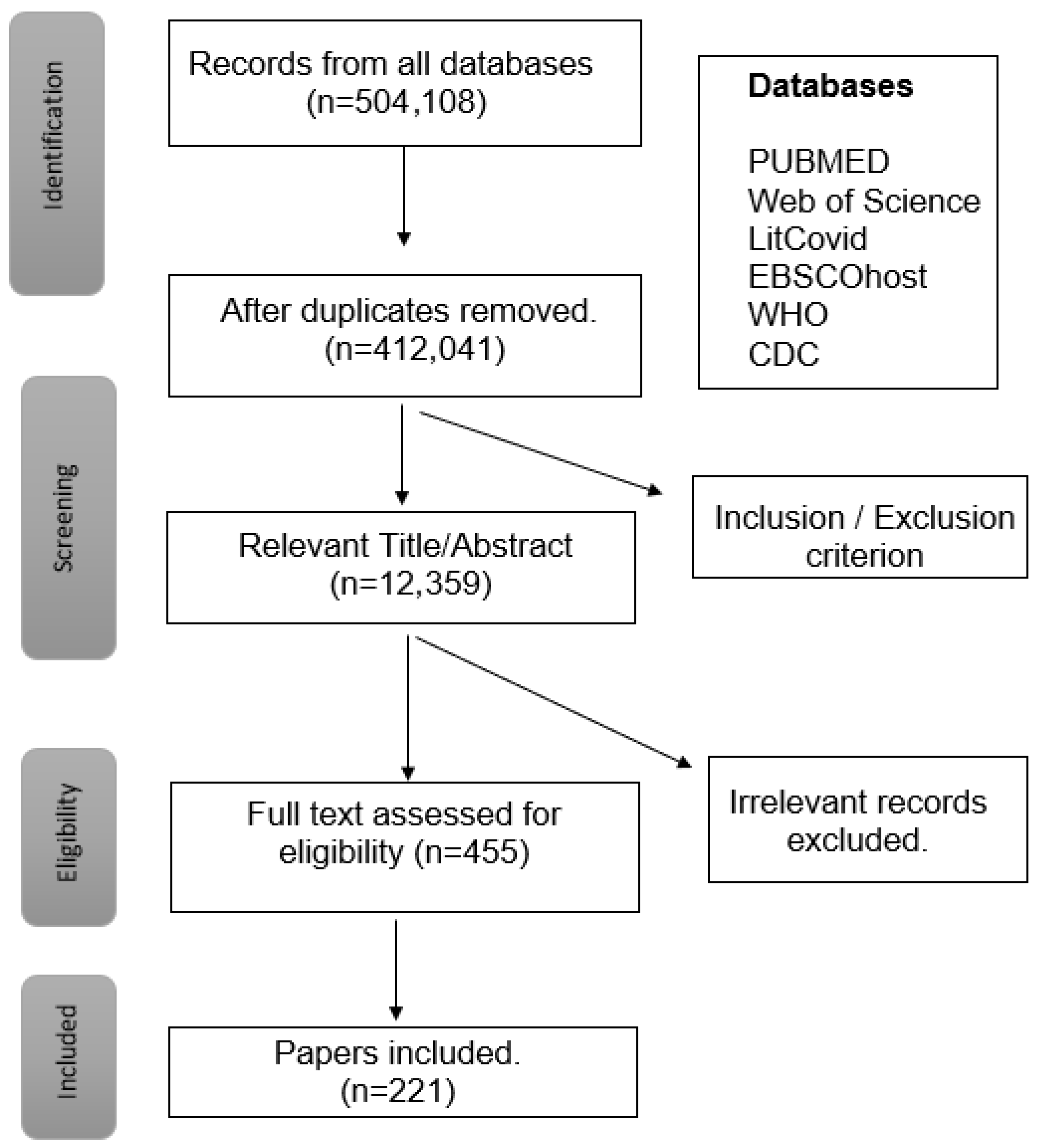
| Symptoms of LC-19 | |
|---|---|
| NEUROLOGICAL [42] | Cognitive Impairment, Neuropsychiatric Symptoms, Depression, Anxiety |
| CARDIOVASCULAR [43] | Cardiomyopathy, Chest Pain, Thrombophilia, Tachycardia, Palpitations |
| RESPIRATORY [44] | Pulmonary Dysfunction, Lung Fibrosis, Cough, Shortness of Breath |
| IMMUNE RESPONSE [45] | Inflammation, Impaired Tissue Repair, Fever |
| MUSCULAR [46] | Fatigue, Myalgia |
| Genes Associated With Symptoms in LC-19 | ||
|---|---|---|
| GENE | OMIM ID | SYMPTOM |
| FOXP4 [56,57] | 608924 | Pulmonary Dysfunction |
| TLR4 [58] | 603030 | Cognitive Impairment |
| IL6 [59] | 147620 | Fatigue, Inflammation, Neuropsychiatric Symptoms |
| TNF-α [60] | 191160 | Fatigue, Inflammation |
| VEGF-A [59] | 192240 | Cardiomyopathy, Pulmonary Dysfunction |
| FOXO1 [60] | 136533 | Cardiomyopathy |
| CXCR4 [60] | 162643 | Cardiomyopathy |
| SMAD4 [60] | 600993 | Cardiomyopathy |
| INF-γ [61,62] | 147569 | Thrombophilia, Inflammation, Impaired Tissue Repair |
| ACE2 [59] | 300335 | Impaired Tissue Repair |
| TMPRSS2 [59] | 602060 | Immune Response |
| IL1 [59] | 147720 | Inflammation, Depression |
| CCL11 [59] | 601156 | Cognitive Impairment |
| NFL [59,62] | 162280 | Cognitive Impairment, Fatigue |
| GFAP [59,62] | 137780 | Cognitive Impairment |
| GSTP1AB [63] | 134660 | Cognitive Impairment, Myalgia |
| GSTO1 [63] | 605482 | Cognitive Impairment, Myalgia |
| RTEL1 [64] | 608833 | Disease severity, Higher liver function indices, Lung Fibrosis |
| NLPR3 [65] | 606416 | Major Depressive Disorder, Fatigue, Cognitive Impairment, Anxiety |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikanth, S.; Boulos, J.R.; Ivankovic, D.; Gonzales, L.; Dean, D.; Boccuto, L. Long COVID-19: A Concept Analysis. Infect. Dis. Rep. 2025, 17, 90. https://doi.org/10.3390/idr17040090
Srikanth S, Boulos JR, Ivankovic D, Gonzales L, Dean D, Boccuto L. Long COVID-19: A Concept Analysis. Infectious Disease Reports. 2025; 17(4):90. https://doi.org/10.3390/idr17040090
Chicago/Turabian StyleSrikanth, Sujata, Jessica R. Boulos, Diana Ivankovic, Lucia Gonzales, Delphine Dean, and Luigi Boccuto. 2025. "Long COVID-19: A Concept Analysis" Infectious Disease Reports 17, no. 4: 90. https://doi.org/10.3390/idr17040090
APA StyleSrikanth, S., Boulos, J. R., Ivankovic, D., Gonzales, L., Dean, D., & Boccuto, L. (2025). Long COVID-19: A Concept Analysis. Infectious Disease Reports, 17(4), 90. https://doi.org/10.3390/idr17040090







