The Role of Nutritional Environment in Cryptococcus gattii Titan Cells’ Ultrastructure, Biophysical Properties, Molecular Features, and Virulence in Cryptococcosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Information
2.2. Growth Conditions
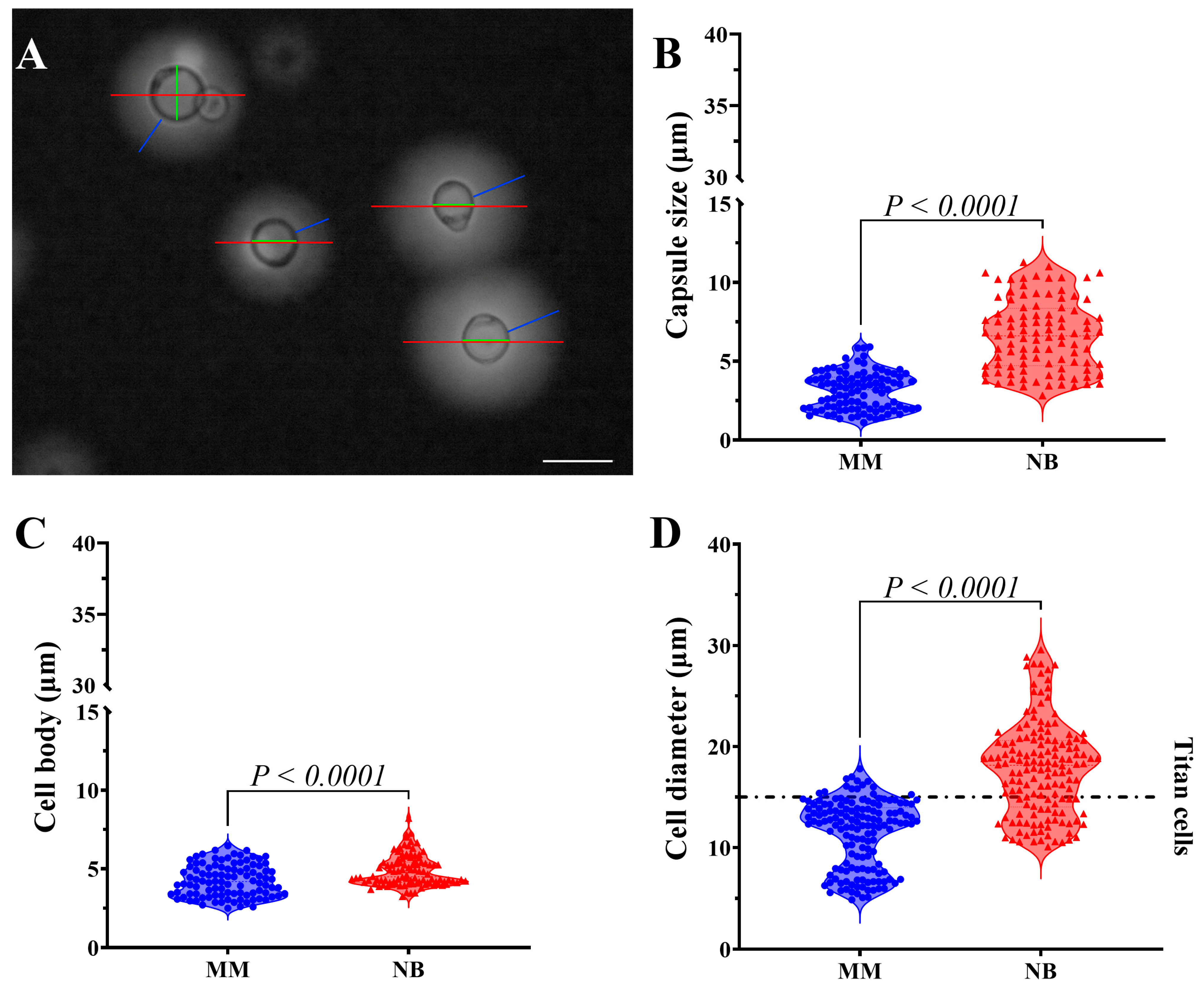
2.3. Morphological Evaluation
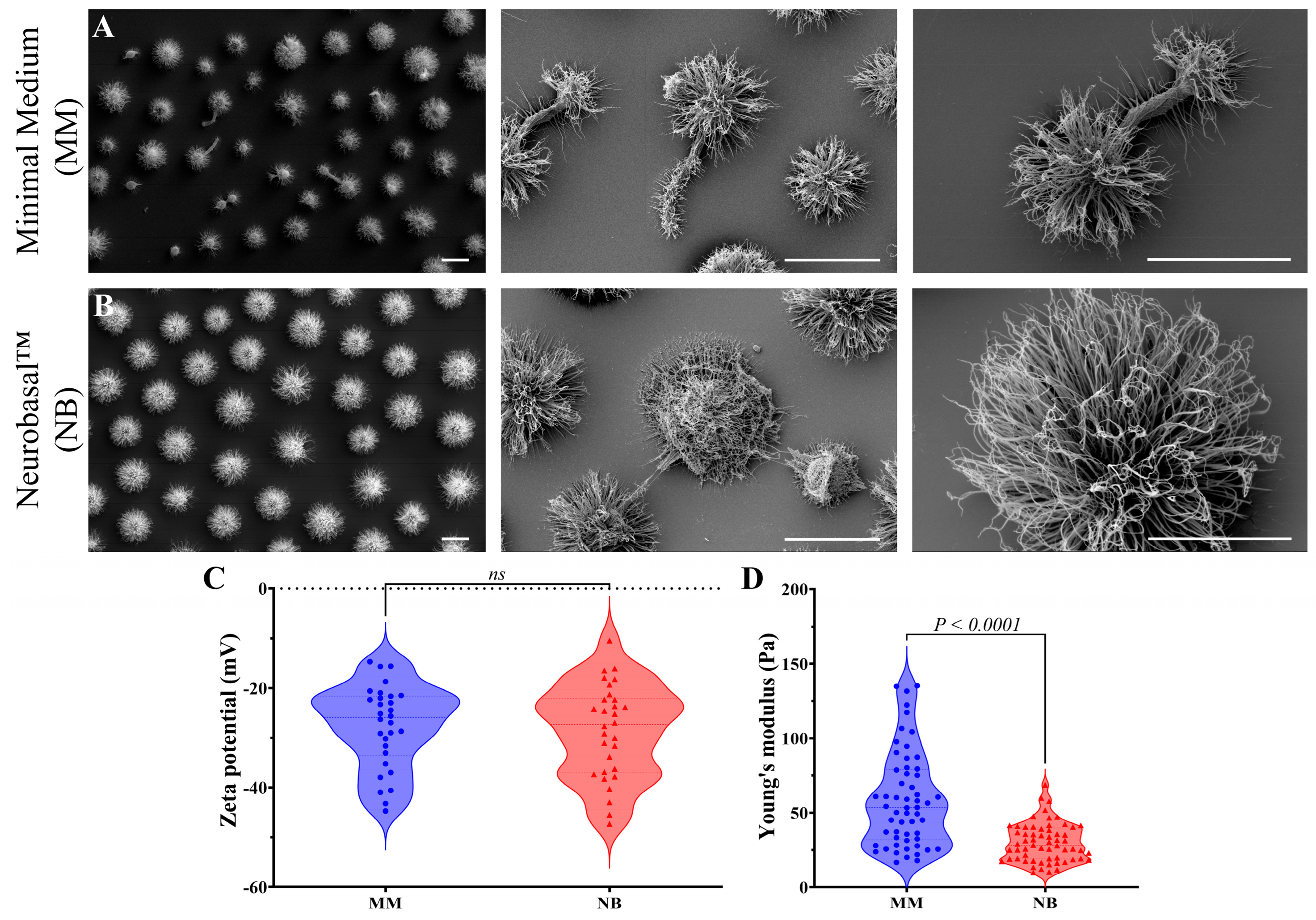
2.4. Scanning Electron Microscopy (SEM)
2.5. Capsular Antigens and Chitin Quantification
2.6. Young’s Modulus Measurements with Optical Tweezers
2.7. Extraction and Concentration of Secreted Polysaccharides
2.8. Dynamic Light Scattering (DLS), Zeta Potential (ζ), and Conductance Measurements
2.9. Passive Micro-Rheology
2.10. Antifungal Activity Assessment
2.11. Mice Survival
2.12. Data Analyses
3. Results
3.1. Nutrition Influences C. gattii Morphology, Capsule Formation, and Titan Cell Development
3.2. Rheological and Structural Alterations in C. gattii Secreted Polysaccharides
3.3. Culture Conditions Affect C. gattii Titan Cell Formation and Antifungal Susceptibility
3.4. Culture Environments Influence C. gattii Titan Cell Formation and Virulence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beardsley, J.; Dao, A.; Keighley, C.; Garnham, K.; Halliday, C.; Chen, S.C.-A.; Sorrell, T.C. What’s new in Cryptococcus gattii: From bench to bedside and beyond. J. Fungi 2022, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W.; Chen, S.C.-A.; Huisingh, C.; Benedict, K.; DeBess, E.E.; Galanis, E.; Jackson, B.R.; MacDougall, L.; Marsden-Haug, N.; Oltean, H.; et al. MSG07: An international cohort study comparing epidemiology and outcomes of patients with Cryptococcus neoformans or Cryptococcus gattii infections. Clin. Infect. Dis. 2021, 73, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.-A.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef]
- Xue, X.; Deng, H.; Zhao, L.; Zang, X.; Asuquo, I.P.; Meng, M.; Ma, X.; Qin, C.; Meng, Y.; Wu, C.; et al. Cryptococcosis caused by Cryptococcus gattii. Medicine 2020, 99, e23213. [Google Scholar] [CrossRef]
- Datta, K.; Bartlett, K.H.; Baer, R.; Byrnes, E.; Galanis, E.; Heitman, J.; Hoang, L.; Leslie, M.J.; MacDougall, L.; Magill, S.S.; et al. Spread of Cryptococcus gattii into Pacific Northwest Region of the United States. Emerg. Infect. Dis. 2009, 15, 1185–1191. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Kidd, S. Current trends in the prevalence of Cryptococcus gattii in the United States and Canada. Infect. Drug Resist. 2015, 8, 89–97. [Google Scholar] [CrossRef]
- Kidd, S.E.; Bach, P.J.; Hingston, A.O.; Mak, S.; Chow, Y.; MacDougall, L.; Kronstad, J.W.; Bartlett, K.H. Cryptococcus gattii Dispersal Mechanisms, British Columbia, Canada. Emerg. Infect. Dis. 2007, 13, 51–57. [Google Scholar] [CrossRef]
- O’hErn, J.A.; Koenen, A.; Janson, S.; Hajkowicz, K.M.; Robertson, I.K.; Kidd, S.E.; Baird, R.W.; Tong, S.Y.; Davis, J.S.; Carson, P.; et al. Epidemiology, management and outcomes of Cryptococcus gattii infections: A 22-year cohort. PLoS Neglected Trop. Dis. 2023, 17, e0011162. [Google Scholar] [CrossRef]
- Casadevall, A.; Coelho, C.; Cordero, R.J.B.; Dragotakes, Q.; Jung, E.; Vij, R.; Wear, M.P. The capsule of Cryptococcus neoformans. Virulence 2019, 10, 822–831. [Google Scholar] [CrossRef]
- Diniz-Lima, I.; da Fonseca, L.M.; da Silva-Junior, E.B.; Guimarães-De-Oliveira, J.C.; Freire-De-Lima, L.; Nascimento, D.O.; Morrot, A.; Previato, J.O.; Mendonça-Previato, L.; Decote-Ricardo, D.; et al. Cryptococcus: History, epidemiology and immune evasion. Appl. Sci. 2022, 12, 7086. [Google Scholar] [CrossRef]
- Freitas, G.J.C.; Santos, D.A. Cryptococcus gattii polysaccharide capsule: An insight on fungal-host interactions and vaccine studies. Eur. J. Immunol. 2021, 51, 2206–2209. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Dwyer, C.; Campbell, L.T.; Carter, D.A.; Mitchell, A.P. Species in the Cryptococcus gattii complex differ in capsule and cell size following growth under capsule-Inducing conditions. mSphere 2016, 1, e00350-16. [Google Scholar] [CrossRef] [PubMed]
- Saidykhan, L.; Correia, J.; Romanyuk, A.; Peacock, A.F.A.; Desanti, G.E.; Taylor-Smith, L.; Makarova, M.; Ballou, E.R.; May, R.C.; Nielsen, K. An in vitro method for inducing titan cells reveals novel features of yeast-to-titan switching in the human fungal pathogen Cryptococcus gattii. PLoS Pathog. 2022, 18, e1010321. [Google Scholar] [CrossRef] [PubMed]
- Dyląg, M.; Colon-Reyes, R.J.; Kozubowski, L. Titan cell formation is unique to Cryptococcus species complex. Virulence 2020, 11, 719–729. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.H.; Machado, M.P.; Kumaresan, P.R.; da Silva, T.A. Titan cells and yeast forms of Cryptococcus neoformans and Cryptococcus gattii are recognized by GXMR-CAR. Microorganisms 2021, 9, 1886. [Google Scholar] [CrossRef]
- Dyląg, M.; Colón-Reyes, R.J.; Loperena-Álvarez, Y.; Kozubowski, L. Establishing minimal conditions sufficient for the development of titan-like cells in Cryptococcus neoformans/gattii species complex. Pathogens 2022, 11, 768. [Google Scholar] [CrossRef]
- Godoy, J.; Avellar-Moura, I.; Soares, J.; Pontes, B.; Frases, S. Neurobasal medium enhances titan cell formation in Cryptococcus spp. Mem. Do Inst. Oswaldo Cruz 2025, 120, e240286. [Google Scholar] [CrossRef]
- Urai, M.; Kaneko, Y.; Ueno, K.; Okubo, Y.; Aizawa, T.; Fukazawa, H.; Sugita, T.; Ohno, H.; Shibuya, K.; Kinjo, Y.; et al. Evasion of innate immune responses by the highly virulent Cryptococcus gattii by altering capsule glucuronoxylomannan structure. Front. Cell Infect. Microbiol. 2016, 5, 101. [Google Scholar] [CrossRef]
- Zaragoza, O.; García-Rodas, R.; Nosanchuk, J.D.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A.; Mitchell, A.P. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010, 6, e1000945. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Zaragoza, O.; Nielsen, K. Titan cells in Cryptococcus neoformans: Cells with a giant impact. Curr. Opin. Microbiol. 2013, 16, 409–413. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; de Oliveira, H.C.; García-Rodas, R.; Rossi, S.A.; Llorente, I.; Zaballos, Á.; Janbon, G.; Ariño, J.; Zaragoza, Ó. Cryptococcus neoformans can form titan-like cells in vitro in response to multiple signals. PLoS Pathog. 2018, 14, e1007007. [Google Scholar] [CrossRef]
- Araújo, G.R.D.S.; Pontes, B.; Frases, S. Electron microscopy of Cryptococcus neoformans: Processing challenges to avoid artifacts. In Cryptococcus neoformans. Methods in Molecular Biology, 1st ed.; McClelland, E., Ed.; Humana: New York, NY, USA, 2024; pp. 141–153. [Google Scholar]
- Araújo, G.R.d.S.; Alcantara, C.d.L.; Rodrigues, N.; de Souza, W.; Pontes, B.; Frases, S. Ultrastructural study of Cryptococcus neoformans surface during budding events. Front. Microbiol. 2021, 12, 609244. [Google Scholar] [CrossRef]
- Araújo, G.R.d.S.; Fontes, G.N.; Leão, D.; Rocha, G.M.; Pontes, B.; Sant’aNna, C.; de Souza, W.; Frases, S. Cryptococcus neoformans capsular polysaccharides form branched and complex filamentous networks viewed by high-resolution microscopy. J. Struct. Biol. 2016, 193, 75–82. [Google Scholar] [CrossRef]
- Frases, S.; Pontes, B.; Nimrichter, L.; Rodrigues, M.L.; Viana, N.B.; Casadevall, A. The elastic properties of the Cryptococcus neoformans capsule. Biophys. J. 2009, 97, 937–945. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.G. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Frases, S.; Nimrichter, L.; Viana, N.B.; Nakouzi, A.; Casadevall, A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot. Cell 2008, 7, 319–327. [Google Scholar] [CrossRef]
- Araujo, G.d.S.; Fonseca, F.L.; Pontes, B.; Torres, A.; Cordero, R.J.B.; Zancopé-Oliveira, R.M.; Casadevall, A.; Viana, N.B.; Nimrichter, L.; Rodrigues, M.L.; et al. Capsules from pathogenic and non-pathogenic Cryptococcus spp. manifest significant differences in structure and ability to protect against phagocytic cells. PLoS ONE 2012, 7, e29561. [Google Scholar] [CrossRef]
- Ayala, Y.A.; Pontes, B.; Ether, D.S.; Pires, L.B.; Araujo, G.R.; Frases, S.; Romão, L.F.; Farina, M.; Moura-Neto, V.; Viana, N.B.; et al. Rheological properties of cells measured by optical tweezers. BMC Biophys. 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, E.; Li, G.; Barr, J.; Plischke, E.; Rabitz, H. Global Sensitivity Analysis with Mixtures: A Generalized Functional ANOVA Approach. Risk Anal. 2021, 42, 304–333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W. p < 0.05, < 0.01, < 0.001, < 0.0001, < 0.00001, < 0.000001, or < 0.0000001 …. J. Sport Health Sci. 2016, 5, 77–79. [Google Scholar] [CrossRef]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chrétien, F.; Heitman, J.; Dromer, F.; Nielsen, K.; Mitchell, A.P. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010, 6, e1000953. [Google Scholar] [CrossRef]
- Dambuza, I.M.; Drake, T.; Chapuis, A.; Zhou, X.; Correia, J.; Taylor-Smith, L.; LeGrave, N.; Rasmussen, T.; Fisher, M.C.; Bicanic, T.; et al. The Cryptococcus neoformans Titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog. 2018, 14, e1006978. [Google Scholar] [CrossRef]
- Gerstein, A.C.; Fu, M.S.; Mukaremera, L.; Li, Z.; Ormerod, K.L.; Fraser, J.A.; Berman, J.; Nielsen, K.; Huffnagle, G.B. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio 2015, 6, e01340-15. [Google Scholar] [CrossRef]
- Kidd, S.E.; Hagen, F.; Tscharke, R.L.; Huynh, M.; Bartlett, K.H.; Fyfe, M.; MacDougall, L.; Boekhout, T.; Kwon-Chung, K.J.; Meyer, W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. USA 2004, 101, 17258–17263. [Google Scholar] [CrossRef]
- Farrer, R.A.; Chang, M.; Davis, M.J.; van Dorp, L.; Yang, D.-H.; Shea, T.; Sewell, T.R.; Meyer, W.; Balloux, F.; Edwards, H.M.; et al. A new lineage of Cryptococcus gattii (VGV) discovered in the central Zambezian miombo woodlands. mBio 2019, 10, e02306-19. [Google Scholar] [CrossRef]
- Hommel, B.; Mukaremera, L.; Cordero, R.J.B.; Coelho, C.; Desjardins, C.A.; Sturny-Leclère, A.; Janbon, G.; Perfect, J.R.; Fraser, J.A.; Casadevall, A.; et al. Titan cells formation in Cryptococcus neoformans is finely tuned by environmental conditions and modulated by positive and negative genetic regulators. PLoS Pathog. 2018, 14, e1006982. [Google Scholar] [CrossRef]
- Trevijano-Contador, N.; Rossi, S.A.; Alves, E.; Landín-Ferreiroa, S.; Zaragoza, O. Capsule enlargement in Cryptococcus neoformans is dependent on mitochondrial Activity. Front. Microbiol. 2017, 8, 1423. [Google Scholar] [CrossRef]
- García-Barbazán, I.; Trevijano-Contador, N.; Rueda, C.; de Andrés, B.; Pérez-Tavárez, R.; Herrero-Fernández, I.; Gaspar, M.L.; Zaragoza, O. The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol. 2016, 18, 111–124. [Google Scholar] [CrossRef]
- Mukaremera, L.; Lee, K.K.; Wagener, J.; Wiesner, D.L.; Gow, N.A.; Nielsen, K. Titan cell production in Cryptococcus neoformans reshapes the cell wall and capsule composition during infection. Cell Surf. 2018, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Homer, C.M.; Summers, D.K.; Goranov, A.I.; Clarke, S.C.; Wiesner, D.L.; Diedrich, J.K.; Moresco, J.J.; Toffaletti, D.; Upadhya, R.; Caradonna, I.; et al. Intracellular action of a secreted peptide required for fungal virulence. Cell Host Microbe 2016, 19, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. Cellular charge of Cryptococcus neoformans: Contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 1997, 65, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Frases, S.; Guimaräes, A.J.; Rivera, J.; Casadevall, A. Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol. Microbiol. 2011, 79, 1101–1117. [Google Scholar] [CrossRef]
- Frases, S.; Pontes, B.; Nimrichter, L.; Viana, N.B.; Rodrigues, M.L.; Casadevall, A. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 1228–1233. [Google Scholar] [CrossRef]
- Cordero, R.J.B.; Pontes, B.; Guimarães, A.J.; Martinez, L.R.; Rivera, J.; Fries, B.C.; Nimrichter, L.; Rodrigues, M.L.; Viana, N.B.; Casadevall, A.; et al. Chronological aging is associated with biophysical and chemical changes in the capsule of Cryptococcus neoformans. Infect. Immun. 2011, 79, 4990–5000. [Google Scholar] [CrossRef]
- Nimrichter, L.; Frases, S.; Cinelli, L.P.; Viana, N.B.; Nakouzi, A.; Travassos, L.R.; Casadevall, A.; Rodrigues, M.L. Self-Aggregation of Cryptococcus neoformans Capsular Glucuronoxylomannan Is Dependent on Divalent Cations. Eukaryot. Cell 2007, 6, 1400–1410. [Google Scholar] [CrossRef]
- Gates-Hollingsworth, M.A.; Kozel, T.R. Phenotypic heterogeneity in expression of epitopes in the Cryptococcus neoformans capsule. Mol. Microbiol. 2009, 74, 126–138. [Google Scholar] [CrossRef]
- Cordero, R.J.B.; Pontes, B.; Frases, S.; Nakouzi, A.S.; Nimrichter, L.; Rodrigues, M.L.; Viana, N.B.; Casadevall, A. Antibody binding to Cryptococcus neoformans impairs budding by altering capsular mechanical properties. J. Immunol. 2013, 190, 317–323. [Google Scholar] [CrossRef]
- García-Rodas, R.; Casadevall, A.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O.; Cramer, R.A. Cryptococcus neoformans Capsular Enlargement and Cellular Gigantism during Galleria mellonella Infection. PLoS ONE 2011, 6, e24485. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in cryptococcus neoformans. Eukaryot. Cell 2007, 6, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O.; Telzak, A.; Bryan, R.A.; Dadachova, E.; Casadevall, A. The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol. Microbiol. 2005, 59, 67–83. [Google Scholar] [CrossRef]
- Zaragoza, O.; Chrisman, C.J.; Castelli, M.V.; Frases, S.; Cuenca-Estrella, M.; Rodríguez-Tudela, J.L.; Casadevall, A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008, 10, 2043–2057. [Google Scholar] [CrossRef]
- Fonseca, F.L.; Nohara, L.L.; Cordero, R.J.B.; Frases, S.; Casadevall, A.; Almeida, I.C.; Nimrichter, L.; Rodrigues, M.L. Immunomodulatory effects of serotype B glucuronoxylomannan from Cryptococcus gattii correlate with polysaccharide diameter. Infect. Immun. 2010, 78, 3861–3870. [Google Scholar] [CrossRef]
- Araújo, G.R.d.S.; Viana, N.B.; Pontes, B.; Frases, S. Rheological properties of cryptococcal polysaccharide change with fiber size, antibody binding and temperature. Futur. Microbiol. 2019, 14, 867–884. [Google Scholar] [CrossRef]
- McFadden, D.C.; De Jesus, M.; Casadevall, A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J. Biol. Chem. 2006, 281, 1868–1875. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 2007, 73, 4592–4601. [Google Scholar] [CrossRef]
- Vecchiarelli, A.; Pericolini, E.; Gabrielli, E.; Kenno, S.; Perito, S.; Cenci, E.; Monari, C. Elucidating the immunological function of the Cryptococcus neoformans capsule. Futur. Microbiol. 2013, 8, 1107–1116. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Crabtree, J.N.; Okagaki, L.H.; Wiesner, D.L.; Strain, A.K.; Nielsen, J.N.; Nielsen, K.; Deepe, G.S. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect. Immun. 2012, 80, 3776–3785. [Google Scholar] [CrossRef]
- Caza, M.; Kronstad, J.W. The cAMP/Protein kinase a pathway regulates virulence and adaptation to host conditions in Cryptococcus neoformans. Front. Cell Infect. Microbiol. 2019, 9, 212. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avellar-Moura, I.; Araujo, G.R.d.S.; Godoy, J.; Alves, V.; Andrade, I.B.d.; Soares, J.; Pontes, B.; Frases, S. The Role of Nutritional Environment in Cryptococcus gattii Titan Cells’ Ultrastructure, Biophysical Properties, Molecular Features, and Virulence in Cryptococcosis. Infect. Dis. Rep. 2025, 17, 101. https://doi.org/10.3390/idr17040101
Avellar-Moura I, Araujo GRdS, Godoy J, Alves V, Andrade IBd, Soares J, Pontes B, Frases S. The Role of Nutritional Environment in Cryptococcus gattii Titan Cells’ Ultrastructure, Biophysical Properties, Molecular Features, and Virulence in Cryptococcosis. Infectious Disease Reports. 2025; 17(4):101. https://doi.org/10.3390/idr17040101
Chicago/Turabian StyleAvellar-Moura, Igor, Glauber R. de S. Araujo, Juliana Godoy, Vinicius Alves, Iara Bastos de Andrade, Juliana Soares, Bruno Pontes, and Susana Frases. 2025. "The Role of Nutritional Environment in Cryptococcus gattii Titan Cells’ Ultrastructure, Biophysical Properties, Molecular Features, and Virulence in Cryptococcosis" Infectious Disease Reports 17, no. 4: 101. https://doi.org/10.3390/idr17040101
APA StyleAvellar-Moura, I., Araujo, G. R. d. S., Godoy, J., Alves, V., Andrade, I. B. d., Soares, J., Pontes, B., & Frases, S. (2025). The Role of Nutritional Environment in Cryptococcus gattii Titan Cells’ Ultrastructure, Biophysical Properties, Molecular Features, and Virulence in Cryptococcosis. Infectious Disease Reports, 17(4), 101. https://doi.org/10.3390/idr17040101





