Sickle Cell Disease and Antimicrobial Resistance: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
2.5. Quality Assessment
3. Results
3.1. Study Selection Process
3.2. Characteristics of Included Studies
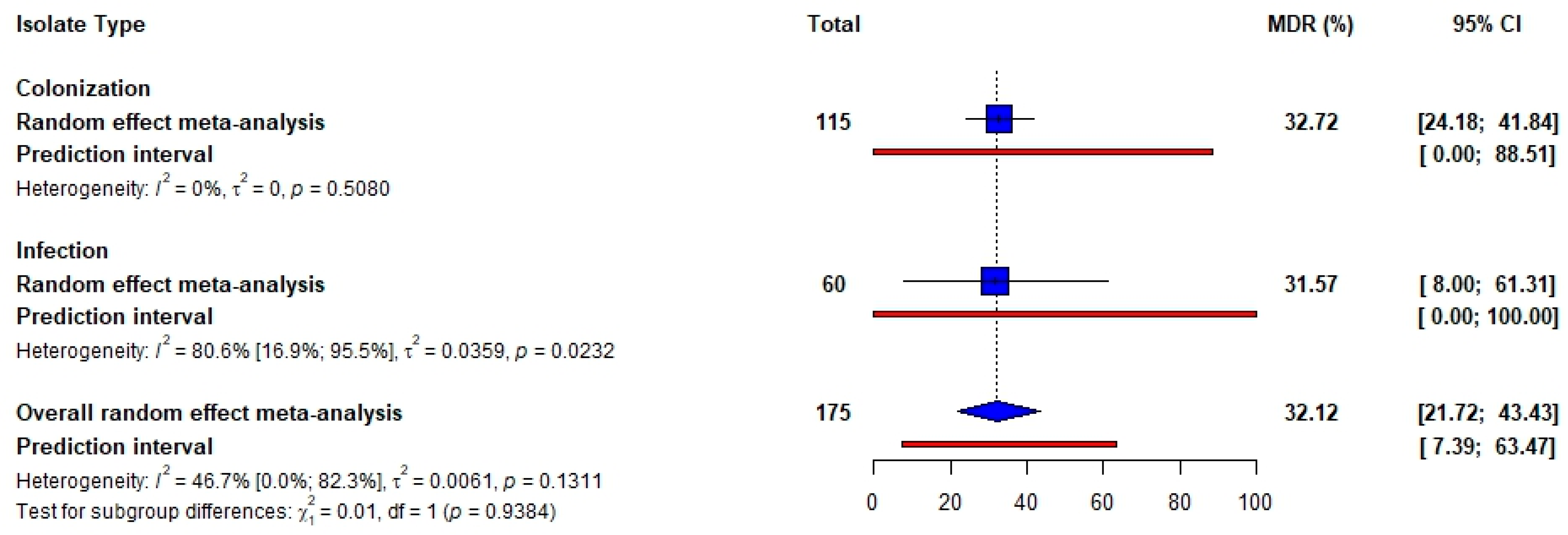
3.3. Antibiotic Resistance in Staphylococcus aureus
3.4. Antibiotic Resistance in Streptococcus pneumoniae
3.5. Antibiotic Resistance in Escherichia coli
3.6. Quality of the Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giacomini, E.; Perrone, V.; Alessandrini, D.; Paoli, D.; Nappi, C.; Degli Esposti, L. Evidence of Antibiotic Resistance from Population-Based Studies: A Narrative Review. Infect. Drug Resist. 2021, 14, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.d.A.; Al-Amin, M.d.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Donkor, E.S.; Odoom, A.; Osman, A.-H.; Darkwah, S.; Kotey, F.C.N. A Systematic Review on Antimicrobial Resistance in Ghana from a One Health Perspective. Antibiotics 2024, 13, 662. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10 (Suppl. S12), S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Inusa, B.; Obaro, S.K. Infection in sickle cell disease: A review. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2010, 14, e2–e12. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Alakwe-Ojimba, C.E.; Elendu, T.C.; Elendu, R.C.; Ayabazu, C.P.; Aina, T.O.; Aborisade, O.; Adenikinju, J.S. Understanding Sickle cell disease: Causes, symptoms, and treatment options. Medicine 2023, 102, e35237. [Google Scholar] [CrossRef]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4336. [Google Scholar] [CrossRef]
- Bailey, M.; Gibbs, M.; Dani, N.; Mendell, A.; Thompson, M. Burden of Illness of Sickle Cell Disease in Countries of the Middle East: A Systematic Literature Review. Blood 2019, 134 (Suppl. S1), 5867. [Google Scholar] [CrossRef]
- GBD 2021 Sickle Cell Disease Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- Williams, T.N. Sickle Cell Disease in Sub-Saharan Africa. Hematol. Oncol. Clin. N. Am. 2016, 30, 343–358. [Google Scholar] [CrossRef]
- Battersby, A.J.; Knox-Macaulay, H.H.M.; Carrol, E.D. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr. Blood Cancer 2010, 55, 401–406. [Google Scholar] [CrossRef]
- Dibbasey, M.; Dahaba, M.; Sarfo, F.; Jallow-Manneh, I.; Ceesay, B.; Umukoro, S.; Diop, M.F.; Amambua-Ngwa, A. Laboratory indices of hospitalized sickle cell disease patients, prevalence and antimicrobial susceptibility of pathogenic bacterial isolates at MRCG ward in the Gambia. BMC Infect. Dis. 2023, 23, 546. [Google Scholar] [CrossRef]
- Abdulmanea, A.A.; Alharbi, N.S.; Somily, A.M.; Khaled, J.M.; Algahtani, F.H. The Prevalence of the Virulence Genes of Staphylococcus aureus in Sickle Cell Disease Patients at KSUMC, Riyadh, Saudi Arabia. Antibiotics 2023, 12, 1221. [Google Scholar] [CrossRef]
- Appiah, V.A.; Pesewu, G.A.; Kotey, F.C.N.; Boakye, A.N.; Duodu, S.; Tette, E.M.A.; Nyarko, M.Y.; Donkor, E.S. Staphylococcus aureus nasal colonization among children with sickle cell disease at the children’s hospital, accra: Prevalence, risk factors, and antibiotic resistance. Pathogens 2020, 9, 329. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Donkor, E.S.; Foster-Nyarko, E.; Enweronu-Laryea, C.C. Relationship between antibiotic resistance and sickle cell anemia: Preliminary evidence from a pediatric carriage study in Ghana. Infect. Drug Resist. 2013, 6, 71–77. [Google Scholar] [CrossRef][Green Version]
- Dayie, N.T.; Sekoh, D.N.; Tetteh-Quarcoo, P.B.; Dayie, A.D.; Osei, M.-M.; Kotey, F.C.; Donkor, E.S. Staphylococcus aureus Nasopharyngeal Carriage and Antimicrobial Resistance among Adults with Sickle Cell Disease at the Korle Bu Teaching Hospital in Accra, Ghana. Microbiol. Insights 2022, 15, 11786361221133959. [Google Scholar] [CrossRef]
- Dayie, N.T.K.D.; Sekoh, D.N.K.; Kotey, F.C.N.; Egyir, B.; Tetteh-Quarcoo, P.B.; Adutwum-Ofosu, K.K.; Ahenkorah, J.; Osei, M.-M.; Donkor, E.S. Nasopharyngeal Carriage of Methicillin-Resistant Staphylococcus aureus (MRSA) among Sickle Cell Disease (SCD) Children in the Pneumococcal Conjugate Vaccine Era. Infect. Dis. Rep. 2021, 13, 191–204. [Google Scholar] [CrossRef]
- Mava, Y.; Bello, M.; Ambe, J.P.; Zailani, S.B. Antimicrobial sensitivity pattern of organisms causing urinary tract infection in children with sickle cell anemia in Maiduguri, Nigeria. Niger. J. Clin. Pract. 2012, 15, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.W.; Hawkins, P.A.; Jibir, B.; Hassan-Hanga, F.; Gambo, M.; Olaosebikan, R.; Olanipekun, G.; Munir, H.; Kocmich, N.; Rezac-Elgohary, A.; et al. Molecular characterization of streptococcus pneumoniae causing disease among children in nigeria during the introduction of pcv10 (Gsk). Microb. Genomics 2023, 9, 001094. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Msanga, D.R.; Mtemisika, C.I.; Silago, V.; Mirambo, M.M.; Mshana, S.E. Extended Spectrum β-Lactamase Producing Lactose Fermenting Bacteria Colonizing Children with Human Immunodeficiency Virus, Sickle Cell Disease and Diabetes Mellitus in Mwanza City, Tanzania: A Cross-Sectional Study. Trop. Med. Infect. Dis. 2022, 7, 144. [Google Scholar] [CrossRef]
- Steele, R.W.; Warrier, R.; Unkel, P.J.; Foch, B.J.; Howes, R.F.; Shah, S.; Williams, K.; Moore, S.; Jue, S.J. Colonization with antibiotic-resistant Streptococcus pneumoniae in children with sickle cell disease. J. Pediatr. 1996, 128, 531–535. [Google Scholar] [CrossRef]
- Dayie, N.T.K.D.; Tetteh-Ocloo, G.; Labi, A.-K.; Olayemi, E.; Slotved, H.-C.; Lartey, M.; Donkor, E.S. Pneumococcal carriage among sickle cell disease patients in Accra, Ghana: Risk factors, serotypes and antibiotic resistance. PLoS ONE 2018, 13, e0206728. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.F.; Smith-Whitley, K.; McGowan, K.L. Positive blood cultures in sickle cell disease: Time to positivity and clinical outcome. J. Pediatr. Hematol. Oncol. 2003, 25, 390–395. [Google Scholar] [CrossRef]
- Miller, M.L.; Obert, C.A.; Gao, G.; Daw, N.C.; Flynn, P.; Tuomanen, E. Cephalosporin-resistant Pneumococci and sickle cell disease. Emerg. Infect. Dis. 2005, 11, 1192–1196. [Google Scholar] [CrossRef]
- Mutagonda, R.F.; Bwire, G.; Sangeda, R.Z.; Kilonzi, M.; Mlyuka, H.; Ndunguru, J.; Jonathan, A.; Makani, J.; Minja, I.K.; Ruggajo, P.; et al. Nasopharyngeal Carriage and Antibiogram of Pneumococcal and Other Bacterial Pathogens from Children with Sickle Cell Disease in Tanzania. Infect. Drug Resist. 2022, 15, 4407–4418. [Google Scholar] [CrossRef]
- Subudhi, M.; Jagatheeswary, P.A.T.; Sahu, S.K.; Das, S.K.; Subudhi, K.B.; Rout, R.R. Incidence and variation of microbiological profile of catheter-associated urinary tract infection in precise comorbidities associated with tribal sickle cell anemic patients of medical intensive care unit in a tribal tertiary care center. J. Appl. Hematol. 2021, 12, 140–146. [Google Scholar] [CrossRef]
- Brown, B.J.; Asinobi, A.O.; Fatunde, O.J.; Osinusi, K.; Fasina, N.A. Antimicrobial sensitivity pattern of organisms causing urinary tract infection in children with sickle cell anaemia in Ibadan, Nigeria. West Afr. J. Med. 2003, 22, 110–113. [Google Scholar] [CrossRef][Green Version]
- Daw, N.C.; Wilimas, J.A.; Wang, W.C.; Presbury, G.J.; Joyner, R.E.; Harris, S.C.; Davis, Y.; Chen, G.; Joan Chesney, P. Nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae in children with sickle cell disease. Pediatrics 1997, 99, 594–595. [Google Scholar] [CrossRef]
- Sangeda, R.; Yohana, J.; Jonathan, A.; Manyanga, V.; Soka, D.; Makani, J. Prevalence of Urinary Tract Infections and Antibiogram of Bacteria Isolated From Children With Sickle Cell Disease in Tanzania. CUREUS J. Med. Sci. 2024, 16, e58786. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Webber, D.M.; Wallace, M.A.; Burnham, C.-A.D. Stop Waiting for Tomorrow: Disk Diffusion Performed on Early Growth Is an Accurate Method for Antimicrobial Susceptibility Testing with Reduced Turnaround Time. J. Clin. Microbiol. 2022, 60, e03007-20. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Antibacterial mechanism of lignin and lignin-based antimicrobial materials in different fields. Int. J. Biol. Macromol. 2023, 252, 126281. [Google Scholar] [CrossRef]
- Gupta, P.; Khare, V.; Kumar, D.; Ahmad, A.; Banerjee, G.; Singh, M. Comparative evaluation of disc diffusion and E-test with broth micro-dilution in Susceptibility testing of amphotericin B, voriconazole and caspofungin against clinical Aspergillus isolates. J. Clin. Diagn. Res. 2015, 9, DC04–DC07. [Google Scholar] [CrossRef] [PubMed]
- Pincus, D.H. Microbial Identification Using the Biomérieux VITEK® 2 System. In Encyclopedia of Rapid Microbiological Methods; BioMérieux, Inc.: Hazelwood, MO, USA, 2006. [Google Scholar]
- Salam, M.d.A.; Al-Amin, M.d.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional methods and future trends in antimicrobial susceptibility testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Cober, M.P.; Phelps, S.J. Penicillin Prophylaxis in Children with Sickle Cell Disease. J. Pediatr. Pharmacol. Ther. JPPT 2010, 15, 152. [Google Scholar] [CrossRef]
- van Aalst, M.; Lötsch, F.; Spijker, R.; van der Meer, J.T.M.; Langendam, M.W.; Goorhuis, A.; Grobusch, M.P.; de Bree, G.J. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2018, 24, 89–100. [Google Scholar] [CrossRef]
- Olaru, I.D.; Tacconelli, E.; Yeung, S.; Ferrand, R.A.; Stabler, R.A.; Hopkins, H.; Aiken, A.M.; Kranzer, K. The association between antimicrobial resistance and HIV infection: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 846–853. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Zachariah, R.; Harries, A.D.; Spielmann, M.P.; Arendt, V.; Nchingula, D.; Mwenda, R.; Courtielle, O.; Kirpach, P.; Mwale, B.; Salaniponi, F.M.L. Changes in Escherichia coli resistance to co-trimoxazole in tuberculosis patients and in relation to co-trimoxazole prophylaxis in Thyolo, Malawi. Malawi Med. J. 2002, 14, 10–12. [Google Scholar] [CrossRef][Green Version]
- Mwenya, D.M.; Charalambous, B.M.; Phillips, P.P.J.; Mwansa, J.C.L.; Batt, S.L.; Nunn, A.J.; Walker, S.; Gibb, D.M.; Gillespie, S.H. Impact of cotrimoxazole on carriage and antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae in HIV-infected children in Zambia. Antimicrob. Agents Chemother. 2010, 54, 3756–3762. [Google Scholar] [CrossRef] [PubMed]
- Ladu, A.I.; Kadaura, M.U.; Dauda, M.; Baba, A.S.; Zango, N.G.; Jeffery, C.; Farate, A.; Adekile, A.; Bates, I. Malaria Infection in Patients with Sickle Cell Disease in Nigeria: Association with Markers of Hyposplenism. Hemoglobin 2024, 48, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mwaiswelo, R.O.; Mawala, W.; Iversen, P.O.; de Montalembert, M.; Luzzatto, L.; Makani, J. Sickle cell disease and malaria: Decreased exposure and asplenia can modulate the risk from Plasmodium falciparum. Malar. J. 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Dowell, S.F.; Nwanyanwu, O.C.; Klugman, K.P.; Kazembe, P.N.; Barat, L.M.; Graf, C.; Bloland, P.B.; Ziba, C.; Huebner, R.E.; et al. Increased carriage of trimethoprim/sulfamethoxazole-resistant Streptococcus pneumoniae in Malawian children after treatment for malaria with sulfadoxine/pyrimethamine. J. Infect. Dis. 2000, 181, 1501–1505. [Google Scholar] [CrossRef]
- Elabbadi, A.; Voiriot, G.; Tristan, A.; Gibelin, A.; Verdet, C.; Djibré, M.; Santin, A.; Jutant, E.-M.; Lopinto, J.; Vandenesch, F.; et al. Lower respiratory tract infection with Staphylococcus aureus in sickle-cell adult patients with severe acute chest syndrome—The STAPHACS Study. Haematologica 2021, 106, 3236–3239. [Google Scholar] [CrossRef]
- Raeispour, M.; Ranjbar, R. Antibiotic resistance, virulence factors and genotyping of Uropathogenic Escherichia coli strains. Antimicrob. Resist. Infect. Control 2018, 7, 118. [Google Scholar] [CrossRef]
- Bunduki, G.K.; Heinz, E.; Phiri, V.S.; Noah, P.; Feasey, N.; Musaya, J. Virulence factors and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from urinary tract infections: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 753. [Google Scholar] [CrossRef] [PubMed]
- Pootong, A.; Mungkornkeaw, N.; Norrapong, B.; Cowawintaweewat, S. Phylogenetic background, drug susceptibility and virulence factors of uropathogenic E. coli isolate in a tertiary university hospital in central Thailand. Trop. Biomed. 2018, 35, 195–204. [Google Scholar] [PubMed]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]

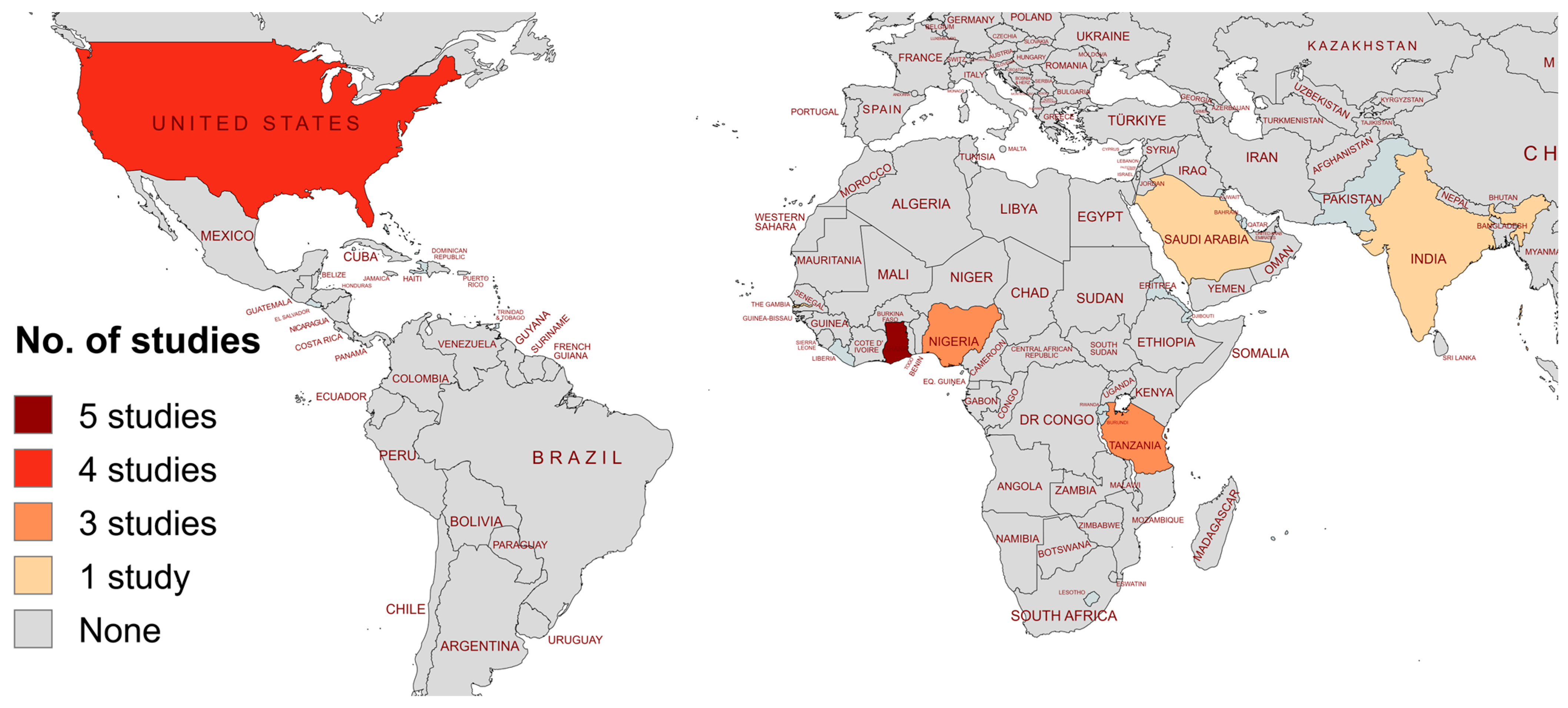

| Authors, Year (Ref) | Country | Study Design | Year of Study | Study Population | Age Category | No. SCD Patients | Isolate Type | Isolate Site | Organism(s) | AST Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdulmanea et al., 2023 [15] | Saudi Arabia | Prospective | 2017–2021 | Sickle cell disease and non-Sickle cell disease patients | All ages | 47 | Infection | Blood | S. aureus | VITEK 2 |
| Donkor et al., 2013 [19] | Ghana | Prospective | 2006–2007 | HbSS+ and HbSS- children | Children | 142 | Carriage | Nasal and Nasopharynx | S. aureus, S. pneumoniae | Disc diffusion |
| Dayie et al., 2022 [20] | Ghana | Prospective | 2016–2017 | Sickle cell disease adults | Adults | 200 | Carriage | Nasopharynx | S. aureus | Disc diffusion |
| Dayie et al., 2021 [21] | Ghana | Prospective | 2016–2017 | Sickle cell disease children | Children | 202 | Carriage | Nasopharynx | S. aureus | Disc diffusion |
| Mava et al., 2012 [22] | Nigeria | Not reported | 2005–2008 | HbSS+ and HbSS- children | Children | 250 | Infection | Urinary tract | E. coli, Proteus spp, Coliforms, Klebsiella spp, S. aureus, Salmonella spp | Disc diffusion |
| Lo et al., 2023 [23] | Nigeria | Not reported | 2014–2018 | HbSS+ and HbSS- children | Children | 192 | Infection | Blood and CSF | S. pneumoniae | Broth microdilution |
| Said et al., 2022 [24] | Tanzania | Prospective | 2021 | HIV, Diabetes Mellitus, and Sickle cell disease children | Children | 404 | Carriage | Rectum | Enterobacteriaceae | Disc diffusion |
| Dibbasey et al., 2023 [14] | Gambia | Retrospective | 2015–2022 | Sickle cell disease patients | All ages | 159 | Infection | Blood | S. pneumoniae, S. aureus | Disc diffusion |
| Steele et al., 1996 [25] | USA | Not reported | 1994–1995 | Sickle cell disease and non-Sickle cell disease patients | Children | 596 | Carriage | Nasopharynx | S. pneumoniae | Disc diffusion |
| Dayie et al., 2018 [26] | Ghana | Prospective | 2016–2017 | Sickle cell disease patients | All ages | 402 | Carriage | Nasopharynx | S. pneumoniae | Disc diffusion |
| Norris et al., 2003 [27] | USA | Prospective | 1993–2001 | Sickle cell disease children | Children | 105 | Infection | Blood | S. pneumoniae | E-test |
| Miller et al., 2005 [28] | USA | Retrospective | 1994–1995 | Sickle cell disease patients | Not reported | 42 | Carriage | Nasopharynx | S. pneumoniae | E-test |
| Appiah et al., 2020 [16] | Ghana | Prospective | 2018 | Sickle cell disease and non-Sickle cell disease patients | Children | 220 | Carriage | Nasal | S. aureus | Disc diffusion |
| Mutagonda et al., 2022 [29] | Tanzania | Prospective | 2021 | Sickle cell disease children | Children | 204 | Carriage | Nasopharynx | S. pneumoniae, S. aureus | Disc diffusion |
| Subudhi et al., 2021 [30] | India | Prospective | 2019–2020 | Sickle cell anemia patients at the MICU | Adults | 190 | Infection | Urinary tract | S. aureus, S. pneumoniae, E. coli, K. pneumoniae, P. aeruginosa, A. baumannii | Disc diffusion |
| Brown et al., 2003 [31] | Nigeria | Prospective | 1999–2000 | Sickle cell disease and non-Sickle cell disease patients | Children | 342 | Infection | Urinary tract | E. coli, K. pneumoniae, Salmonellae spp., S. aureus | Disc diffusion |
| Daw et al., 1997 [32] | USA | Not reported | 1994–1995 | Sickle cell disease children | Children | 312 | Carriage | Nasopharynx | S. pneumoniae | E-test |
| Sangeda et al., 2024 [33] | Tanzania | Prospective | 2015 | Sickle cell disease children | Children | 250 | Infection | Urinary tract | E. coli, Staphylococcus spp., Klebsiella spp., Proteus spp., Pseudomonas spp. | Disc diffusion |
| Organism | Antibiotic Class | Antibiotic | No. Studies | Pooled Resistance (%) [95% CI] | Heterogeneity I2 (%), p-Value |
|---|---|---|---|---|---|
| S. aureus (Infection) | Cephalosporins | Cefuroxime | 2 | 34.82 [2.51; 76.56] | 61.4, p = 0.1074 |
| Fluoroquinolones | Ciprofloxacin | 3 | 16.10 [7.03; 27.31] | 0, p = 0.8855 | |
| Penicillins | Penicillin | 2 | 99.99 [94.87; 100.00] | 0, p = 0.8184 | |
| Ampicillin | 3 | 98.15 [49.83; 100.00] | 80.4, p = 0.0060 | ||
| Amoxicillin | 3 | 77.82 [61.93; 91.16] | 0, p = 0.8369 | ||
| Aminoglycosides | Gentamicin | 3 | 42.48 [3.02; 87.98] | 50.7, p = 0.1313 | |
| Macrolides | Erythromycin | 2 | 53.94 [15.57; 89.98] | 80.4, p = 0.0241 | |
| Sulfonamides | Co-trimoxazole | 3 | 24.78 [0.00; 72.08] | 81.3, p = 0.0011 | |
| S. aureus (Colonization) | Fluoroquinolones | Ciprofloxacin | 3 | 15.57 [7.50; 25.78] | 80.5, p = 0.0059 |
| Penicillins | Penicillin | 5 | 90.47 [57.19; 100.00] | 98.3, p < 0.0001 | |
| Aminoglycosides | Gentamicin | 4 | 16.89 [8.76; 26.89] | 80.3, p = 0.0016 | |
| Lincosamides | Clindamycin | 3 | 11.02 [2.08; 25.13] | 90.5, p < 0.0001 | |
| Macrolides | Erythromycin | 5 | 30.25 [10.92; 53.98] | 95.4, p < 0.0001 | |
| Tetracyclines | Tetracycline | 3 | 30.93 [16.01; 48.17] | 89.7, p < 0.0001 | |
| Sulfonamides | Co-trimoxazole | 3 | 31.61 [14.47; 51.62] | 77.6, p = 0.0114 | |
| S. pneumoniae (Infection) | Penicillins | Penicillin | 3 | 46.61 [24.58; 69.21] | 52.6, p = 0.1214 |
| Macrolides | Erythromycin | 2 | 6.50 [0.00; 49.50] | 62.7, p = 0.1018 | |
| Sulfonamides | Co-trimoxazole | 3 | 74.26 [7.14; 100.00] | 95.5, p < 0.0001 | |
| S. pneumoniae (Colonization) | Penicillins | Penicillin | 5 | 47.27 [36.13; 58.55] | 66.6, p = 0.0175 |
| Macrolides | Erythromycin | 3 | 18.75 [6.01; 35.48] | 54.1, p = 0.1133 | |
| Sulfonamides | Co-trimoxazole | 3 | 84.99 [70.32; 95.75] | 48.1, p = 0.1455 | |
| E. coli (Infection) | Cephalosporins | Cefuroxime | 3 | 30.43 [11.52; 52.5] | 30, p = 0.24 |
| Ceftriaxone | 4 | 24.89 [5.94; 49.18] | 66, p = 0.03 | ||
| Sulfonamides | Co-trimoxazole | 4 | 93.17 [72.10; 100] | 70, p = 0.02 |
| S. aureus | Antibiotic Class | Antibiotic | No. Studies | OR [95% CI] | Heterogeneity I2 (%), p-Value |
|---|---|---|---|---|---|
| Infection | Penicillins | Penicillin | 1 | 0.94 [0.04; 24.22] | Not applicable |
| Ampicillin | 3 | 0.20 [0.02; 2.39] | Not applicable | ||
| Amoxicillin | 3 | 1.47 [0.04; 52.77] | 84.9, p = 0.0102 | ||
| Macrolides | Erythromycin | 2 | 2.30 [0.73; 7.18] | 0, p = 0.8686 | |
| Sulfonamides | Co-trimoxazole | 3 | 0.70 [0.16; 2.99] | 0, p = 0.9647 | |
| Colonization | Penicillins | Penicillin | 2 | 7.62 [0.37; 155.87] | Not applicable |
| Ampicillin | 1 | 7.62 [0.37; 155.87] | Not applicable | ||
| Macrolides | Erythromycin | 2 | 2.64 [0.87; 8.02] | 0, p = 0.5066 | |
| Sulfonamides | Co-trimoxazole | 2 | 0.60 [0.12; 2.97] | 56.4, p = 0.1301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opoku-Asare, B.; Ntim, O.K.; Awere-Duodu, A.; Donkor, E.S. Sickle Cell Disease and Antimicrobial Resistance: A Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2025, 17, 32. https://doi.org/10.3390/idr17020032
Opoku-Asare B, Ntim OK, Awere-Duodu A, Donkor ES. Sickle Cell Disease and Antimicrobial Resistance: A Systematic Review and Meta-Analysis. Infectious Disease Reports. 2025; 17(2):32. https://doi.org/10.3390/idr17020032
Chicago/Turabian StyleOpoku-Asare, Bismark, Onyansaniba K. Ntim, Aaron Awere-Duodu, and Eric S. Donkor. 2025. "Sickle Cell Disease and Antimicrobial Resistance: A Systematic Review and Meta-Analysis" Infectious Disease Reports 17, no. 2: 32. https://doi.org/10.3390/idr17020032
APA StyleOpoku-Asare, B., Ntim, O. K., Awere-Duodu, A., & Donkor, E. S. (2025). Sickle Cell Disease and Antimicrobial Resistance: A Systematic Review and Meta-Analysis. Infectious Disease Reports, 17(2), 32. https://doi.org/10.3390/idr17020032






