SARS-CoV-2 Did Not Spread Through Dental Clinics During the COVID-19 Pandemic in Japan
Abstract
1. Introduction
2. Materials and Methods
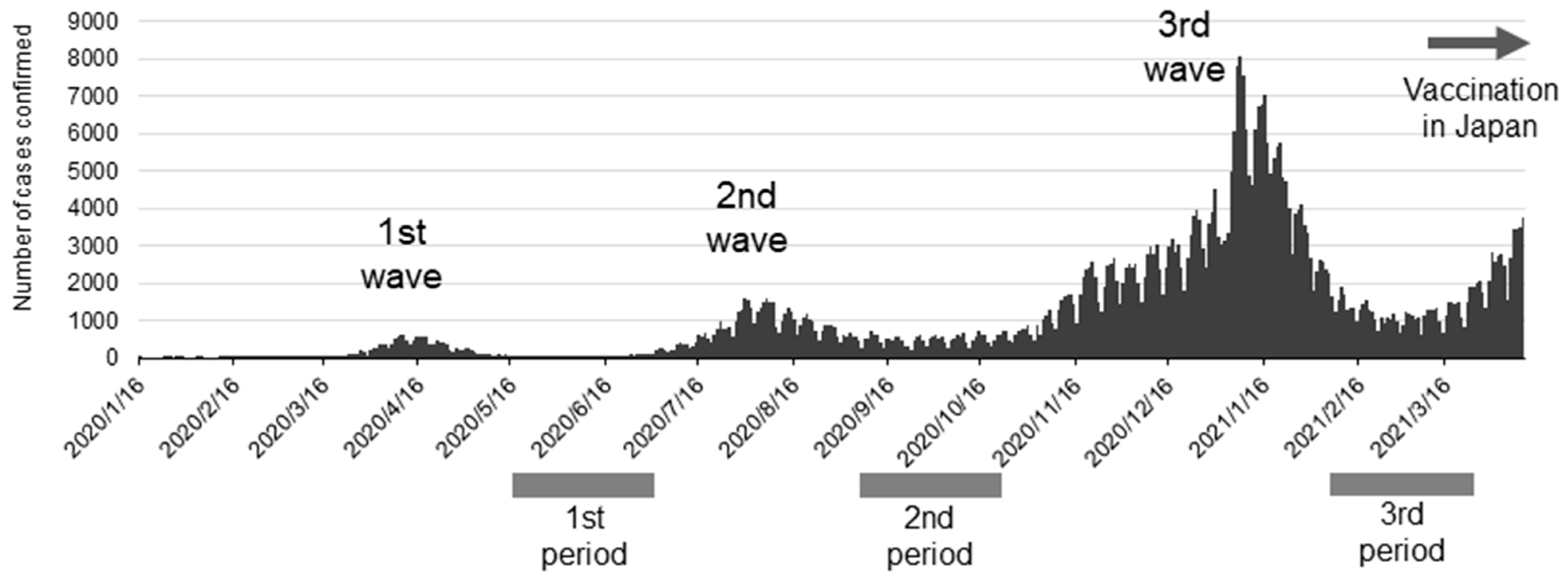
2.1. Determination of IgG/IgM Antibody Titer Against SARS-CoV-2 in the Blood (Evidence for Previous Infections) in Dental Professionals and Their Families During the SARS-CoV-2 Wave
2.2. Sample Collection of Saliva and Aerosol During Oral Management for Hospitalized COVID-19 Patients
2.3. Detection of SARS-CoV-2 Viral Genome with RT-qPCR
2.4. Statistical Analysis
3. Results
3.1. Antibody-Positive Rate for SARS-CoV-2 in Dental Professionals
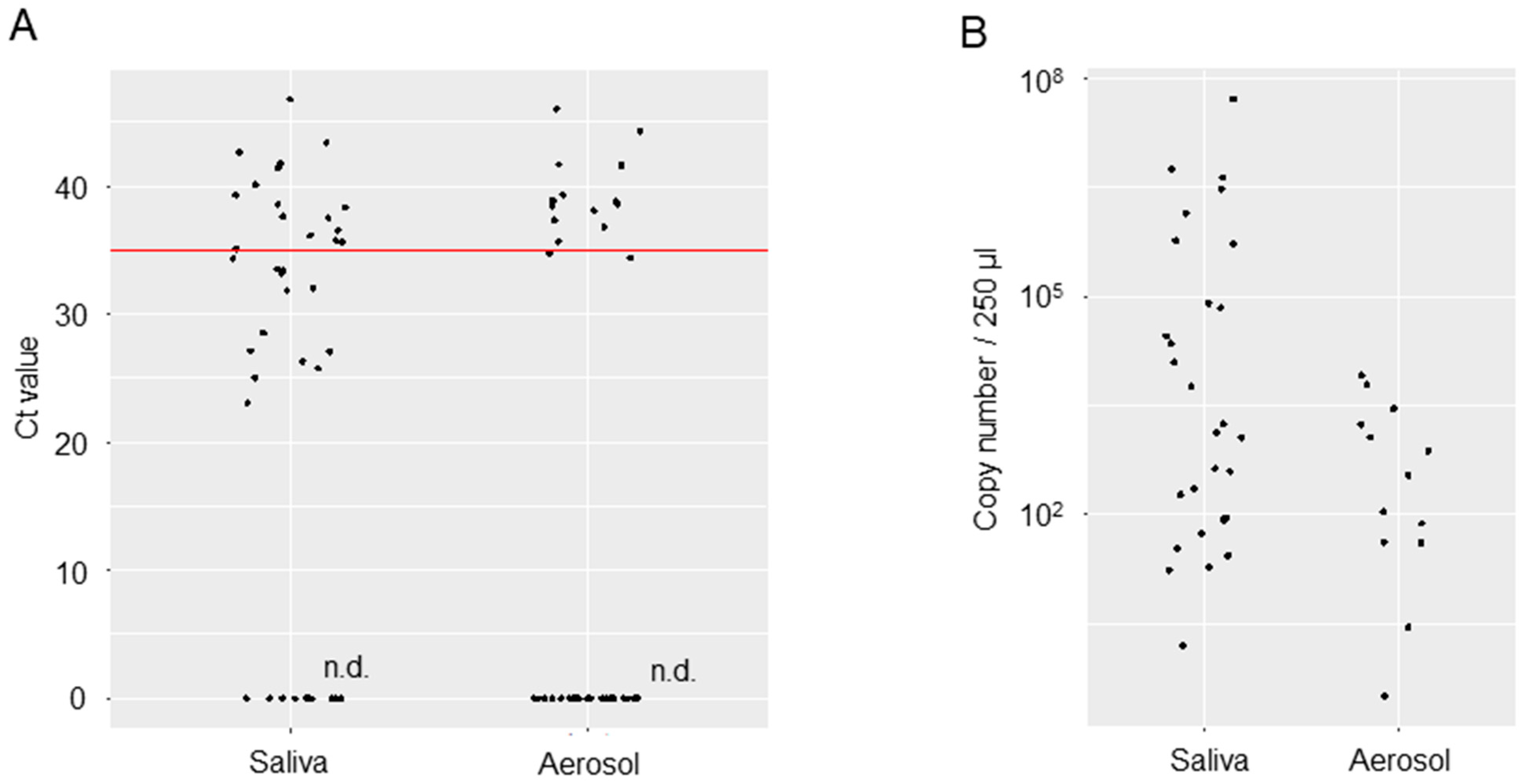
3.2. Viral Loads in the Saliva or Oral Management-Related Aerosol of COVID-19 Patients
4. Discussion
5. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamio, L. The Workers Who Face the Greatest Coronavirus Risk. The New York Times. 15 March 2020. Available online: https://www.nytimes.com/2020/03/15/business/economy/coronavirus-economy-impact.html (accessed on 28 July 2021).
- Japan Ministry of Health Labor and Welfare. 45th Novel Coronavirus Infection Disease Advisary Board material 3—1 Dai 45 kai Shingata Corona Uirusu Kansensyou Taisaku Advisary Board Shiryou 3-1 (in Japanese). 2021. Available online: https://www.mhlw.go.jp/content/10900000/000812896.pdf (accessed on 28 July 2021).
- House of Counsillors, The Natinal Diet of Japan. The 204th National Diet. House of Councillors Budget Committee Meeting Minutes No. 14 Dai 204 kai kokkai. Sangiin yosan iinkai kaigiroku dai 14 gou (in Japanese). 2021. Available online: https://kokkai.ndl.go.jp/minutes/api/v1/detailPDF/img/120415261X01420210319 (accessed on 19 March 2021).
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.Y.; Yang, L.M.; Xia, J.J.; Fu, X.H.; Zhang, Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B. 2020, 21, 361–368. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Center for Disease Control. Research Use Only 2019-Novel Coronavirus (2019-nCoV) Real-time RT-PCR Primers and Probes. CDC’s Diagnostic Test COVID-19 Only Supplies. 2019. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/virus-requests.html (accessed on 17 April 2025).
- Japan Ministry of Health Labor and Welfare. First Antibody Possession Test Daiikkai Koutai Hoyuu Chyousa (in Japanese). 2022. Available online: https://www.mhlw.go.jp/content/10906000/000640184.pdf (accessed on 17 April 2025).
- Japan Ministry of Health Labor and Welfare. Second Antibody Possession Test. Dainikai Koutai Hoyuu Kensa (in Japanese). 2023. Available online: https://www.mhlw.go.jp/content/000761671.pdf (accessed on 17 April 2025).
- Softbank Group Inc. Preliminary Reports for Antibody Test Koutai Kennsa Kekka Sokuhoutitou ni Tuite (in Japanese). 2024. Available online: https://group.softbank/system/files/pdf/antibodytest.pdf (accessed on 17 April 2025).
- Santigli, E.; Lindner, M.; Kessler, H.H.; Jakse, N.; Fakheran, O. Seroprevalence of SARS-CoV-2 antibodies among oral health care workers with natural seroconversion: A systematic review and meta-analysis. Sci Rep. 2025, 15, 7848. [Google Scholar] [CrossRef] [PubMed]
- Chiramana, S.; Hima, B.O.S.; Kishore, K.K.; Prakash, M.; Durga, P.T.; Chaitanya, S.K. Evaluation of Minimum Required Safe Distance between Two Consecutive Dental Chairs for Optimal Asepsis. J. Orofac. Res. 2013, 3, 12–15. [Google Scholar] [CrossRef]
- Seyedalinaghi, S.; Karimi, A.; Mojdeganlou, H.; Pashaei, Z.; Mirzapour, P.; Shamsabadi, A.; Barzegary, A.; Afroughi, F.; Dehghani, S.; Janfaza, N.; et al. Minimum infective dose of severe acute respiratory syndrome coronavirus 2 based on the current evidence: A systematic review. SAGE Open Med. 2022, 10, 20503121221115053. [Google Scholar] [CrossRef] [PubMed]
- Usami, Y.; Hirose, K.; Okumura, M.; Toyosawa, S.; Sakai, T. Brief communication: Immunohistochemical detection of ACE2 in human salivary gland. Oral Sci. Int. 2021, 18, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Saldi, T.K.; Gonzales, P.K.; Lasda, E.; Decker, C.J.; Tat, K.L.; Fink, M.R.; Hager, C.R.; Davis, J.C.; Ozeroff, C.D.; et al. Just 2% of SARS-CoV-2-positive indivisuals carry 90% of the Virus Circulating in Communities. Proc. Natl. Acad. Sci. USA 2021, 118, e2104547118. [Google Scholar]
- Meethil, A.P.; Saraswat, S.; Chaudhary, P.P.; Dabdoub, S.M.; Kumar, P.S. Sources of SARS-CoV-2 and Other Microorganisms in Dental Aerosols. J. Dent. Res. 2021, 100, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, M.; Yamada, K.; Matsubara, H.; Narita, M.; Kawamura, K.; Tamura, S. Considerations on SARS-CoV-2 virus copy number and viral titer in specimens. Infect. Agents Surv. Rep. 2021, 42, 22–24. [Google Scholar]
| Characteristic | |
|---|---|
| Age—y (range) † | 39.8 (20–77) |
| Age—y, no./total no. (%) | |
| 20 to 29 | 74/304 (24.3) |
| 30 to 39 | 83/304 (27.3) |
| 40 to 49 | 76/304 (25.0) |
| 50 to 59 | 48/304 (15.8) |
| 60 to 69 | 18/304 (5.9) |
| 70 to 79 | 4/304 (1.3) |
| Not specified | 1/304 (0.3) |
| Sex—no./total no. (%) | |
| Male | 80/304 (26.3) |
| Female | 224/304 (73.7) |
| Occupation—no./total no. (%) | |
| Dental hygienist | 116/304 (38.2) |
| Dentist/oral surgeon | 97/304 (31.9) |
| Dental assistant | 40/304 (13.2) |
| Medical clerk | 28/304 (9.2) |
| Family member | 9/304 (3.0) |
| Dental technician | 8/304 (2.6) |
| Nurse | 5/304 (1.6) |
| Others | 1/304 (0.3) |
| Location of healthcare facility—no./total no. (%) | |
| Tochigi | 162/304 (53.3) |
| Gunma | 42/304 (13.8) |
| Saitama | 34/304 (11.2) |
| Tokyo | 34/304 (11.2) |
| Kanagawa | 22/304 (7.2) |
| Fukushima | 9/304 (3.0) |
| Ibaraki | 1/304 (0.3) |
| Chiba | 1/304 (0.3) |
| Others | 6/304 (2.0) |
| 1st Period | 2nd Period | 3rd Period | Total | |
|---|---|---|---|---|
| All participants | 2/291 (0.69%) | 0/31 (0%) | 0/102 (0%) | 2/424 (0.47%) |
| Dentist | 1/92 (1.09%) | 0/30 (0%) | 0/46 (0%) | 1/168 (0.60%) |
| Dental hygienist | 0/112 (0%) | 0/0 (0%) | 0/40 (0%) | 0/152 (0%) |
| Dental assistant | 0/40 (0%) | 0/0 (0%) | 0/5 (0%) | 0/45 (0%) |
| Medical clerk | 0/25 (0%) | 0/0 (0%) | 0/8 (0%) | 0/33 (0%) |
| Family | 1/9 (11.11%) | 0/1 (0%) | 0/1 (0%) | 1/11 (9.09%) |
| Dental technician | 0/8 (0%) | 0/0 (0%) | 0/1 (0%) | 0/9 (0%) |
| Nurse | 0/5 (0%) | 0/0 (0%) | 0/0 (0%) | 0/5 (0%) |
| Others | 0/0 (0%) | 0/0 (0%) | 0/1 (0%) | 0/1 (0%) |
| Patients Number | Age (y) | Sex | SARS-CoV-2 Strain | Days of Sampling from Positive Confirmation | Type of Respiratory Management | Method of Oral Management | Ct Values (Saliva) | Ct Values (Aerosol) |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | alpha | 5 | IPPV (intraoral intubation) | Brushing, Scaling | 25.8 | 35.67 |
| 2 | 66 | M | alpha | 4 | IPPV (tracheotomy) | Brushing, Scaling | 37.665 | - |
| 3 | 64 | M | alpha | 12 | HFOT | Brushing, Scaling | 41.41 | - |
| 4 | 53 | M | alpha | 13 | IPPV (tracheotomy) | Brushing, Scaling | 41.765 | - |
| 5 | 59 | M | alpha | 16 | NIPPV | Brushing, Scaling | - | 37.35 |
| 6 | 53 | M | alpha | 7 | IPPV (intraoral intubation) with ECMO | Brushing, Scaling | 26.33 | - |
| 7 | 72 | M | alpha | 8 | NIPPV | Brushing, Scaling | - | - |
| 8 | 53 | M | alpha | 5 | IPPV (tracheotomy) | Brushing, Scaling | 31.89 | - |
| 9 | 73 | M | alpha | 7 | IPPV (intraoral intubation) | Brushing, Scaling | 35.62 | - |
| 10 | 54 | M | alpha | 2 | IPPV (intraoral intubation) | Brushing, Scaling | 39.32 | - |
| 11 | 53 | M | alpha | 9 | IPPV (tracheotomy) | Brushing, Scaling | - | - |
| 12 | 53 | M | alpha | 5 | IPPV (tracheotomy) | Brushing, Scaling | - | - |
| 13 | 47 | M | delta | 4 | IPPV (intraoral intubation) | Brushing, Scaling | - | - |
| 14 | 52 | M | alpha | 16 | NIPPV | Brushing, Scaling | 36.125 | 38.91 |
| 15 | 48 | F | alpha | 47 | IPPV (intraoral intubation) | Brushing, Scaling | 36.555 | - |
| 16 | 50 | M | delta | 5 | HFOT | Brushing | 46.79 | - |
| 17 | 42 | M | delta | 4 | IPPV (intraoral intubation) with ECMO | Brushing | 40.135 | 34.415 |
| 18 | 42 | M | delta | 12 | IPPV (intraoral intubation) with ECMO | Brushing | 25.05 | - |
| 19 | 46 | M | alpha | 13 | IPPV (intraoral intubation) | Brushing | 35.755 | 46.05 |
| 20 | 52 | M | delta | 16 | IPPV (intraoral intubation) | Brushing | 33.4 | - |
| 21 | 89 | M | delta | 7 | IPPV (tracheotomy) | Brushing, Scaling | 27.1 | - |
| 22 | 62 | M | alpha | 8 | IPPV (tracheotomy) | Brushing | 32.01 | - |
| 23 | 42 | M | delta | 5 | IPPV (tracheotomy) | Brushing | 35.095 | 38.435 |
| 24 | 76 | M | delta | 7 | IPPV (intraoral intubation) | Brushing | 23.095 | - |
| 25 | 48 | M | delta | 2 | IPPV (tracheotomy) | Brushing, Scaling | - | - |
| 26 | 42 | M | delta | 9 | IPPV (tracheotomy) | Brushing, Scaling | - | - |
| 27 | 62 | M | alpha | 5 | IPPV (tracheotomy) | Brushing, Scaling | 38.35 | - |
| 28 | 62 | F | - | 4 | IPPV (intraoral intubation) | Brushing | 33.14 | - |
| 29 | 76 | M | delta | 16 | IPPV (intraoral intubation) | Brushing, Scaling | 28.56 | - |
| 30 | 57 | M | delta | 47 | IPPV (intraoral intubation) | Brushing | 34.4 | - |
| 31 | 76 | M | delta | 5 | IPPV (intraoral intubation) | Brushing, Scaling | 33.5 | 38.085 |
| 32 | 57 | M | delta | 4 | IPPV (tracheotomy) | 43.425 | 36.805 | |
| 33 | 62 | M | alpha | 12 | IPPV (tracheotomy) | - | 41.645 | |
| 34 | 47 | M | delta | 13 | NIPPV | Brushing, Scaling | 42.62 | 44.295 |
| 35 | 47 | M | delta | 16 | NIPPV | Brushing, Scaling | - | - |
| 36 | 62 | M | alpha | 7 | IPPV (tracheotomy) | Brushing, Scaling | - | - |
| 37 | 57 | M | delta | 8 | IPPV (tracheotomy) | Brushing, Scaling | 27.12 | 38.805 |
| 38 | 57 | M | delta | 5 | IPPV (tracheotomy) | Brushing, Scaling | 37.49 | 41.61 |
| 39 | 58 | M | delta | 7 | HFOT | Brushing, Scaling | 38.595 | 34.762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsubura, Y.; Komiyama, Y.; Ohtani, S.; Hyodo, T.; Shiraishi, R.; Yagisawa, S.; Yaguchi, E.; Tsubura-Okubo, M.; Houzumi, H.; Nemoto, M.; et al. SARS-CoV-2 Did Not Spread Through Dental Clinics During the COVID-19 Pandemic in Japan. Infect. Dis. Rep. 2025, 17, 70. https://doi.org/10.3390/idr17030070
Tsubura Y, Komiyama Y, Ohtani S, Hyodo T, Shiraishi R, Yagisawa S, Yaguchi E, Tsubura-Okubo M, Houzumi H, Nemoto M, et al. SARS-CoV-2 Did Not Spread Through Dental Clinics During the COVID-19 Pandemic in Japan. Infectious Disease Reports. 2025; 17(3):70. https://doi.org/10.3390/idr17030070
Chicago/Turabian StyleTsubura, Yasuhiro, Yuske Komiyama, Saori Ohtani, Toshiki Hyodo, Ryo Shiraishi, Shuma Yagisawa, Erika Yaguchi, Maki Tsubura-Okubo, Hajime Houzumi, Masato Nemoto, and et al. 2025. "SARS-CoV-2 Did Not Spread Through Dental Clinics During the COVID-19 Pandemic in Japan" Infectious Disease Reports 17, no. 3: 70. https://doi.org/10.3390/idr17030070
APA StyleTsubura, Y., Komiyama, Y., Ohtani, S., Hyodo, T., Shiraishi, R., Yagisawa, S., Yaguchi, E., Tsubura-Okubo, M., Houzumi, H., Nemoto, M., Kikuchi, J., Fukumoto, C., Izumi, S., Wakui, T., Wake, K., & Kawamata, H. (2025). SARS-CoV-2 Did Not Spread Through Dental Clinics During the COVID-19 Pandemic in Japan. Infectious Disease Reports, 17(3), 70. https://doi.org/10.3390/idr17030070






