Immune Alterations and Viral Reservoir Atlas in SIV-Infected Chinese Rhesus Macaques
Abstract
1. Non-Human Primates and SIV Infections
2. Viral Dynamics and Persistence Despite ART
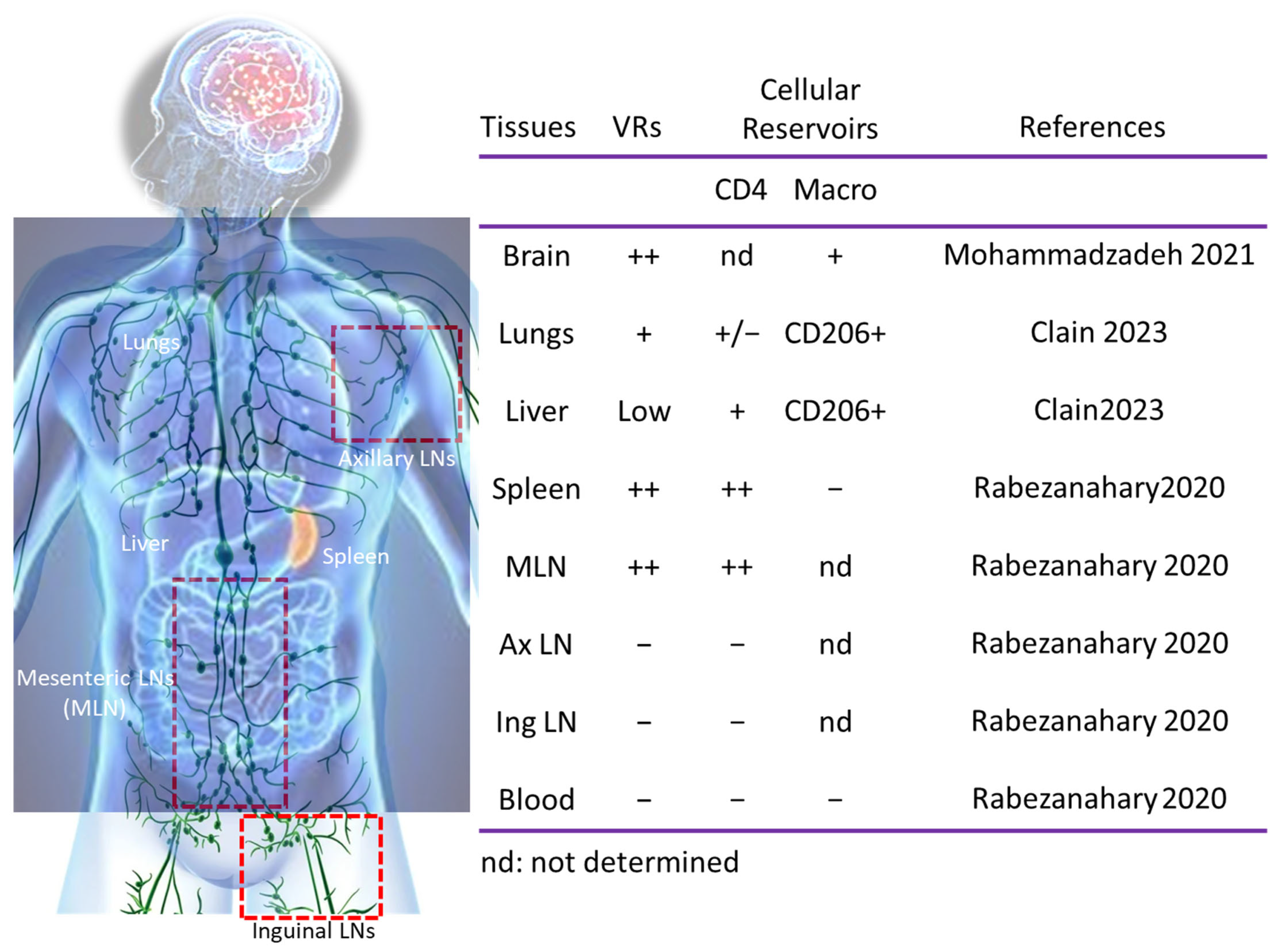
3. Myeloid Cells and VRs
4. CD4 T Cell Subsets and VRs
5. Viral Dissemination After ART Interruption
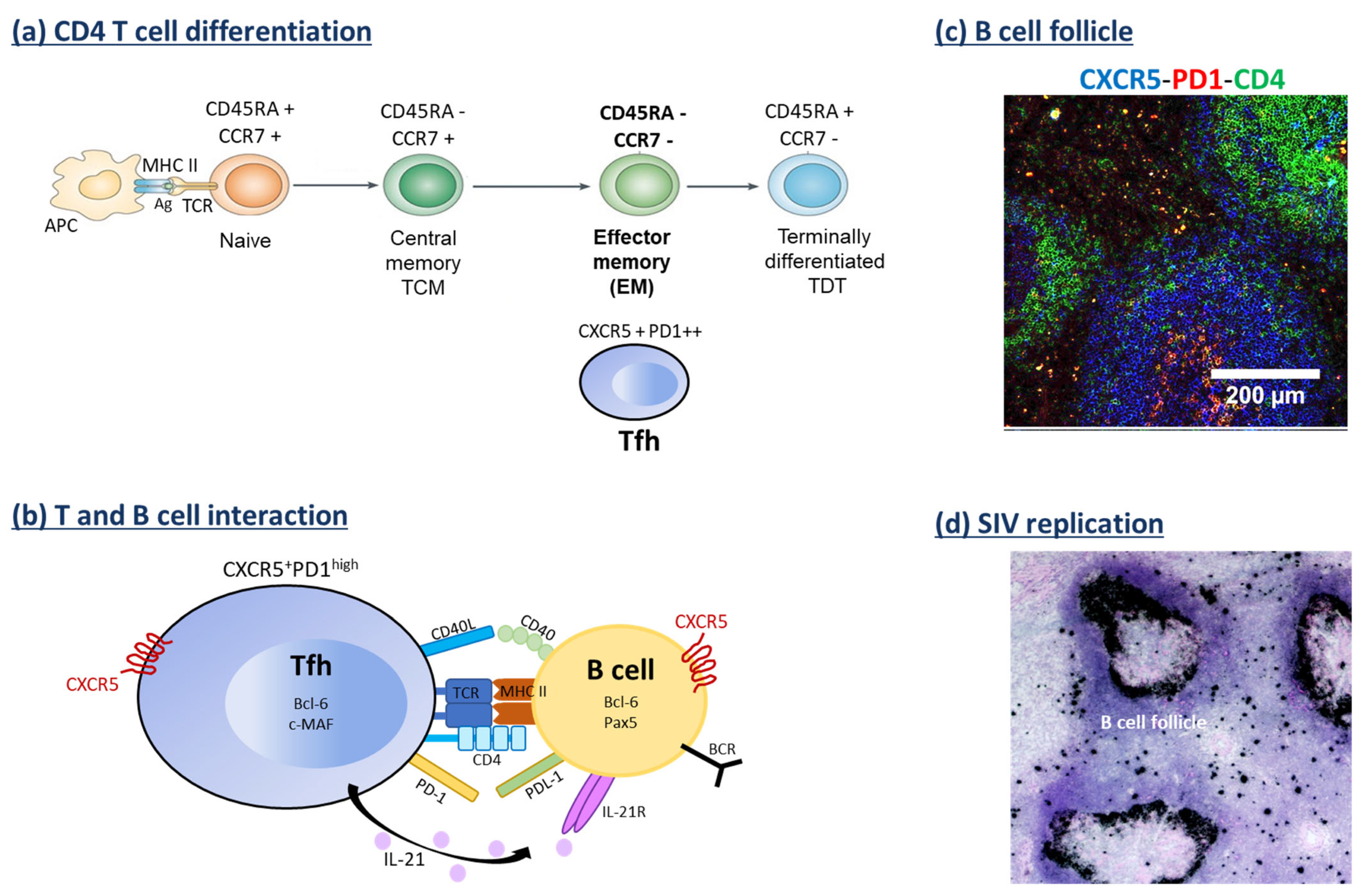
6. B Cell Response and VRs
7. Apoptosis, Caspases and Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peeters, M.; Honoré, C.; Huet, T.; Bedjabaga, L.; Ossari, S.; Bussi, P.; Cooper, R.W.; Delaporte, E. Isolation and partial characterization of an HIV-related virus occurring naturally in chimpanzees in Gabon. Aids 1989, 3, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Kanki, P.J.; Alroy, J.; Essex, M. Isolation of T-lymphotropic retrovirus related to HTLV-III/LAV from wild-caught African green monkeys. Science 1985, 230, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, M.; Miura, T.; Hasegawa, A.; Morikawa, S.; Tsujimoto, H.; Miki, K.; Kitamura, T.; Hayami, M. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature 1988, 333, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.M.; Olmsted, R.A.; Murphey-Corb, M.; Purcell, R.H.; Johnson, P.R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 1989, 339, 389–392. [Google Scholar] [CrossRef]
- Estaquier, J.; Peeters, M.; Bedjabaga, L.; Honoré, C.; Bussi, P.; Dixson, A.; Delaporte, E. Prevalence and transmission of simian immunodeficiency virus and simian T-cell leukemia virus in a semi-free-range breeding colony of mandrills in Gabon. Aids 1991, 5, 1385–1386. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Hasegawa, A.; Maki, N.; Fukasawa, M.; Miura, T.; Speidel, S.; Cooper, R.W.; Moriyama, E.N.; Gojobori, T.; Hayami, M. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 1989, 341, 539–541. [Google Scholar] [CrossRef]
- Van Heuverswyn, F.; Li, Y.; Neel, C.; Bailes, E.; Keele, B.F.; Liu, W.; Loul, S.; Butel, C.; Liegeois, F.; Bienvenue, Y.; et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 2006, 444, 164. [Google Scholar] [CrossRef]
- Estaquier, J.; Idziorek, T.; de Bels, F.; Barre-Sinoussi, F.; Hurtrel, B.; Aubertin, A.M.; Venet, A.; Mehtali, M.; Muchmore, E.; Michel, P.; et al. Programmed cell death and AIDS: Significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 1994, 91, 9431–9435. [Google Scholar] [CrossRef]
- Apetrei, C.; Robertson, D.L.; Marx, P.A. The history of SIVS and AIDS: Epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front. Biosci. 2004, 9, 225–254. [Google Scholar] [CrossRef]
- Reimann, K.A.; Parker, R.A.; Seaman, M.S.; Beaudry, K.; Beddall, M.; Peterson, L.; Williams, K.C.; Veazey, R.S.; Montefiori, D.C.; Mascola, J.R.; et al. Pathogenicity of simian-human immunodeficiency virus SHIV-89.6P and SIVmac is attenuated in cynomolgus macaques and associated with early T-lymphocyte responses. J. Virol. 2005, 79, 8878–8885. [Google Scholar] [CrossRef]
- Ling, B.; Veazey, R.S.; Luckay, A.; Penedo, C.; Xu, K.; Lifson, J.D.; Marx, P.A. SIVmac pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans. AIDS 2002, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.C.; Penedo, M.C.; Lanigan, C.; Hall, D.; Watry, D.D.; Zandonatti, M.; Fox, H.S. Simian immunodeficiency virus-induced CD4+ T cell deficits in cytokine secretion profile are dependent on monkey origin. Viral Immunol. 2006, 19, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Monceaux, V.; Viollet, L.; Petit, F.; Cumont, M.C.; Kaufmann, G.R.; Aubertin, A.M.; Hurtrel, B.; Silvestri, G.; Estaquier, J. CD4+ CCR5+ T-cell dynamics during simian immunodeficiency virus infection of Chinese rhesus macaques. J. Virol. 2007, 81, 13865–13875. [Google Scholar] [CrossRef] [PubMed]
- Elbim, C.; Monceaux, V.; Mueller, Y.M.; Lewis, M.G.; Francois, S.; Diop, O.; Akarid, K.; Hurtrel, B.; Gougerot-Pocidalo, M.A.; Levy, Y.; et al. Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Immunol. 2008, 181, 8613–8623. [Google Scholar] [CrossRef]
- Campillo-Gimenez, L.; Laforge, M.; Fay, M.; Brussel, A.; Cumont, M.C.; Monceaux, V.; Diop, O.; Levy, Y.; Hurtrel, B.; Zaunders, J.; et al. Nonpathogenesis of simian immunodeficiency virus infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J. Virol. 2010, 84, 1838–1846. [Google Scholar] [CrossRef]
- Cumont, M.C.; Diop, O.; Vaslin, B.; Elbim, C.; Viollet, L.; Monceaux, V.; Lay, S.; Silvestri, G.; Le Grand, R.; Muller-Trutwin, M.; et al. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Virol. 2008, 82, 1175–1184. [Google Scholar] [CrossRef]
- Ostrowski, M.A.; Justement, S.J.; Catanzaro, A.; Hallahan, C.A.; Ehler, L.A.; Mizell, S.B.; Kumar, P.N.; Mican, J.A.; Chun, T.W.; Fauci, A.S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 1998, 161, 3195–3201. [Google Scholar] [CrossRef]
- Reynes, J.; Baillat, V.; Portales, P.; Clot, J.; Corbeau, P. Relationship between CCR5 density and viral load after discontinuation of antiretroviral therapy. Jama 2004, 291, 46. [Google Scholar] [CrossRef]
- Reynes, J.; Portales, P.; Segondy, M.; Baillat, V.; Andre, P.; Reant, B.; Avinens, O.; Couderc, G.; Benkirane, M.; Clot, J.; et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J. Infect. Dis. 2000, 181, 927–932. [Google Scholar] [CrossRef]
- Deeks, S.G.; Kitchen, C.M.; Liu, L.; Guo, H.; Gascon, R.; Narvaez, A.B.; Hunt, P.; Martin, J.N.; Kahn, J.O.; Levy, J.; et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004, 104, 942–947. [Google Scholar] [CrossRef]
- Picker, L.J.; Hagen, S.I.; Lum, R.; Reed-Inderbitzin, E.F.; Daly, L.M.; Sylwester, A.W.; Walker, J.M.; Siess, D.C.; Piatak, M., Jr.; Wang, C.; et al. Insufficient Production and Tissue Delivery of CD4+ Memory T Cells in Rapidly Progressive Simian Immunodeficiency Virus Infection. J. Exp. Med. 2004, 200, 1299–1314. [Google Scholar] [CrossRef] [PubMed]
- Pandrea, I.; Apetrei, C.; Gordon, S.; Barbercheck, J.; Dufour, J.; Bohm, R.; Sumpter, B.; Roques, P.; Marx, P.A.; Hirsch, V.M.; et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood 2007, 109, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Paiardini, M.; Cervasi, B.; Reyes-Aviles, E.; Micci, L.; Ortiz, A.M.; Chahroudi, A.; Vinton, C.; Gordon, S.N.; Bosinger, S.E.; Francella, N.; et al. Low levels of SIV infection in sooty mangabey central memory CD⁴⁺ T cells are associated with limited CCR5 expression. Nat. Med. 2011, 17, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Viollet, L.; Monceaux, V.; Petit, F.; Ho Tsong Fang, R.; Cumont, M.C.; Hurtrel, B.; Estaquier, J. Death of CD4+ T cells from lymph nodes during primary SIVmac251 infection predicts the rate of AIDS progression. J. Immunol. 2006, 177, 6685–6694. [Google Scholar] [CrossRef] [PubMed]
- Klatt, N.R.; Canary, L.A.; Vanderford, T.H.; Vinton, C.L.; Engram, J.C.; Dunham, R.M.; Cronise, H.E.; Swerczek, J.M.; Lafont, B.A.; Picker, L.J.; et al. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J. Virol. 2012, 86, 1203–1213. [Google Scholar] [CrossRef]
- Hirsch, V.M.; Santra, S.; Goldstein, S.; Plishka, R.; Buckler-White, A.; Seth, A.; Ourmanov, I.; Brown, C.R.; Engle, R.; Montefiori, D.; et al. Immune failure in the absence of profound CD4+ T-lymphocyte depletion in simian immunodeficiency virus-infected rapid progressor macaques. J. Virol. 2004, 78, 275–284. [Google Scholar] [CrossRef]
- Clements, J.E.; Mankowski, J.L.; Gama, L.; Zink, M.C. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: From mechanism to treatment. J. Neurovirology 2008, 14, 309–317. [Google Scholar] [CrossRef]
- Xu, Y.; Weatherall, C.; Bailey, M.; Alcantara, S.; De Rose, R.; Estaquier, J.; Wilson, K.; Suzuki, K.; Corbeil, J.; Cooper, D.A.; et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J. Virol. 2013, 87, 3760–3773. [Google Scholar] [CrossRef]
- Jasinska, A.J.; Apetrei, C.; Pandrea, I. Walk on the wild side: SIV infection in African non-human primate hosts-from the field to the laboratory. Front. Immunol. 2022, 13, 1060985. [Google Scholar] [CrossRef]
- Allen, T.M.; Mothé, B.R.; Sidney, J.; Jing, P.; Dzuris, J.L.; Liebl, M.E.; Vogel, T.U.; O’Connor, D.H.; Wang, X.; Wussow, M.C.; et al. CD8+ lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: Implications for vaccine design and testing. J. Virol. 2001, 75, 738–749. [Google Scholar] [CrossRef]
- Loffredo, J.T.; Sidney, J.; Piaskowski, S.; Szymanski, A.; Furlott, J.; Rudersdorf, R.; Reed, J.; Peters, B.; Hickman-Miller, H.D.; Bardet, W.; et al. The high frequency Indian rhesus macaque MHC class I molecule, Mamu-B*01, does not appear to be involved in CD8+ T lymphocyte responses to SIVmac239. J. Immunol. 2005, 175, 5986–5997. [Google Scholar] [CrossRef] [PubMed]
- Mothé, B.R.; Weinfurter, J.; Wang, C.; Rehrauer, W.; Wilson, N.; Allen, T.M.; Allison, D.B.; Watkins, D.I. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 2003, 77, 2736–2740. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, J.T.; Sidney, J.; Wojewoda, C.; Dodds, E.; Reynolds, M.R.; Napoé, G.; Mothé, B.R.; O’Connor, D.H.; Wilson, N.A.; Watkins, D.I.; et al. Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J. Immunol. 2004, 173, 5064–5076. [Google Scholar] [CrossRef] [PubMed]
- Budde, M.L.; Wiseman, R.W.; Karl, J.A.; Hanczaruk, B.; Simen, B.B.; O’Connor, D.H. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics 2010, 62, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.A.; Graham, M.E.; Wiseman, R.W.; Heimbruch, K.E.; Gieger, S.M.; Doxiadis, G.G.; Bontrop, R.E.; O’Connor, D.H. Major histocompatibility complex haplotyping and long-amplicon allele discovery in cynomolgus macaques from Chinese breeding facilities. Immunogenetics 2017, 69, 211–229. [Google Scholar] [CrossRef]
- Karl, J.A.; Wiseman, R.W.; Campbell, K.J.; Blasky, A.J.; Hughes, A.L.; Ferguson, B.; Read, D.S.; O’Connor, D.H. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics 2008, 60, 37–46. [Google Scholar] [CrossRef]
- Solomon, C.; Southwood, S.; Hoof, I.; Rudersdorf, R.; Peters, B.; Sidney, J.; Pinilla, C.; Marcondes, M.C.; Ling, B.; Marx, P.; et al. The most common Chinese rhesus macaque MHC class I molecule shares peptide binding repertoire with the HLA-B7 supertype. Immunogenetics 2010, 62, 451–464. [Google Scholar] [CrossRef][Green Version]
- Otting, N.; Heijmans, C.M.C.; Noort, R.C.; de Groot, N.G.; Doxiadis, G.G.M.; van Rood, J.J.; Watkins, D.I.; Bontrop, R.E. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc. Natl. Acad. Sci. USA 2005, 102, 1626–1631. [Google Scholar] [CrossRef]
- Otting, N.; de Vos-Rouweler, A.J.; Heijmans, C.M.; de Groot, N.G.; Doxiadis, G.G.; Bontrop, R.E. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics 2007, 59, 367–375. [Google Scholar] [CrossRef]
- Kaizu, M.; Borchardt, G.J.; Glidden, C.E.; Fisk, D.L.; Loffredo, J.T.; Watkins, D.I.; Rehrauer, W.M. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics 2007, 59, 693–703. [Google Scholar] [CrossRef]
- Southwood, S.; Solomon, C.; Hoof, I.; Rudersdorf, R.; Sidney, J.; Peters, B.; Wahl, A.; Hawkins, O.; Hildebrand, W.; Mothe, B.R.; et al. Functional analysis of frequently expressed Chinese rhesus macaque MHC class I molecules Mamu-A1*02601 and Mamu-B*08301 reveals HLA-A2 and HLA-A3 supertypic specificities. Immunogenetics 2011, 63, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Sidney, J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 1999, 50, 201–212. [Google Scholar] [CrossRef] [PubMed]
- de Groot, N.G.; Heijmans, C.M.; Koopman, G.; Verschoor, E.J.; Bogers, W.M.; Bontrop, R.E. TRIM5 allelic polymorphism in macaque species/populations of different geographic origins: Its impact on SIV vaccine studies. Tissue Antigens 2011, 78, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, J.T.; Sidney, J.; Bean, A.T.; Beal, D.R.; Bardet, W.; Wahl, A.; Hawkins, O.E.; Piaskowski, S.; Wilson, N.A.; Hildebrand, W.H.; et al. Two MHC class I molecules associated with elite control of immunodeficiency virus replication, Mamu-B*08 and HLA-B*2705, bind peptides with sequence similarity. J. Immunol. 2009, 182, 7763–7775. [Google Scholar] [CrossRef]
- Loffredo, J.T.; Maxwell, J.; Qi, Y.; Glidden, C.E.; Borchardt, G.J.; Soma, T.; Bean, A.T.; Beal, D.R.; Wilson, N.A.; Rehrauer, W.M.; et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J. Virol. 2007, 81, 8827–8832. [Google Scholar] [CrossRef]
- O’Connor, D.H.; Mothe, B.R.; Weinfurter, J.T.; Fuenger, S.; Rehrauer, W.M.; Jing, P.; Rudersdorf, R.R.; Liebl, M.E.; Krebs, K.; Vasquez, J.; et al. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 2003, 77, 9029–9040. [Google Scholar] [CrossRef]
- Yant, L.J.; Friedrich, T.C.; Johnson, R.C.; May, G.E.; Maness, N.J.; Enz, A.M.; Lifson, J.D.; O’Connor, D.H.; Carrington, M.; Watkins, D.I. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 2006, 80, 5074–5077. [Google Scholar] [CrossRef]
- McLaren, P.J.; Coulonges, C.; Ripke, S.; van den Berg, L.; Buchbinder, S.; Carrington, M.; Cossarizza, A.; Dalmau, J.; Deeks, S.G.; Delaneau, O.; et al. Association study of common genetic variants and HIV-1 acquisition in 6,300 infected cases and 7,200 controls. PLoS Pathog. 2013, 9, e1003515. [Google Scholar] [CrossRef]
- Hendel, H.; Caillat-Zucman, S.; Lebuanec, H.; Carrington, M.; O’Brien, S.; Andrieu, J.M.; Schächter, F.; Zagury, D.; Rappaport, J.; Winkler, C.; et al. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J. Immunol. 1999, 162, 6942–6946. [Google Scholar] [CrossRef]
- Marcilla, M.; Alvarez, I.; Ramos-Fernández, A.; Lombardía, M.; Paradela, A.; Albar, J.P. Comparative Analysis of the Endogenous Peptidomes Displayed by HLA-B*27 and Mamu-B*08: Two MHC Class I Alleles Associated with Elite Control of HIV/SIV Infection. J. Proteome Res. 2016, 15, 1059–1069. [Google Scholar] [CrossRef]
- Muhl, T.; Krawczak, M.; Ten Haaft, P.; Hunsmann, G.; Sauermann, U. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 2002, 169, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef] [PubMed]
- Migueles, S.A.; Laborico, A.C.; Imamichi, H.; Shupert, W.L.; Royce, C.; McLaughlin, M.; Ehler, L.; Metcalf, J.; Liu, S.; Hallahan, C.W.; et al. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. J. Virol. 2003, 77, 6889–6898. [Google Scholar] [CrossRef]
- Miura, T.; Brockman, M.A.; Schneidewind, A.; Lobritz, M.; Pereyra, F.; Rathod, A.; Block, B.L.; Brumme, Z.L.; Brumme, C.J.; Baker, B.; et al. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J. Virol. 2009, 83, 2743–2755. [Google Scholar] [CrossRef]
- Budde, M.L.; Greene, J.M.; Chin, E.N.; Ericsen, A.J.; Scarlotta, M.; Cain, B.T.; Pham, N.H.; Becker, E.A.; Harris, M.; Weinfurter, J.T.; et al. Specific CD8+ T cell responses correlate with control of simian immunodeficiency virus replication in Mauritian cynomolgus macaques. J. Virol. 2012, 86, 7596–7604. [Google Scholar] [CrossRef]
- Mee, E.T.; Berry, N.; Ham, C.; Sauermann, U.; Maggiorella, M.T.; Martinon, F.; Verschoor, E.J.; Heeney, J.L.; Le Grand, R.; Titti, F.; et al. Mhc haplotype H6 is associated with sustained control of SIVmac251 infection in Mauritian cynomolgus macaques. Immunogenetics 2009, 61, 327–339. [Google Scholar] [CrossRef]
- Bruel, T.; Hamimi, C.; Dereuddre-Bosquet, N.; Cosma, A.; Shin, S.Y.; Corneau, A.; Versmisse, P.; Karlsson, I.; Malleret, B.; Targat, B.; et al. Long-term control of simian immunodeficiency virus (SIV) in cynomolgus macaques not associated with efficient SIV-specific CD8+ T-cell responses. J. Virol. 2015, 89, 3542–3556. [Google Scholar] [CrossRef]
- Lim, S.Y.; Chan, T.; Gelman, R.S.; Whitney, J.B.; O’Brien, K.L.; Barouch, D.H.; Goldstein, D.B.; Haynes, B.F.; Letvin, N.L. Contributions of Mamu-A*01 status and TRIM5 allele expression, but not CCL3L copy number variation, to the control of SIVmac251 replication in Indian-origin rhesus monkeys. PLoS Genet. 2010, 6, e1000997. [Google Scholar] [CrossRef]
- Fenizia, C.; Keele, B.F.; Nichols, D.; Cornara, S.; Binello, N.; Vaccari, M.; Pegu, P.; Robert-Guroff, M.; Ma, Z.M.; Miller, C.J.; et al. TRIM5α does not affect simian immunodeficiency virus SIV(mac251) replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J. Virol. 2011, 85, 12399–12409. [Google Scholar] [CrossRef]
- Mellors, J.W.; Rinaldo, C.R., Jr.; Gupta, P.; White, R.M.; Todd, J.A.; Kingsley, L.A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996, 272, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Lifson, J.D.; Nowak, M.A.; Goldstein, S.; Rossio, J.L.; Kinter, A.; Vasquez, G.; Wiltrout, T.A.; Brown, C.; Schneider, D.; Wahl, L.; et al. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 1997, 71, 9508–9514. [Google Scholar] [CrossRef]
- Pantaleo, G.; Graziosi, C.; Demarest, J.F.; Butini, L.; Montroni, M.; Fox, C.H.; Orenstein, J.M.; Kotler, D.P.; Fauci, A.S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993, 362, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, R.C.; Hansen-Moosa, A.; Mori, K.; Bouvier, D.P.; King, N.W.; Daniel, M.D.; Ringler, D.J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am. J. Pathol. 1991, 139, 29–35. [Google Scholar]
- Xu, L.; Pegu, A.; Rao, E.; Doria-Rose, N.; Beninga, J.; McKee, K.; Lord, D.M.; Wei, R.R.; Deng, G.; Louder, M.; et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 2017, 358, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kestler, H.W., 3rd; Ringler, D.J.; Mori, K.; Panicali, D.L.; Sehgal, P.K.; Daniel, M.D.; Desrosiers, R.C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 1991, 65, 651–662. [Google Scholar] [CrossRef]
- Baba, T.W.; Jeong, Y.S.; Pennick, D.; Bronson, R.; Greene, M.F.; Ruprecht, R.M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 1995, 267, 1820–1825. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Vlasak, J.; Williams, A.L.; Chenine, A.L.; McClure, H.M.; Anderson, D.C.; O’Neil, S.; Ruprecht, R.M. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. Aids 2003, 17, 157–166. [Google Scholar] [CrossRef]
- Ho Tsong Fang, R.; Khatissian, E.; Monceaux, V.; Cumont, M.C.; Beq, S.; Ameisen, J.C.; Aubertin, A.M.; Israël, N.; Estaquier, J.; Hurtrel, B. Disease progression in macaques with low SIV replication levels: On the relevance of TREC counts. Aids 2005, 19, 663–673. [Google Scholar] [CrossRef]
- Chun, T.W.; Moir, S.; Fauci, A.S. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat. Immunol. 2015, 16, 584–589. [Google Scholar] [CrossRef]
- Chun, T.W.; Davey, R.T., Jr.; Engel, D.; Lane, H.C.; Fauci, A.S. Re-emergence of HIV after stopping therapy. Nature 1999, 401, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.T., Jr.; Bhat, N.; Yoder, C.; Chun, T.W.; Metcalf, J.A.; Dewar, R.; Natarajan, V.; Lempicki, R.A.; Adelsberger, J.W.; Miller, K.D.; et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 1999, 96, 15109–15114. [Google Scholar] [CrossRef] [PubMed]
- Rothenberger, M.K.; Keele, B.F.; Wietgrefe, S.W.; Fletcher, C.V.; Beilman, G.J.; Chipman, J.G.; Khoruts, A.; Estes, J.D.; Anderson, J.; Callisto, S.P.; et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc. Natl. Acad. Sci. USA 2015, 112, E1126–E1134. [Google Scholar] [CrossRef] [PubMed]
- Henrich, T.J.; Hatano, H.; Bacon, O.; Hogan, L.E.; Rutishauser, R.; Hill, A.; Kearney, M.F.; Anderson, E.M.; Buchbinder, S.P.; Cohen, S.E.; et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017, 14, e1002417. [Google Scholar] [CrossRef]
- Colby, D.J.; Trautmann, L.; Pinyakorn, S.; Leyre, L.; Pagliuzza, A.; Kroon, E.; Rolland, M.; Takata, H.; Buranapraditkun, S.; Intasan, J.; et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med. 2018, 24, 923–926. [Google Scholar] [CrossRef]
- Whitney, J.B.; Hill, A.L.; Sanisetty, S.; Penaloza-MacMaster, P.; Liu, J.; Shetty, M.; Parenteau, L.; Cabral, C.; Shields, J.; Blackmore, S.; et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014, 512, 74–77. [Google Scholar] [CrossRef]
- Okoye, A.A.; Hansen, S.G.; Vaidya, M.; Fukazawa, Y.; Park, H.; Duell, D.M.; Lum, R.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 2018, 24, 1430–1440. [Google Scholar] [CrossRef]
- Bender, A.M.; Simonetti, F.R.; Kumar, M.R.; Fray, E.J.; Bruner, K.M.; Timmons, A.E.; Tai, K.Y.; Jenike, K.M.; Antar, A.A.R.; Liu, P.T.; et al. The Landscape of Persistent Viral Genomes in ART-Treated SIV, SHIV, and HIV-2 Infections. Cell Host Microbe 2019, 26, 73–85 e74. [Google Scholar] [CrossRef]
- Keele, B.F.; Okoye, A.A.; Fennessey, C.M.; Varco-Merth, B.; Immonen, T.T.; Kose, E.; Conchas, A.; Pinkevych, M.; Lipkey, L.; Newman, L.; et al. Early antiretroviral therapy in SIV-infected rhesus macaques reveals a multiphasic, saturable dynamic accumulation of the rebound competent viral reservoir. PLoS Pathog. 2024, 20, e1012135. [Google Scholar] [CrossRef]
- Rabezanahary, H.; Moukambi, F.; Palesch, D.; Clain, J.; Racine, G.; Andreani, G.; Benmadid-Laktout, G.; Zghidi-Abouzid, O.; Soundaramourty, C.; Tremblay, C.; et al. Despite early antiretroviral therapy effector memory and follicular helper CD4 T cells are major reservoirs in visceral lymphoid tissues of SIV-infected macaques. Mucosal Immunol. 2020, 13, 149–160. [Google Scholar] [CrossRef]
- Rabezanahary, H.; Clain, J.; Racine, G.; Andreani, G.; Benmadid-Laktout, G.; Borde, C.; Mammano, F.; Mesplèdes, T.; Ancuta, P.; Zghidi-Abouzid, O.; et al. Early Antiretroviral Therapy Prevents Viral Infection of Monocytes and Inflammation in Simian Immunodeficiency Virus-Infected Rhesus Macaques. J. Virol. 2020, 94, e01478-20. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Roda, W.; Branton, W.G.; Clain, J.; Rabezanahary, H.; Zghidi-Abouzid, O.; Gelman, B.B.; Angel, J.B.; Cohen, E.A.; Gill, M.J.; et al. Lentiviral Infections Persist in Brain despite Effective Antiretroviral Therapy and Neuroimmune Activation. mBio 2021, 12, e02784-21. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.A.; Rabezanahary, H.; Racine, G.; Boutrais, S.; Soundaramourty, C.; Joly Beauparlant, C.; Jenabian, M.A.; Droit, A.; Ancuta, P.; Zghidi-Abouzid, O.; et al. Early ART reduces viral seeding and innate immunity in liver and lungs of SIV-infected macaques. JCI Insight 2023, 8, e167856. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.A.; Boutrais, S.; Dewatines, J.; Racine, G.; Rabezanahary, H.; Droit, A.; Zghidi-Abouzid, O.; Estaquier, J. Lipid metabolic reprogramming of hepatic CD4+ T cells during SIV infection. Microbiol. Spectr. 2023, 11, e01687-23. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.B.; Silva, I.T.; Oliveira, T.Y.; Rosales, R.A.; Parrish, E.H.; Learn, G.H.; Hahn, B.H.; Czartoski, J.L.; McElrath, M.J.; Lehmann, C.; et al. HIV-1 integration landscape during latent and active infection. Cell 2015, 160, 420–432. [Google Scholar] [CrossRef]
- Bruner, K.M.; Murray, A.J.; Pollack, R.A.; Soliman, M.G.; Laskey, S.B.; Capoferri, A.A.; Lai, J.; Strain, M.C.; Lada, S.M.; Hoh, R.; et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016, 22, 1043–1049. [Google Scholar] [CrossRef]
- Pollack, R.A.; Jones, R.B.; Pertea, M.; Bruner, K.M.; Martin, A.R.; Thomas, A.S.; Capoferri, A.A.; Beg, S.A.; Huang, S.H.; Karandish, S.; et al. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe 2017, 21, 494–506.e494. [Google Scholar] [CrossRef]
- Imamichi, H.; Dewar, R.L.; Adelsberger, J.W.; Rehm, C.A.; O’Doherty, U.; Paxinos, E.E.; Fauci, A.S.; Lane, H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 8783–8788. [Google Scholar] [CrossRef]
- Kuniholm, J.; Armstrong, E.; Bernabe, B.; Coote, C.; Berenson, A.; Patalano, S.D.; Olson, A.; He, X.; Lin, N.H.; Fuxman Bass, J.I.; et al. Intragenic proviral elements support transcription of defective HIV-1 proviruses. PLoS Pathog. 2021, 17, e1009982. [Google Scholar] [CrossRef]
- Kuniholm, J.; Coote, C.; Henderson, A.J. Defective HIV-1 genomes and their potential impact on HIV pathogenesis. Retrovirology 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Fray, E.J.; Wu, F.; Simonetti, F.R.; Zitzmann, C.; Sambaturu, N.; Molina-Paris, C.; Bender, A.M.; Liu, P.T.; Ventura, J.D.; Wiseman, R.W.; et al. Antiretroviral therapy reveals triphasic decay of intact SIV genomes and persistence of ancestral variants. Cell Host Microbe 2023, 31, 356–372.e355. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, P.J.; Rogers, K.A.; Zurla, C.; Blanchard, E.L.; Gumber, S.; Strait, K.; Connor-Stroud, F.; Schuster, D.M.; Amancha, P.K.; Hong, J.J.; et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat. Methods 2015, 12, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, E.K.; Spicer, L.; Smith, S.A.; Lee, D.; Fast, R.; Paganini, S.; Lawson, B.O.; Nega, M.; Easley, K.; Schmitz, J.E.; et al. CD8+ Lymphocytes Are Required for Maintaining Viral Suppression in SIV-Infected Macaques Treated with Short-Term Antiretroviral Therapy. Immunity 2016, 45, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Samer, S.; Thomas, Y.; Araínga, M.; Carter, C.; Shirreff, L.M.; Arif, M.S.; Avita, J.M.; Frank, I.; McRaven, M.D.; Thuruthiyil, C.T.; et al. Blockade of TGF-β signaling reactivates HIV-1/SIV reservoirs and immune responses in vivo. JCI Insight 2022, 7, e162290. [Google Scholar] [CrossRef]
- Cumont, M.C.; Monceaux, V.; Viollet, L.; Lay, S.; Parker, R.; Hurtrel, B.; Estaquier, J. TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ. 2007, 14, 1747–1758. [Google Scholar] [CrossRef]
- Pereira Ribeiro, S.; Strongin, Z.; Soudeyns, H.; Ten-Caten, F.; Ghneim, K.; Pacheco Sanchez, G.; Xavier de Medeiros, G.; Del Rio Estrada, P.M.; Pelletier, A.N.; Hoang, T.; et al. Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption. Nat. Immunol. 2024, 25, 1900–1912. [Google Scholar] [CrossRef]
- Estaquier, J.; Tanaka, M.; Suda, T.; Nagata, S.; Golstein, P.; Ameisen, J.C. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: Differential in vitro preventive effect of cytokines and protease antagonists. Blood 1996, 87, 4959–4966. [Google Scholar] [CrossRef]
- Clerici, M.; Sarin, A.; Coffman, R.L.; Wynn, T.A.; Blatt, S.P.; Hendrix, C.W.; Wolf, S.F.; Shearer, G.M.; Henkart, P.A. Type 1/type 2 cytokine modulation of T-cell programmed cell death as a model for human immunodeficiency virus pathogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 11811–11815. [Google Scholar] [CrossRef]
- Estaquier, J.; Idziorek, T.; Zou, W.; Emilie, D.; Farber, C.M.; Bourez, J.M.; Ameisen, J.C. T helper type 1/T helper type 2 cytokines and T cell death: Preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med. 1995, 182, 1759–1767. [Google Scholar] [CrossRef]
- Huot, N.; Jacquelin, B.; Garcia-Tellez, T.; Rascle, P.; Ploquin, M.J.; Madec, Y.; Reeves, R.K.; Derreudre-Bosquet, N.; Müller-Trutwin, M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 2017, 23, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Naif, H.M.; Li, S.; Alali, M.; Sloane, A.; Wu, L.; Kelly, M.; Lynch, G.; Lloyd, A.; Cunningham, A.L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 1998, 72, 830–836. [Google Scholar] [CrossRef]
- Tuttle, D.L.; Harrison, J.K.; Anders, C.; Sleasman, J.W.; Goodenow, M.M. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J. Virol. 1998, 72, 4962–4969. [Google Scholar] [CrossRef] [PubMed]
- Gartner, S.; Markovits, P.; Markovitz, D.M.; Kaplan, M.H.; Gallo, R.C.; Popovic, M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986, 233, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, H.E.; Orenstein, J.M.; Martin, M.A.; Ferrua, C.; Mitra, R.; Phipps, T.; Wahl, L.A.; Lane, H.C.; Fauci, A.S.; Burke, D.S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 1988, 167, 1428–1441. [Google Scholar] [CrossRef]
- Perno, C.F.; Yarchoan, R.; Cooney, D.A.; Hartman, N.R.; Webb, D.S.; Hao, Z.; Mitsuya, H.; Johns, D.G.; Broder, S. Replication of human immunodeficiency virus in monocytes. Granulocyte/macrophage colony-stimulating factor (GM-CSF) potentiates viral production yet enhances the antiviral effect mediated by 3’-azido-2’3’-dideoxythymidine (AZT) and other dideoxynucleoside congeners of thymidine. J. Exp. Med. 1989, 169, 933–951. [Google Scholar] [CrossRef]
- Pomerantz, R.J.; Feinberg, M.B.; Trono, D.; Baltimore, D. Lipopolysaccharide is a potent monocyte/macrophage-specific stimulator of human immunodeficiency virus type 1 expression. J. Exp. Med. 1990, 172, 253–261. [Google Scholar] [CrossRef]
- Igarashi, T.; Brown, C.R.; Endo, Y.; Buckler-White, A.; Plishka, R.; Bischofberger, N.; Hirsch, V.; Martin, M.A. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 2001, 98, 658–663. [Google Scholar] [CrossRef]
- Micci, L.; Alvarez, X.; Iriele, R.I.; Ortiz, A.M.; Ryan, E.S.; McGary, C.S.; Deleage, C.; McAtee, B.B.; He, T.; Apetrei, C.; et al. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014, 10, e1004467. [Google Scholar] [CrossRef]
- Ho, D.D.; Rota, T.R.; Hirsch, M.S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J. Clin. Investig. 1986, 77, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
- McElrath, M.J.; Steinman, R.M.; Cohn, Z.A. Latent HIV-1 infection in enriched populations of blood monocytes and T cells from seropositive patients. J. Clin. Investig. 1991, 87, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.; Campillo-Gimenez, L.; Monceaux, V.; Cumont, M.C.; Hurtrel, B.; Corbeil, J.; Zaunders, J.; Elbim, C.; Estaquier, J. HIV/SIV infection primes monocytes and dendritic cells for apoptosis. PLoS Pathog. 2011, 7, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.R.; Callaway, D.S.; Phair, J.P.; Kunstman, K.J.; Stanton, J.L.; Macken, C.A.; Perelson, A.S.; Wolinsky, S.M. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 1999, 340, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Lambotte, O.; Taoufik, Y.; de Goër, M.G.; Wallon, C.; Goujard, C.; Delfraissy, J.F. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2000, 23, 114–119. [Google Scholar] [CrossRef]
- Zhu, T.; Muthui, D.; Holte, S.; Nickle, D.; Feng, F.; Brodie, S.; Hwangbo, Y.; Mullins, J.I.; Corey, L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 2002, 76, 707–716. [Google Scholar] [CrossRef]
- Cattin, A.; Wiche Salinas, T.R.; Gosselin, A.; Planas, D.; Shacklett, B.; Cohen, E.A.; Ghali, M.P.; Routy, J.P.; Ancuta, P. HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. Aids 2019, 33, 1293–1306. [Google Scholar] [CrossRef]
- Honeycutt, J.B.; Wahl, A.; Baker, C.; Spagnuolo, R.A.; Foster, J.; Zakharova, O.; Wietgrefe, S.; Caro-Vegas, C.; Madden, V.; Sharpe, G.; et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig. 2016, 126, 1353–1366. [Google Scholar] [CrossRef]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef]
- Iijima, N.; Mattei, L.M.; Iwasaki, A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 284–289. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Guilliams, M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; Metcalf Pate, K.A.; Beck, S.E.; et al. Infectious Virus Persists in CD4+ T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2019, 93, e00065-19. [Google Scholar] [CrossRef] [PubMed]
- Costiniuk, C.T.; Salahuddin, S.; Farnos, O.; Olivenstein, R.; Pagliuzza, A.; Orlova, M.; Schurr, E.; De Castro, C.; Bourbeau, J.; Routy, J.P.; et al. HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. Aids 2018, 32, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Meziane, O.; Alexandrova, Y.; Olivenstein, R.; Dupuy, F.P.; Salahuddin, S.; Thomson, E.; Orlova, M.; Schurr, E.; Ancuta, P.; Durand, M.; et al. Peculiar Phenotypic and Cytotoxic Features of Pulmonary Mucosal CD8 T Cells in People Living with HIV Receiving Long-Term Antiretroviral Therapy. J. Immunol. 2021, 206, 641–651. [Google Scholar] [CrossRef]
- Alexandrova, Y.; Yero, A.; Olivenstein, R.; Orlova, M.; Schurr, E.; Estaquier, J.; Costiniuk, C.T.; Jenabian, M.A. Dynamics of pulmonary mucosal cytotoxic CD8 T-cells in people living with HIV under suppressive antiretroviral therapy. Respir. Res. 2024, 25, 240. [Google Scholar] [CrossRef]
- Koenig, S.; Gendelman, H.E.; Orenstein, J.M.; Dal Canto, M.C.; Pezeshkpour, G.H.; Yungbluth, M.; Janotta, F.; Aksamit, A.; Martin, M.A.; Fauci, A.S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science 1986, 233, 1089–1093. [Google Scholar] [CrossRef]
- Williams, K.C.; Corey, S.; Westmoreland, S.V.; Pauley, D.; Knight, H.; deBakker, C.; Alvarez, X.; Lackner, A.A. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: Implications for the neuropathogenesis of AIDS. J. Exp. Med. 2001, 193, 905–915. [Google Scholar] [CrossRef]
- Murray, E.A.; Rausch, D.M.; Lendvay, J.; Sharer, L.R.; Eiden, L.E. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science 1992, 255, 1246–1249. [Google Scholar] [CrossRef]
- Lamers, S.L.; Rose, R.; Maidji, E.; Agsalda-Garcia, M.; Nolan, D.J.; Fogel, G.B.; Salemi, M.; Garcia, D.L.; Bracci, P.; Yong, W.; et al. HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J. Virol. 2016, 90, 8968–8983. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Avalos, C.R.; Abreu, C.M.; Queen, S.E.; Li, M.; Price, S.; Shirk, E.N.; Engle, E.L.; Forsyth, E.; Bullock, B.T.; Mac Gabhann, F.; et al. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: A Functional Latent Reservoir. mBio 2017, 8, e01186-17. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Johnson, A.M.; Xiang, S.H.; Li, J.; Foley, B.T.; Doyle-Meyers, L.; Panganiban, A.; Kaur, A.; Veazey, R.S.; Wu, Y.; et al. Persistence of SIV in the brain of SIV-infected Chinese rhesus macaques with or without antiretroviral therapy. J. Neurovirology 2018, 24, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.S.; Green, R.R.; Brown, R.R.; Wood, M.P.; Hensley-McBain, T.; Fisher, C.; Chang, J.; Miller, A.D.; Bosche, W.J.; Lifson, J.D.; et al. Liver macrophage-associated inflammation correlates with SIV burden and is substantially reduced following cART. PLoS Pathog. 2018, 14, e1006871. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Zhang, N.; Branton, W.G.; Zghidi-Abouzid, O.; Cohen, E.A.; Gelman, B.B.; Estaquier, J.; Kong, L.; Power, C. The HIV Restriction Factor Profile in the Brain Is Associated with the Clinical Status and Viral Quantities. Viruses 2023, 15, 316. [Google Scholar] [CrossRef]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef]
- Tenorio, A.R.; Zheng, Y.; Bosch, R.J.; Krishnan, S.; Rodriguez, B.; Hunt, P.W.; Plants, J.; Seth, A.; Wilson, C.C.; Deeks, S.G.; et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis. 2014, 210, 1248–1259. [Google Scholar] [CrossRef]
- Sereti, I.; Krebs, S.J.; Phanuphak, N.; Fletcher, J.L.; Slike, B.; Pinyakorn, S.; O’Connell, R.J.; Rupert, A.; Chomont, N.; Valcour, V.; et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin. Infect. Dis. 2017, 64, 124–131. [Google Scholar] [CrossRef]
- Strategies for Management of Antiretroviral Therapy Study Group; El-Sadr, W.M.; Lundgren, J.; Neaton, J.D.; Gordin, F.; Abrams, D.; Arduino, R.C.; Babiker, A.; Burman, W.; Clumeck, N.; et al. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006, 355, 2283–2296. [Google Scholar] [CrossRef]
- Balagopal, A.; Gupte, N.; Shivakoti, R.; Cox, A.L.; Yang, W.T.; Berendes, S.; Mwelase, N.; Kanyama, C.; Pillay, S.; Samaneka, W.; et al. Continued Elevation of Interleukin-18 and Interferon-gamma After Initiation of Antiretroviral Therapy and Clinical Failure in a Diverse Multicountry Human Immunodeficiency Virus Cohort. Open Forum Infect. Dis. 2016, 3, ofw118. [Google Scholar] [CrossRef]
- Bandera, A.; Masetti, M.; Fabbiani, M.; Biasin, M.; Muscatello, A.; Squillace, N.; Clerici, M.; Gori, A.; Trabattoni, D. The NLRP3 Inflammasome Is Upregulated in HIV-Infected Antiretroviral Therapy-Treated Individuals with Defective Immune Recovery. Front. Immunol. 2018, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Matsui, K.; Kawada, N.; Hyodo, Y.; Hayashi, N.; Okamura, H.; Higashino, K.; Nakanishi, K. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol. 1997, 159, 3961–3967. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, W.; Osaki, T.; Okamura, H.; Robbins, P.D.; Kurimoto, M.; Nagata, S.; Lotze, M.T.; Tahara, H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J. Immunol. 1999, 163, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Kuida, K.; Tsutsui, H.; Ku, G.; Hsiao, K.; Fleming, M.A.; Hayashi, N.; Higashino, K.; Okamura, H.; Nakanishi, K.; et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 1997, 275, 206–209. [Google Scholar] [CrossRef]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M.; et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997, 386, 619–623. [Google Scholar] [CrossRef]
- Laforge, M.; Silvestre, R.; Rodrigues, V.; Garibal, J.; Campillo-Gimenez, L.; Mouhamad, S.; Monceaux, V.; Cumont, M.C.; Rabezanahary, H.; Pruvost, A.; et al. The anti-caspase inhibitor Q-VD-OPH prevents AIDS disease progression in SIV-infected rhesus macaques. J. Clin. Investig. 2018, 128, 1627–1640. [Google Scholar] [CrossRef]
- Alam, A.; Cohen, L.Y.; Aouad, S.; Sekaly, R.P. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 1999, 190, 1879–1890. [Google Scholar] [CrossRef]
- Mouhamad, S.; Arnoult, D.; Auffredou, M.T.; Estaquier, J.; Vazquez, A. Differential modulation of interleukin-2-and interleukin-4-mediated early activation of normal human B lymphocytes by the caspase inhibitor zVAD-fmk. Eur. Cytokine Netw. 2002, 13, 439–445. [Google Scholar]
- Stack, J.H.; Beaumont, K.; Larsen, P.D.; Straley, K.S.; Henkel, G.W.; Randle, J.C.; Hoffman, H.M. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J. Immunol. 2005, 175, 2630–2634. [Google Scholar] [CrossRef]
- André, S.; Picard, M.; Cezar, R.; Roux-Dalvai, F.; Alleaume-Butaux, A.; Soundaramourty, C.; Cruz, A.S.; Mendes-Frias, A.; Gotti, C.; Leclercq, M.; et al. T cell apoptosis characterizes severe Covid-19 disease. Cell Death Differ. 2022, 29, 1486–1499. [Google Scholar] [CrossRef]
- Yero, A.; Bouassa, R.M.; Ancuta, P.; Estaquier, J.; Jenabian, M.A. Immuno-metabolic control of the balance between Th17-polarized and regulatory T-cells during HIV infection. Cytokine Growth Factor Rev. 2023, 69, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Buzon, M.J.; Sun, H.; Li, C.; Shaw, A.; Seiss, K.; Ouyang, Z.; Martin-Gayo, E.; Leng, J.; Henrich, T.J.; Li, J.Z.; et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 2014, 20, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, A.; Wiche Salinas, T.R.; Planas, D.; Wacleche, V.S.; Zhang, Y.; Fromentin, R.; Chomont, N.; Cohen, É.A.; Shacklett, B.; Mehraj, V.; et al. HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. Aids 2017, 31, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Stieh, D.J.; Matias, E.; Xu, H.; Fought, A.J.; Blanchard, J.L.; Marx, P.A.; Veazey, R.S.; Hope, T.J. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe 2016, 19, 529–540. [Google Scholar] [CrossRef]
- McGary, C.S.; Deleage, C.; Harper, J.; Micci, L.; Ribeiro, S.P.; Paganini, S.; Kuri-Cervantes, L.; Benne, C.; Ryan, E.S.; Balderas, R.; et al. CTLA-4+PD-1- Memory CD4+ T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 2017, 47, 776–788.e775. [Google Scholar] [CrossRef]
- Cecchinato, V.; Trindade, C.J.; Laurence, A.; Heraud, J.M.; Brenchley, J.M.; Ferrari, M.G.; Zaffiri, L.; Tryniszewska, E.; Tsai, W.P.; Vaccari, M.; et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008, 1, 279–288. [Google Scholar] [CrossRef]
- Favre, D.; Lederer, S.; Kanwar, B.; Ma, Z.M.; Proll, S.; Kasakow, Z.; Mold, J.; Swainson, L.; Barbour, J.D.; Baskin, C.R.; et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009, 5, e1000295. [Google Scholar] [CrossRef]
- Yero, A.; Farnos, O.; Rabezanahary, H.; Racine, G.; Estaquier, J.; Jenabian, M.A. Differential Dynamics of Regulatory T-Cell and Th17 Cell Balance in Mesenteric Lymph Nodes and Blood following Early Antiretroviral Initiation during Acute Simian Immunodeficiency Virus Infection. J. Virol. 2019, 93, e00371-19. [Google Scholar] [CrossRef]
- Fromentin, R.; Bakeman, W.; Lawani, M.B.; Khoury, G.; Hartogensis, W.; DaFonseca, S.; Killian, M.; Epling, L.; Hoh, R.; Sinclair, E.; et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog. 2016, 12, e1005761. [Google Scholar] [CrossRef]
- Rasmussen, T.A.; Zerbato, J.M.; Rhodes, A.; Tumpach, C.; Dantanarayana, A.; McMahon, J.H.; Lau, J.S.Y.; Chang, J.J.; Gubser, C.; Brown, W.; et al. Memory CD4+ T cells that co-express PD1 and CTLA4 have reduced response to activating stimuli facilitating HIV latency. Cell Rep Med 2022, 3, 100766. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Mattis, A.E.; Kremmer, E.; Wolf, E.; Brem, G.; Lipp, M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 1996, 87, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Loetscher, M.; Roos, R.S.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998, 187, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Förster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef]
- Perreau, M.; Savoye, A.L.; De Crignis, E.; Corpataux, J.M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C.; Pantaleo, G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013, 210, 143–156. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Lum, R.; Okoye, A.A.; Park, H.; Matsuda, K.; Bae, J.Y.; Hagen, S.I.; Shoemaker, R.; Deleage, C.; Lucero, C.; et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015, 21, 132–139. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Malam, N.; Lackner, A.A.; Veazey, R.S. Persistent Simian Immunodeficiency Virus Infection Causes Ultimate Depletion of Follicular Th Cells in AIDS. J. Immunol. 2015, 195, 4351–4357. [Google Scholar] [CrossRef]
- Kohler, S.L.; Pham, M.N.; Folkvord, J.M.; Arends, T.; Miller, S.M.; Miles, B.; Meditz, A.L.; McCarter, M.; Levy, D.N.; Connick, E. Germinal Center T Follicular Helper Cells Are Highly Permissive to HIV-1 and Alter Their Phenotype during Virus Replication. J. Immunol. 2016, 196, 2711–2722. [Google Scholar] [CrossRef]
- Boritz, E.A.; Darko, S.; Swaszek, L.; Wolf, G.; Wells, D.; Wu, X.; Henry, A.R.; Laboune, F.; Hu, J.; Ambrozak, D.; et al. Multiple Origins of Virus Persistence during Natural Control of HIV Infection. Cell 2016, 166, 1004–1015. [Google Scholar] [CrossRef]
- Moukambi, F.; Rabezanahary, H.; Rodrigues, V.; Racine, G.; Robitaille, L.; Krust, B.; Andreani, G.; Soundaramourty, C.; Silvestre, R.; Laforge, M.; et al. Early Loss of Splenic Tfh Cells in SIV-Infected Rhesus Macaques. PLoS Pathog. 2015, 11, e1005287. [Google Scholar] [CrossRef] [PubMed]
- Niessl, J.; Baxter, A.E.; Morou, A.; Brunet-Ratnasingham, E.; Sannier, G.; Gendron-Lepage, G.; Richard, J.; Delgado, G.G.; Brassard, N.; Turcotte, I.; et al. Persistent expansion and Th1-like skewing of HIV-specific circulating T follicular helper cells during antiretroviral therapy. EBioMedicine 2020, 54, 102727. [Google Scholar] [CrossRef] [PubMed]
- Morou, A.; Brunet-Ratnasingham, E.; Dubé, M.; Charlebois, R.; Mercier, E.; Darko, S.; Brassard, N.; Nganou-Makamdop, K.; Arumugam, S.; Gendron-Lepage, G.; et al. Altered differentiation is central to HIV-specific CD4+ T cell dysfunction in progressive disease. Nat. Immunol. 2019, 20, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Lynch, R.M.; Gautam, R.; Matus-Nicodemos, R.; Schmidt, S.D.; Boswell, K.L.; Darko, S.; Wong, P.; Sheng, Z.; Petrovas, C.; et al. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci. Transl. Med. 2015, 7, 298ra120. [Google Scholar] [CrossRef]
- Moukambi, F.; Rabezanahary, H.; Fortier, Y.; Rodrigues, V.; Clain, J.; Benmadid-Laktout, G.; Zghidi-Abouzid, O.; Soundaramourty, C.; Laforge, M.; Estaquier, J. Mucosal T follicular helper cells in SIV-infected rhesus macaques: Contributing role of IL-27. Mucosal Immunol. 2019, 12, 1038–1054. [Google Scholar] [CrossRef]
- Bauquet, A.T.; Jin, H.; Paterson, A.M.; Mitsdoerffer, M.; Ho, I.C.; Sharpe, A.H.; Kuchroo, V.K. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009, 10, 167–175. [Google Scholar] [CrossRef]
- Kroenke, M.A.; Eto, D.; Locci, M.; Cho, M.; Davidson, T.; Haddad, E.K.; Crotty, S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J. Immunol. 2012, 188, 3734–3744. [Google Scholar] [CrossRef]
- Oestreich, K.J.; Mohn, S.E.; Weinmann, A.S. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat. Immunol. 2012, 13, 405–411. [Google Scholar] [CrossRef]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef]
- Lee, J.Y.; Skon, C.N.; Lee, Y.J.; Oh, S.; Taylor, J.J.; Malhotra, D.; Jenkins, M.K.; Rosenfeld, M.G.; Hogquist, K.A.; Jameson, S.C. The transcription factor KLF2 restrains CD4+ T follicular helper cell differentiation. Immunity 2015, 42, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.P.; Fuhrmann, F.; Feist, R.K.; Lahmann, A.; Al Baz, M.S.; Gentz, L.J.; Vu Van, D.; Mages, H.W.; Haftmann, C.; Riedel, R.; et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J. Exp. Med. 2015, 212, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Fabre, S.; Carrette, F.; Chen, J.; Lang, V.; Semichon, M.; Denoyelle, C.; Lazar, V.; Cagnard, N.; Dubart-Kupperschmitt, A.; Mangeney, M.; et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J. Immunol. 2008, 181, 2980–2989. [Google Scholar] [CrossRef]
- Kerdiles, Y.M.; Beisner, D.R.; Tinoco, R.; Dejean, A.S.; Castrillon, D.H.; DePinho, R.A.; Hedrick, S.M. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat. Immunol. 2009, 10, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Choi, Y.S.; Diamond, J.A.; Yang, J.A.; Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012, 209, 243–250. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Podd, A.; Chen, Y.; Alekseev, A.M.; Yu, M.; Qi, X.; Huang, H.; Wen, R.; Wang, J.; Li, H.S.; et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J. Biol. Chem. 2012, 287, 11234–11239. [Google Scholar] [CrossRef]
- Ballesteros-Tato, A.; Leon, B.; Graf, B.A.; Moquin, A.; Adams, P.S.; Lund, F.E.; Randall, T.D. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012, 36, 847–856. [Google Scholar] [CrossRef]
- Boswell, K.L.; Paris, R.; Boritz, E.; Ambrozak, D.; Yamamoto, T.; Darko, S.; Wloka, K.; Wheatley, A.; Narpala, S.; McDermott, A.; et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014, 10, e1003853. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Vinton, C.; Tabb, B.; Hao, X.P.; Connick, E.; Paiardini, M.; Lifson, J.D.; Silvestri, G.; Estes, J.D. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood 2012, 120, 4172–4181. [Google Scholar] [CrossRef]
- Cubas, R.A.; Mudd, J.C.; Savoye, A.L.; Perreau, M.; van Grevenynghe, J.; Metcalf, T.; Connick, E.; Meditz, A.; Freeman, G.J.; Abesada-Terk, G., Jr.; et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 2013, 19, 494–499. [Google Scholar] [CrossRef]
- Petrovas, C.; Yamamoto, T.; Gerner, M.Y.; Boswell, K.L.; Wloka, K.; Smith, E.C.; Ambrozak, D.R.; Sandler, N.G.; Timmer, K.J.; Sun, X.; et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Investig. 2012, 122, 3281–3294. [Google Scholar] [CrossRef] [PubMed]
- Velu, V.; Mylvaganam, G.H.; Gangadhara, S.; Hong, J.J.; Iyer, S.S.; Gumber, S.; Ibegbu, C.C.; Villinger, F.; Amara, R.R. Induction of Th1-Biased T Follicular Helper (Tfh) Cells in Lymphoid Tissues during Chronic Simian Immunodeficiency Virus Infection Defines Functionally Distinct Germinal Center Tfh Cells. J. Immunol. 2016, 197, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Monceaux, V.; Estaquier, J.; Fevrier, M.; Cumont, M.C.; Riviere, Y.; Aubertin, A.M.; Ameisen, J.C.; Hurtrel, B. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. Aids 2003, 17, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Dykhuizen, M.; Mitchen, J.L.; Montefiori, D.C.; Thomson, J.; Acker, L.; Lardy, H.; Pauza, C.D. Determinants of disease in the simian immunodeficiency virus-infected rhesus macaque: Characterizing animals with low antibody responses and rapid progression. J. Gen. Virol. 1998, 79 (Pt 10), 2461–2467. [Google Scholar] [CrossRef][Green Version]
- Levesque, M.C.; Moody, M.A.; Hwang, K.K.; Marshall, D.J.; Whitesides, J.F.; Amos, J.D.; Gurley, T.C.; Allgood, S.; Haynes, B.B.; Vandergrift, N.A.; et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009, 6, e1000107. [Google Scholar] [CrossRef]
- Moir, S.; Malaspina, A.; Pickeral, O.K.; Donoghue, E.T.; Vasquez, J.; Miller, N.J.; Krishnan, S.R.; Planta, M.A.; Turney, J.F.; Justement, J.S.; et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J. Exp. Med. 2004, 200, 587–599. [Google Scholar] [CrossRef]
- Li, S.; Rouphael, N.; Duraisingham, S.; Romero-Steiner, S.; Presnell, S.; Davis, C.; Schmidt, D.S.; Johnson, S.E.; Milton, A.; Rajam, G.; et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 2014, 15, 195–204. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Moir, S.; Buckner, C.M.; Ho, J.; Wang, W.; Chen, J.; Waldner, A.J.; Posada, J.G.; Kardava, L.; O’Shea, M.A.; Kottilil, S.; et al. B cells in early and chronic HIV infection: Evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 2010, 116, 5571–5579. [Google Scholar] [CrossRef]
- Wada, N.I.; Jacobson, L.P.; Margolick, J.B.; Breen, E.C.; Macatangay, B.; Penugonda, S.; Martinez-Maza, O.; Bream, J.H. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015, 29, 463–471. [Google Scholar] [CrossRef]
- Fontaine, J.; Chagnon-Choquet, J.; Valcke, H.S.; Poudrier, J.; Roger, M.; Montreal Primary, H.I.V.I.; Long-Term Non-Progressor Study, G. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 2011, 117, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.; Binley, J.M.; Clas, B.A.; Bonhoeffer, S.; Astill, T.P.; Kost, R.; Hurley, A.; Cao, Y.; Markowitz, M.; Ho, D.D.; et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 1998, 188, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Titanji, K.; De Milito, A.; Cagigi, A.; Thorstensson, R.; Grutzmeier, S.; Atlas, A.; Hejdeman, B.; Kroon, F.P.; Lopalco, L.; Nilsson, A.; et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 2006, 108, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Schacker, T.W.; Nguyen, P.L.; Martinez, E.; Reilly, C.; Gatell, J.M.; Horban, A.; Bakowska, E.; Berzins, B.; van Leeuwen, R.; Wolinsky, S.; et al. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. J. Infect. Dis. 2002, 186, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Schacker, T.W.; Nguyen, P.L.; Beilman, G.J.; Wolinsky, S.; Larson, M.; Reilly, C.; Haase, A.T. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Investig. 2002, 110, 1133–1139. [Google Scholar] [CrossRef]
- Zaunders, J.; Danta, M.; Bailey, M.; Mak, G.; Marks, K.; Seddiki, N.; Xu, Y.; Templeton, D.J.; Cooper, D.A.; Boyd, M.A.; et al. CD4+ T Follicular Helper and IgA+ B Cell Numbers in Gut Biopsies from HIV-Infected Subjects on Antiretroviral Therapy Are Similar to HIV-Uninfected Individuals. Front. Immunol. 2016, 7, 438. [Google Scholar] [CrossRef]
- Kityo, C.; Makamdop, K.N.; Rothenberger, M.; Chipman, J.G.; Hoskuldsson, T.; Beilman, G.J.; Grzywacz, B.; Mugyenyi, P.; Ssali, F.; Akondy, R.S.; et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J. Clin. Investig. 2018, 128, 2763–2773. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Parmigiani, A.; Silva, S.Y.; George, V.K.; Fischl, M.; Pahwa, R.; Pahwa, S. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood 2012, 120, 985–993. [Google Scholar] [CrossRef]
- Pallikkuth, S.; de Armas, L.R.; Rinaldi, S.; George, V.K.; Pan, L.; Arheart, K.L.; Pahwa, R.; Pahwa, S. Dysfunctional peripheral T follicular helper cells dominate in people with impaired influenza vaccine responses: Results from the FLORAH study. PLoS Biol. 2019, 17, e3000257. [Google Scholar] [CrossRef]
- Muro-Cacho, C.A.; Pantaleo, G.; Fauci, A.S. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 1995, 154, 5555–5566. [Google Scholar] [CrossRef]
- Finkel, T.H.; Tudor-Williams, G.; Banda, N.K.; Cotton, M.F.; Curiel, T.; Monks, C.; Baba, T.W.; Ruprecht, R.M.; Kupfer, A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1995, 1, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, M.L.; Lecoeur, H.; Dulioust, A.; Enouf, M.G.; Crouvoiser, M.; Goujard, C.; Debord, T.; Montagnier, L. Programmed cell death in peripheral lymphocytes from HIV-infected persons: Increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 1996, 156, 3509–3520. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.; Sodora, D.L.; Koup, R.A.; Paiardini, M.; O’Neil, S.P.; McClure, H.M.; Staprans, S.I.; Feinberg, M.B. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 2003, 18, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Petit, F.; Lelièvre, J.D.; Lecossier, D.; Hance, A.; Monceaux, V.; Hurtrel, B.; Ho Tsong Fang, R.; Ameisen, J.C.; Estaquier, J. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: Relationship to disease progression. Cell Death Differ. 2003, 10, 1240–1252. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Douek, D.C.; Hill, B.; Nishimura, Y.; Martin, M.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef]
- Li, Q.; Duan, L.; Estes, J.D.; Ma, Z.M.; Rourke, T.; Wang, Y.; Reilly, C.; Carlis, J.; Miller, C.J.; Haase, A.T. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 2005, 434, 1148–1152. [Google Scholar] [CrossRef]
- Terai, C.; Kornbluth, R.S.; Pauza, C.D.; Richman, D.D.; Carson, D.A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J. Clin. Investig. 1991, 87, 1710–1715. [Google Scholar] [CrossRef]
- Petit, F.; Arnoult, D.; Lelièvre, J.D.; Moutouh-de Parseval, L.; Hance, A.J.; Schneider, P.; Corbeil, J.; Ameisen, J.C.; Estaquier, J. Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to a caspase-independent cell death. J. Biol. Chem. 2002, 277, 1477–1487. [Google Scholar] [CrossRef]
- Laforge, M.; Limou, S.; Harper, F.; Casartelli, N.; Rodrigues, V.; Silvestre, R.; Haloui, H.; Zagury, J.F.; Senik, A.; Estaquier, J. DRAM triggers lysosomal membrane permeabilization and cell death in CD4+ T cells infected with HIV. PLoS Pathog. 2013, 9, e1003328. [Google Scholar] [CrossRef]
- Hutcheson, J.; Scatizzi, J.C.; Siddiqui, A.M.; Haines, G.K., 3rd; Wu, T.; Li, Q.Z.; Davis, L.S.; Mohan, C.; Perlman, H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity 2008, 28, 206–217. [Google Scholar] [CrossRef]
- Hughes, P.D.; Belz, G.T.; Fortner, K.A.; Budd, R.C.; Strasser, A.; Bouillet, P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 2008, 28, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, L.A.; Lewin, S.R.; Zhang, L.; Gettie, A.; Luckay, A.; Martin, L.N.; Skulsky, E.; Ho, D.D.; Cheng-Mayer, C.; Marx, P.A. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 2000, 74, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Monceaux, V.; Ho Tsong Fang, R.; Cumont, M.C.; Hurtrel, B.; Estaquier, J. Distinct cycling CD4+- and CD8+-T-cell profiles during the asymptomatic phase of simian immunodeficiency virus SIVmac251 infection in rhesus macaques. J. Virol. 2003, 77, 10047–10059. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Wunderlich, E.S.; Smith, C.A.; Herzenberg, L.A.; Herzenberg, L.A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J. Exp. Med. 1995, 181, 2029–2036. [Google Scholar] [CrossRef]
- Banda, N.K.; Bernier, J.; Kurahara, D.K.; Kurrle, R.; Haigwood, N.; Sekaly, R.P.; Finkel, T.H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 1992, 176, 1099–1106. [Google Scholar] [CrossRef]
- Desbarats, J.; Freed, J.H.; Campbell, P.A.; Newell, M.K. Fas (CD95) expression and death-mediating function are induced by CD4 cross-linking on CD4+ T cells. Proc. Natl. Acad. Sci. USA 1996, 93, 11014–11018. [Google Scholar] [CrossRef]
- Berndt, C.; Mopps, B.; Angermuller, S.; Gierschik, P.; Krammer, P.H. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc. Natl. Acad. Sci. USA 1998, 95, 12556–12561. [Google Scholar] [CrossRef]
- Cicala, C.; Arthos, J.; Rubbert, A.; Selig, S.; Wildt, K.; Cohen, O.J.; Fauci, A.S. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4+ T cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1178–1183. [Google Scholar] [CrossRef]
- Vlahakis, S.R.; Algeciras-Schimnich, A.; Bou, G.; Heppelmann, C.J.; Villasis-Keever, A.; Collman, R.G.; Paya, C.V. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Investig. 2001, 107, 207–215. [Google Scholar] [CrossRef]
- Petit, F.; Corbeil, J.; Lelievre, J.D.; Moutouh-de Parseval, L.; Pinon, G.; Green, D.R.; Ameisen, J.C.; Estaquier, J. Role of CD95-activated caspase-1 processing of IL-1beta in TCR-mediated proliferation of HIV-infected CD4+ T cells. Eur. J. Immunol. 2001, 31, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Estaquier, J.; Lelievre, J.D.; Petit, F.; Brunner, T.; Moutouh-De Parseval, L.; Richman, D.D.; Ameisen, J.C.; Corbeil, J. Effects of antiretroviral drugs on human immunodeficiency virus type 1-induced CD4+ T-cell death. J. Virol. 2002, 76, 5966–5973. [Google Scholar] [CrossRef] [PubMed]
- Holm, G.H.; Zhang, C.; Gorry, P.R.; Peden, K.; Schols, D.; De Clercq, E.; Gabuzda, D. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 2004, 78, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Lelievre, J.D.; Mammano, F.; Arnoult, D.; Petit, F.; Grodet, A.; Estaquier, J.; Ameisen, J.C. A novel mechanism for HIV1-mediated bystander CD4+ T-cell death: Neighboring dying cells drive the capacity of HIV1 to kill noncycling primary CD4+ T cells. Cell Death Differ. 2004, 11, 1017–1027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lelievre, J.D.; Petit, F.; Arnoult, D.; Ameisen, J.C.; Estaquier, J. Interleukin 7 increases human immunodeficiency virus type 1 LAI-mediated Fas-induced T-cell death. J. Virol. 2005, 79, 3195–3199. [Google Scholar] [CrossRef]
- Doitsh, G.; Cavrois, M.; Lassen, K.G.; Zepeda, O.; Yang, Z.; Santiago, M.L.; Hebbeler, A.M.; Greene, W.C. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 2010, 143, 789–801. [Google Scholar] [CrossRef]
- Espert, L.; Denizot, M.; Grimaldi, M.; Robert-Hebmann, V.; Gay, B.; Varbanov, M.; Codogno, P.; Biard-Piechaczyk, M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J. Clin. Investig. 2006, 116, 2161–2172. [Google Scholar] [CrossRef]
- Wang, Q.; Clark, K.M.; Tiwari, R.; Raju, N.; Tharp, G.K.; Rogers, J.; Harris, R.A.; Raveendran, M.; Bosinger, S.E.; Burdo, T.H.; et al. The CARD8 inflammasome dictates HIV/SIV pathogenesis and disease progression. Cell 2024, 187, 1223–1237.e1216. [Google Scholar] [CrossRef]
- Benveniste, O.; Flahault, A.; Rollot, F.; Elbim, C.; Estaquier, J.; Pedron, B.; Duval, X.; Dereuddre-Bosquet, N.; Clayette, P.; Sterkers, G.; et al. Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J. Infect. Dis. 2005, 191, 1670–1679. [Google Scholar] [CrossRef][Green Version]
- Fernandez, S.; Tanaskovic, S.; Helbig, K.; Rajasuriar, R.; Kramski, M.; Murray, J.M.; Beard, M.; Purcell, D.; Lewin, S.R.; Price, P.; et al. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J. Infect. Dis. 2011, 204, 1927–1935. [Google Scholar] [CrossRef]
- Merlini, E.; Luzi, K.; Suardi, E.; Barassi, A.; Cerrone, M.; Martinez, J.S.; Bai, F.; D’Eril, G.V.; Monforte, A.D.; Marchetti, G. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS ONE 2012, 7, e46073. [Google Scholar] [CrossRef] [PubMed]
- Janossy, G.; Borthwick, N.; Lomnitzer, R.; Medina, E.; Squire, S.B.; Phillips, A.N.; Lipman, M.; Johnson, M.A.; Lee, C.; Bofill, M. Lymphocyte activation in HIV-1 infection. I. Predominant proliferative defects among CD45R0+ cells of the CD4 and CD8 lineages. Aids 1993, 7, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Sarin, A.; Berzofsky, J.A.; Landay, A.L.; Kessler, H.A.; Hashemi, F.; Hendrix, C.W.; Blatt, S.P.; Rusnak, J.; Dolan, M.J.; et al. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. Aids 1996, 10, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.S.; Lackner, A.A.; Lang, S.M.; Simon, M.A.; Sehgal, P.K.; Daniel, M.D.; Desrosiers, R.C. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 1995, 69, 2378–2383. [Google Scholar] [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Segeral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef]

| Features | RMs of Indian Origin | RMs of Chinese Origin | Pigtail Macaques (United States) | Cynomolgus (Mauritian) | African Green Monkeys | PLWH | |
|---|---|---|---|---|---|---|---|
| Virus and Disease progression | Blood Viremia PLN Viremia | +++ +++ | ++ ++ | +++ +++ | ++ + | ++ No | ++ ++ |
| Progression to AIDS | Rapid Less than 1 year | Moderate 1 to 5 years | Rapid Less than 6 Months | Low Controller | No Controller | Moderate 5 to 10 years | |
| Immunological parameters | CD4 T depletion | Fast | Moderate | Fast | Low | No | Gradual depletion |
| Immune activation | Low | Moderate to High | Low | Low | Low | Moderate to high | |
| CCR5 expression and after infection | High Full depletion | Low Increase | High Full depletion | Low No increase | Low No increase | Low Increase | |
| SIV-specific antibodies | Low | High | Low | Low | Low | High | |
| MHC molecules (most frequent) | Mamu-A*01, Mamu-B*01, Mamu-B*17 | Mamu-A*02 Mamu-B*08 | Mane-A* 10 | Mafa, M1 to M7 | Chae-A and Chae-B | HLA-A2, HLA-A3 and HLA-B7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clain, J.A.; Picard, M.; Rabezanahary, H.; André, S.; Boutrais, S.; Goma Matsetse, E.; Dewatines, J.; Dueymes, Q.; Thiboutot, E.; Racine, G.; et al. Immune Alterations and Viral Reservoir Atlas in SIV-Infected Chinese Rhesus Macaques. Infect. Dis. Rep. 2025, 17, 12. https://doi.org/10.3390/idr17010012
Clain JA, Picard M, Rabezanahary H, André S, Boutrais S, Goma Matsetse E, Dewatines J, Dueymes Q, Thiboutot E, Racine G, et al. Immune Alterations and Viral Reservoir Atlas in SIV-Infected Chinese Rhesus Macaques. Infectious Disease Reports. 2025; 17(1):12. https://doi.org/10.3390/idr17010012
Chicago/Turabian StyleClain, Julien A., Morgane Picard, Henintsoa Rabezanahary, Sonia André, Steven Boutrais, Ella Goma Matsetse, Juliette Dewatines, Quentin Dueymes, Elise Thiboutot, Gina Racine, and et al. 2025. "Immune Alterations and Viral Reservoir Atlas in SIV-Infected Chinese Rhesus Macaques" Infectious Disease Reports 17, no. 1: 12. https://doi.org/10.3390/idr17010012
APA StyleClain, J. A., Picard, M., Rabezanahary, H., André, S., Boutrais, S., Goma Matsetse, E., Dewatines, J., Dueymes, Q., Thiboutot, E., Racine, G., Soundaramourty, C., Mammano, F., Corbeau, P., Zghidi-Abouzid, O., & Estaquier, J. (2025). Immune Alterations and Viral Reservoir Atlas in SIV-Infected Chinese Rhesus Macaques. Infectious Disease Reports, 17(1), 12. https://doi.org/10.3390/idr17010012






