Current Progress and Future Perspectives in Contact and Releasing-Type Antimicrobial Coatings of Orthopaedic Implants: A Systematic Review Analysis Emanated from In Vitro and In Vivo Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Research Strategy
2.3. Inclusion Criteria and Study Selection
2.4. Data Extraction
2.5. Quality Assessment
3. Results
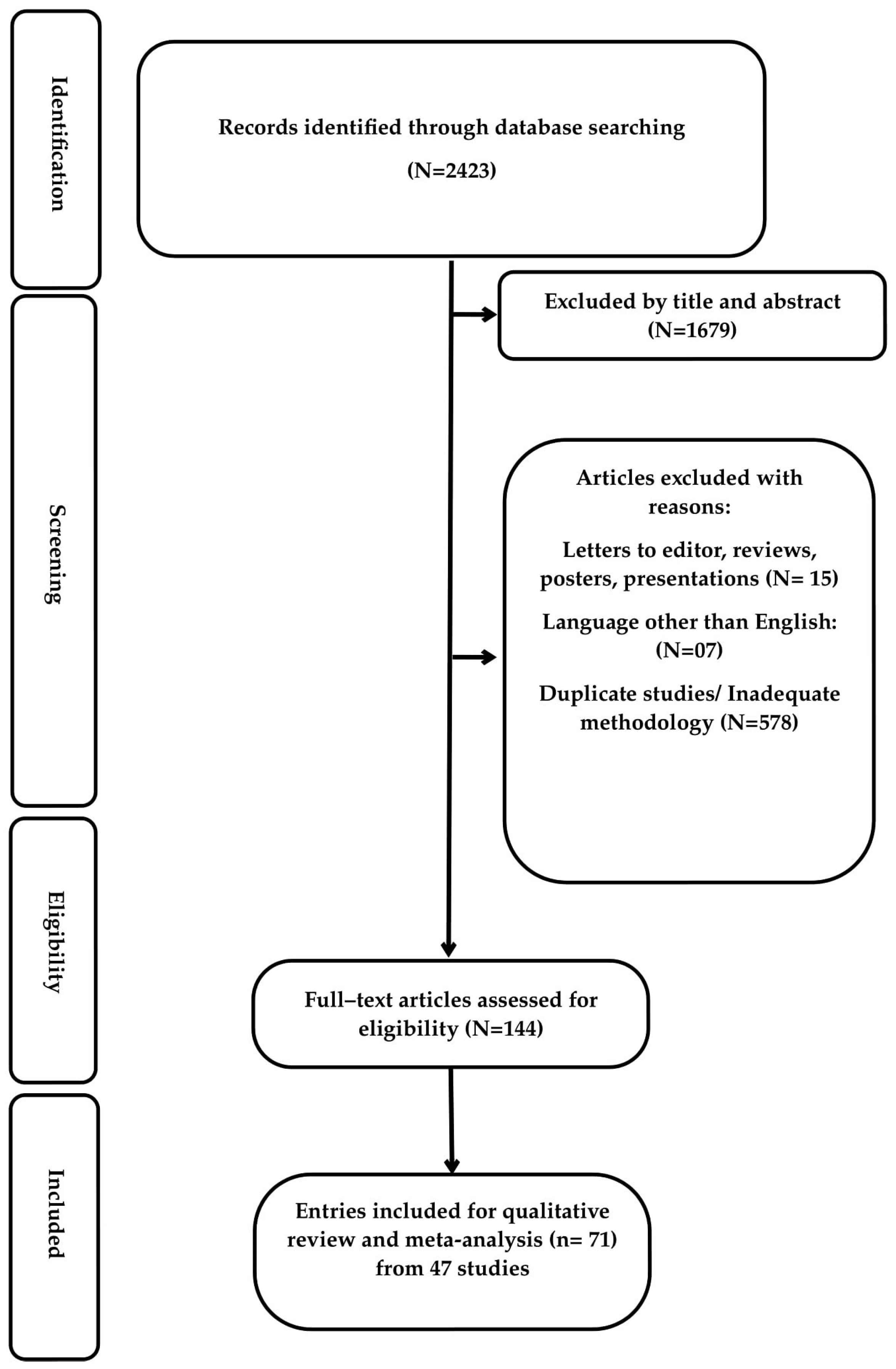
3.1. Search Results
3.2. Study Design and Content
3.2.1. In Vitro Studies and Cell Lines
3.2.2. In Vivo Studies and Implants
3.2.3. Bacterial Strains and Antimicrobial Effectiveness
3.2.4. Osteointegration Ability and Biocompatibility
3.2.5. Quality Assessment
4. Discussion
4.1. Evaluation of Antibiotic-Based Coatings
4.2. Evaluation of Ag-Based Coatings
4.3. Evaluation of Iodine-Based and Other Novel Coatings
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stavrakis, A.I.; Zhu, S.; Hegde, V.; Loftin, A.H.; Ashbaugh, A.G.; Niska, J.A.; Miller, L.S.; Segura, T.; Bernthal, N.M. In Vivo Efficacy of a “Smart” Antimicrobial Implant Coating. J. Bone Jt. Surg. Am. 2016, 98, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Eto, S.; Miyamoto, H.; Shobuike, T.; Noda, I.; Akiyama, T.; Tsukamoto, M.; Ueno, M.; Someya, S.; Kawano, S.; Sonohata, M.; et al. Silver oxide-containing hydroxyapatite coating supports osteoblast function and enhances implant anchorage strength in rat femur. J. Orthop. Res. 2015, 33, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Nast, S.; Fassbender, M.; Bormann, N.; Beck, S.; Montali, A.; Lucke, M.; Schmidmaier, G.; Wildemann, B. In vivo quantification of gentamicin released from an implant coating. J. Biomater. Appl. 2016, 31, 45–54. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, G.; Xu, K.; Wang, L.; Yu, L.; Xing, M.M.Q.; Qiu, X. Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kucharikova, S.; Gerits, E.; De Brucker, K.; Braem, A.; Ceh, K.; Majdic, G.; Španič, T.; Pogorevc, E.; Verstraeten, N.; Tournu, H.; et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016, 71, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Bisland, S.K.; Chien, C.; Wilson, B.C.; Burch, S. Pre-clinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis. Photochem. Photobiol. Sci. 2006, 5, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Vester, H.; Wildemann, B.; Schmidmaier, G.; Stockle, U.; Lucke, M. Gentamycin delivered from a PDLLA coating of metallic implants: In vivo and in vitro characterisation for local prophylaxis of implant-related osteomyelitis. Injury 2010, 41, 1053–1059. [Google Scholar] [CrossRef]
- Honda, M.; Kawanobe, Y.; Ishii, K.; Konishi, T.; Mizumoto, M.; Kanzawa, N.; Matsumoto, M.; Aizawa, M. In vitro and in vivo antimicrobial properties of silver-containing hydroxyapatite prepared via ultrasonic spray pyrolysis route. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 5008–5018. [Google Scholar] [CrossRef]
- Bergemann, C.; Zaatreh, S.; Wegner, K.; Arndt, K.; Podbielski, A.; Bader, R.; Prinz, C.; Lembke, U.; Nebe, J.B. Copper as an alternative antimicrobial coating for implants—An in vitro study. World J. Transplant. 2017, 7, 193–202. [Google Scholar] [CrossRef]
- Gerits, E.; Kucharikova, S.; Van Dijck, P.; Erdtmann, M.; Krona, A.; Lovenklev, M.; Fröhlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A.; et al. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res. 2016, 34, 2191–2198. [Google Scholar] [CrossRef]
- Svensson, S.; Suska, F.; Emanuelsson, L.; Palmquist, A.; Norlindh, B.; Trobos, M.; Bäckros, H.; Persson, L.; Rydja, G.; Ohrlander, M.; et al. Osseointegration of titanium with an antimicrobial nanostructured noble metal coating. Nanomedicine 2013, 9, 1048–1056. [Google Scholar] [CrossRef]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040304. [Google Scholar] [CrossRef] [PubMed]
- Chimutengwende-Gordon, M.; Pendegrass, C.; Blunn, G. The in vivo effect of a porous titanium alloy flange with hydroxyapatite, silver and fibronectin coatings on soft-tissue integration of intraosseous transcutaneous amputation prostheses. Bone Jt. J. 2017, 99 Pt B, 393–400. [Google Scholar] [CrossRef]
- Petrochenko, P.E.; Zheng, J.; Casey, B.J.; Bayati, M.R.; Narayan, R.J.; Goering, P.L. Nanosilver-PMMA composite coating optimized to provide robust antibacterial efficacy while minimizing human bone marrow stromal cell toxicity. Toxicol. In Vitro 2017, 44, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, O.D.; Kaspiris, A.; Goumenos, S.; Trikoupis, I.; Melissaridou, D.; Kalogeropoulos, A.; Serenidis, D.; Georgoulis, J.D.; Lianou, I.; Koulouvaris, P.; et al. Knee Arthrodesis with a Modular Silver-Coated Endoprosthesis for Infected Total Knee Arthroplasty with Extensive Bone Loss: A Retrospective Case-Series Study. J. Clin. Med. 2023, 12, 3600. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2022, S0022-391300345-6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar] [CrossRef]
- Harris, M.A.; Beenken, K.E.; Smeltzer, M.S.; Haggard, W.O.; Jennings, J.A. Phosphatidylcholine Coatings Deliver Local Antimicrobials and Reduce Infection in a Murine Model: A Preliminary Study. Clin. Orthop. Relat. Res. 2017, 475, 1847–1853. [Google Scholar] [CrossRef][Green Version]
- Metsemakers, W.J.; Emanuel, N.; Cohen, O.; Reichart, M.; Potapova, I.; Schmid, T.; Segal, D.; Riool, M.; Kwakman, P.H.; de Boer, L.; et al. A doxycycline-loaded polymer-lipid encapsulation matrix coating for the prevention of implant-related osteomyelitis due to doxycycline-resistant methicillin-resistant Staphylococcus aureus. J. Control. Release 2015, 209, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Harjai, K.; Chhibber, S. Bacteriophage mediated killing of Staphylococcus aureus in vitro on orthopaedic K wires in presence of linezolid prevents implant colonization. PLoS ONE 2014, 9, e90411. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; Dirks, A.J.; Jaspers, V.; de Boer, L.; Loontjens, T.J.; van der Loos, C.M.; Florquin, S.; Apachitei, I.; Rijk, L.N.; Keul, H.A.; et al. A chlorhexidine-releasing epoxy-based coating on titanium implants prevents Staphylococcus aureus experimental biomaterial-associated infection. Eur. Cell Mater. 2017, 33, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Kälicke, T.; Schierholz, J.; Schlegel, U.; Frangen, T.M.; Köller, M.; Printzen, G.; Seybold, D.; Klöckner, S.; Muhr, G.; Arens, S. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: An in vitro and in vivo study. J. Orthop. Res. 2006, 24, 1622–1640. [Google Scholar] [CrossRef]
- Miao, Q.; Sun, J.L.; Huang, F.; Wang, J.; Wang, P.; Zheng, Y.F.; Wang, F.; Ma, C.F. Antibacterial Peptide HHC-36 Sustained-Release Coating Promotes Antibacterial Property of Percutaneous Implant. Front. Bioeng. Biotechnol. 2021, 9, 735889. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liao, X.; Chen, H. Antibiotic-Loaded MMT/PLL-Based Coating on the Surface of Endosseous Implants to Suppress Bacterial Infections. Int. J. Nanomed. 2021, 16, 2983–2994. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Garcia-Casas, A.; Mediero, A.; Romera, D.; Mulero, F.; Cuevas-Lopez, I.; Jiménez-Morales, A.; Esteban, J. A New Antibiotic-Loaded Sol-Gel Can Prevent Bacterial Prosthetic Joint Infection: From in vitro Studies to an in vivo Model. Front. Microbiol. 2019, 10, 2935. [Google Scholar] [CrossRef]
- Bai, H.; Jing, D.; Guo, A.; Yin, S. Randomized controlled trial of zoledronic acid for treatment of osteoporosis in women. J. Int. Med. Res. 2013, 41, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Yu, X.; Yu, H.; Huang, J.; Zhang, B.; Xiao, J. Development of an anti-infective coating on the surface of intraosseous implants responsive to enzymes and bacteria. J. Nanobiotechnol. 2021, 19, 241. [Google Scholar] [CrossRef]
- Devlin-Mullin, A.; Todd, N.M.; Golrokhi, Z.; Geng, H.; Konerding, M.A.; Ternan, N.G.; Hunt, J.A.; Potter, R.J.; Sutcliffe, C.; Jones, E.; et al. Atomic Layer Deposition of a Silver Nanolayer on Advanced Titanium Orthopedic Implants Inhibits Bacterial Colonization and Supports Vascularized de Novo Bone Ingrowth. Adv. Healthc. Mater. 2017, 6, 1700033. [Google Scholar] [CrossRef]
- Xie, K.; Zhou, Z.; Guo, Y.; Wang, L.; Li, G.; Zhao, S.; Liu, X.; Li, J.; Jiang, W.; Wu, S.; et al. Long-Term Prevention of Bacterial Infection and Enhanced Osteoinductivity of a Hybrid Coating with Selective Silver Toxicity. Adv. Healthc. Mater. 2019, 8, e1801465. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Yudintceva, N.M.; Blinova, M.I.; Voronkina, I.V.; Suslov, D.N.; Galibin, O.V.; Gavrilov, D.V.; Akkaoui, M.; Raykhtsaum, G.; Albul, A.V.; et al. Evaluation of the temporary effect of physical vapor deposition silver coating on resistance to infection in transdermal skin and bone integrated pylon with deep porosity. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 169–177. [Google Scholar] [CrossRef]
- Funao, H.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Tsuji, T.; Okada, Y.; Koyasu, S.; Toyama, Y.; Nakamura, M.; Aizawa, M.; et al. A novel hydroxyapatite film coated with ionic silver via inositol hexaphosphate chelation prevents implant-associated infection. Sci. Rep. 2016, 6, 23238. [Google Scholar] [CrossRef]
- Tran, N.; Tran, P.A.; Jarrell, J.D.; Engiles, J.B.; Thomas, N.P.; Young, M.D.; Hayda, R.A.; Born, C.T. In vivo caprine model for osteomyelitis and evaluation of biofilm-resistant intramedullary nails. BioMed Res. Int. 2013, 2013, 674378. [Google Scholar] [CrossRef][Green Version]
- Kuo, Y.J.; Chen, C.H.; Dash, P.; Lin, Y.C.; Hsu, C.W.; Shih, S.J.; Chung, R.J. Angiogenesis, Osseointegration, and Antibacterial Applications of Polyelectrolyte Multilayer Coatings Incorporated with Silver/Strontium Containing Mesoporous Bioactive Glass on 316L Stainless Steel. Front. Bioeng. Biotechnol. 2022, 10, 818137. [Google Scholar] [CrossRef]
- Hu, C.C.; Chang, C.H.; Chang, Y.; Hsieh, J.H.; Ueng, S.W. Beneficial Effect of TaON-Ag Nanocomposite Titanium on Antibacterial Capacity in Orthopedic Application. Int. J. Nanomed. 2020, 15, 7889–7900. [Google Scholar] [CrossRef]
- Inoue, D.; Kabata, T.; Ohtani, K.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Inhibition of biofilm formation on iodine-supported titanium implants. Int. Orthop. 2017, 41, 1093–1099.I. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv. Mater. 2018, 30, e1801808. [Google Scholar] [CrossRef]
- Gomez-Barrena, E.; Esteban, J.; Molina-Manso, D.; Adames, H.; Martinez-Morlanes, M.J.; Terriza, A.; Yubero, F.; Puértolas, J.A. Bacterial adherence on UHMWPE with vitamin E: An in vitro study. J. Mater. Sci. Mater. Med. 2011, 22, 1701–1706. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, W.; Zhang, C.; Gao, B.; Guan, H.; Cheng, H.; Fu, J.; Li, F. Enhanced osseointegration and antibacterial action of zinc-loaded titania-nanotube-coated titanium substrates: In vitro and in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 3939–3950. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional sulfonated polyetheretherketone coating with beta-defensin-14 for yielding durable and broad-spectrum antibacterial activity and osseointegration. Acta Biomater. 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Noordin, S.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Hancock, R.E.; Wang, R. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1344–1352. [Google Scholar] [CrossRef]
- Williams, D.L.; Epperson, R.T.; Ashton, N.N.; Taylor, N.B.; Kawaguchi, B.; Olsen, R.E.; Haussener, T.J.; Sebahar, P.R.; Allyn, G.; Looper, R.E. In vivo analysis of a first-in-class tri-alkyl norspermidine-biaryl antibiotic in an active release coating to reduce the risk of implant-related infection. Acta Biomater. 2019, 93, 36–49. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Martin, V.T.; Wang, L.; Zeng, R.; You, D.; Zhang, X.; Elodie, W.H.; Yu, B. Carboxymethyl chitosan-zinc coating for prevention of pin tract infection: An animal model. J. Orthop. Surg. 2018, 26, 2309499017749981. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Bottagisio, M.; Maraldi, S.; Violatto, M.B.; Bortolin, M.; De Vecchi, E.; Bigini, P.; Drago, L.; Romanò, C.L. Vitamin E Phosphate Coating Stimulates Bone Deposition in Implant-related Infections in a Rat Model. Clin. Orthop. Relat. Res. 2018, 476, 1324–1338. [Google Scholar] [CrossRef]
- Peeters, E.; Hooyberghs, G.; Robijns, S.; De Weerdt, A.; Kucharikova, S.; Tournu, H.; Braem, A.; Čeh, K.; Majdič, G.; Španič, T.; et al. An antibiofilm coating of 5-aryl-2-aminoimidazole covalently attached to a titanium surface. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1908–1919. [Google Scholar] [CrossRef]
- Shiels, S.M.; Bouchard, M.; Wang, H.; Wenke, J.C. Chlorhexidine-releasing implant coating on intramedullary nail reduces infection in a rat model. Eur. Cell Mater. 2018, 35, 178–194. [Google Scholar] [CrossRef]
- Liu, D.; He, C.; Liu, Z.; Xu, W. Gentamicin coating of nanotubular anodized titanium implant reduces implant-related osteomyelitis and enhances bone biocompatibility in rabbits. Int. J. Nanomed. 2017, 12, 5461–5471. [Google Scholar] [CrossRef]
- Mauerer, A.; Stenglein, S.; Schulz-Drost, S.; Schorner, C.; Taylor, D.; Krinner, S.; Heidenau, F.; Adler, W.; Forst, R. Antibacterial Effect of a 4x Cu-TiO(2) Coating Simulating Acute Periprosthetic Infection-An Animal Model. Molecules 2017, 22, 1042. [Google Scholar] [CrossRef]
- Song, W.; Yu, X.; Markel, D.C.; Shi, T.; Ren, W. Coaxial PCL/PVA electrospun nanofibers: Osseointegration enhancer and controlled drug release device. Biofabrication 2013, 5, 035006. [Google Scholar] [CrossRef]
- Jennings, J.A.; Beenken, K.E.; Skinner, R.A.; Meeker, D.G.; Smeltzer, M.S.; Haggard, W.O.; Troxel, K.S. Antibiotic-loaded phosphatidylcholine inhibits staphylococcal bone infection. World J. Orthop. 2016, 7, 467–474. [Google Scholar] [CrossRef]
- Kose, N.; Caylak, R.; Peksen, C.; Kiremitci, A.; Burukoglu, D.; Koparal, S.; Doğan, A. Silver ion doped ceramic nano-powder coated nails prevent infection in open fractures: In vivo study. Injury 2016, 47, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Fabritius, M.; Al-Munajjed, A.A.; Freytag, C.; Julke, H.; Zehe, M.; Lemarchand, T.; Arts, J.J.; Schumann, D.; Alt, V.; Sternberg, K. Antimicrobial Silver Multilayer Coating for Prevention of Bacterial Colonization of Orthopedic Implants. Materials 2020, 13, 1415. [Google Scholar] [CrossRef]
- Gulcu, A.; Akman, A.; Demirkan, A.F.; Yorukoglu, A.C.; Kaleli, I.; Bir, F. Fosfomycin Addition to Poly(D,L-Lactide) Coating Does Not Affect Prophylaxis Efficacy in Rat Implant-Related Infection Model, But That of Gentamicin Does. PLoS ONE 2016, 11, e0165544. [Google Scholar] [CrossRef]
- Mauerer, A.; Lange, B.; Welsch, G.H.; Heidenau, F.; Adler, W.; Forst, R.; Richter, R.H. Release of Cu2+ from a copper-filled TiO2 coating in a rabbit model for total knee arthroplasty. J. Mater. Sci. Mater. Med. 2014, 25, 813–821. [Google Scholar] [CrossRef]
- Alt, V.; Kirchhof, K.; Seim, F.; Hrubesch, I.; Lips, K.S.; Mannel, H.; Domann, E.; Schnettler, R. Rifampicin-fosfomycin coating for cementless endoprostheses: Antimicrobial effects against methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA). Acta Biomater. 2014, 10, 4518–4524. [Google Scholar] [CrossRef]
- Giavaresi, G.; Meani, E.; Sartori, M.; Ferrari, A.; Bellini, D.; Sacchetta, A.C.; Meraner, J.; Sambri, A.; Vocale, C.; Sambri, V.; et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int. Orthop. 2014, 38, 1505–1512. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Miyamoto, H.; Ando, Y.; Noda, I.; Eto, S.; Akiyama, T.; Yonekura, Y.; Sonohata, M.; Mawatari, M. Acute and subacute toxicity in vivo of thermal-sprayed silver containing hydroxyapatite coating in rat tibia. BioMed Res. Int. 2014, 2014, 902343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, Y.; Huo, K.; Gao, B.; Xiong, W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles. J. Biomed. Mater. Res. A 2014, 102, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Miyamoto, H.; Yonekura, Y.; Tsukamoto, M.; Ando, Y.; Noda, I.; Sonohata, M.; Mawatari, M. Silver oxide-containing hydroxyapatite coating has in vivo antibacterial activity in the rat tibia. J. Orthop. Res. 2013, 31, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Moojen, D.J.; Vogely, H.C.; Fleer, A.; Nikkels, P.G.; Higham, P.A.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J. Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants-an experimental study in the rabbit. J. Orthop. Res. 2009, 27, 710–716. [Google Scholar] [CrossRef]
- Han, J.; Yang, Y.; Lu, J.; Wang, C.; Xie, Y.; Zheng, X.; Yao, Z.; Zhang, C. Sustained release vancomycin-coated titanium alloy using a novel electrostatic dry powder coating technique may be a potential strategy to reduce implant-related infection. Biosci. Trends 2017, 11, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.; Butcher, I.; Grigoris, P.; Murnaghan, C.; Seaton, R.A.; Tobin, C.M. Linezolid penetration into osteo-articular tissues. J. Antimicrob. Chemother. 2002, 50, 747–750. [Google Scholar] [CrossRef]
- Lovering, A.M.; Zhang, J.; Bannister, G.C.; Lankester, B.J.; Brown, J.H.; Narendra, G.; MacGowan, A.P. Penetration of linezolid into bone, fat, muscle and haematoma of patients undergoing routine hip replacement. J. Antimicrob. Chemother. 2002, 50, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Kutscha-Lissberg, F.; Hebler, U.; Muhr, G.; Koller, M. Linezolid penetration into bone and joint tissues infected with methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 2003, 47, 3964–3966. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Mai, H.N.; Kim, D.Y.; Hyun, D.C.; Park, J.H.; Lee, S.M.; Lee, D.H. A New Antibacterial Agent-Releasing Polydimethylsiloxane Coating for Polymethyl Methacrylate Dental Restorations. J. Clin. Med. 2019, 8, 1831. [Google Scholar] [CrossRef]
- Lu, M.M.; Wang, Q.J.; Chang, Z.M.; Wang, Z.; Zheng, X.; Shao, D.; Dong, W.F.; Zhou, Y.M. Synergistic bactericidal activity of chlorhexidine-loaded, silver-decorated mesoporous silica nanoparticles. Int. J. Nanomed. 2017, 12, 3577–3589. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhang, B.; Tang, J. Mussel-inspired functionalization of graphene for synthesizing Ag-polydopamine-graphene nanosheets as antibacterial materials. Nanoscale 2013, 5, 118–123. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Riduan, S.N.; Zhang, Y. Recent Advances of Zinc-based Antimicrobial Materials. Chem. Asian J. 2021, 16, 2588–2595. [Google Scholar] [CrossRef]
| Author Year | Coating Technology and Type of Implant a | Release Profile Burst Release | Antimicrobial Activity | ||
|---|---|---|---|---|---|
| Pathogens | Outcome | Cells | |||
| Vester et al. [7], 2010 * | Gentamicin (10% w/w) PDLLA (10% w/w) Ti IMnails and K-wires | Yes (60% within 1 min, 85% after 6 w) | B. subtilis, S. aureus, S. epidermidis | Bactericidal effect, adhesion inhibition, no development of resistance | Saos-2 |
| Zhang et al. [19], 2014 * | VA-coated Ti implants | Yes (~50% on d1, ~80% through d28) | S. aureus | Growth inhibition | MC3T3-E1 |
| Harris et al. [20], 2017 * | Amikacin and C2DA (5–25% w/w) PC-coated stainless steel K-wires | Yes (mainly for 1–2 d, 40–50% through d4-7) | P. aeruginosa, S. aureus | Growth inhibition (S. aureus: 25% amikacin or 15% amikacin + C2DA, P. aeruginosa: all tested eluates; no inhibition of S. aureus for 15% amikacin alone or 5% amikacin + C2DA) | - |
| Metsemakers et al. [21], 2015 * | Doxy-loaded PLEX-coated TAN rectangular implants or IM nails | Yes (25% on d1, >95% through 4 w) | doxyR MRSA, doxyS MSSA | Growth inhibition | - |
| Kaur et al. [22], 2014 ** | Phage and Linezolid (5% w/w) HPMC (4% w/v)-coated K-wires | Max. elution (linezolid, within 30 min; phage, after d1, both through d4) | MRSA | Adhesion inhibition, no development of resistance | - |
| Riool et al. [23], 2017 * | CHX (5, 10 wt %)/dopamine/epoxy-based Al sheets and Ti implants | Yes (>80% within d1, through d4) | S. aureus | Bactericidal effect | - |
| Kalicke et al. [24], 2006 * | RFP (3%) and fusidic acid (7%) or Octenidin (2%) and Irgasan (8%) PLLA-coated Ti plates | Yes (~60% within 1 h, ~80% after 42 d) | S. aureus | Bactericidal effect, adhesion inhibition (more pronounced in the antiseptic-coated plate) | - |
| Miao, et al. [25], 2021 * | HHC36-PDLLA/PLGA implants | Yes (present in the first hours, 30% for the PDLLA group and 21% for the PLGA group on d1, 47, and 33%, respectively, after 15 d) | S. aureus | Bactericidal effect, adhesion inhibition | - |
| Yu, et al. [26], 2021 * | (MMT/PLL-VA)8 K-wires | Yes (CMS degradation accelerates multilayer degradation and VA release) | S. aureus | Bactericidal effect | Osteoblasts |
| Aguilera-Correra, et al. [27], 2019 * | Moxifloxacin-loaded organic–inorganic sol-gel Ti K-wires | Yes (linear release with max rate at 48 h) | E. coli, S. aureus, S. epidermidis | Biofilm formation inhibition | MC3T3-E1 |
| Bai, et al. [28], 2013 * | (MMT/HA-RFP)10 K-wires (RFP: 1 mg/mL) | - | S. aureus | Growth inhibition | - |
| Liao, et al. [29], 2021 * | (MMT/PLL-CHX)10 K-wires | Slow CHX release in PBS, increased release in the presence of S. aureus | S. aureus (CMS) | Bactericidal effect | Osteoblasts |
| Author Year | Coating Technology and Type of Implant a | Release Profile Burst Release | Antimicrobial Activity | Biocompatibility | ||
|---|---|---|---|---|---|---|
| Pathogens | Outcome | Cells | Outcome | |||
| Xu et al. [4], 2018 * | AgNPs/PDA-coated PEGda hydrogel | - | E. coli, S. aureus | Bacteriostatic effect (more pronounced in E. coli) | MC3T3-E1 | No effect on morphology and adhesion, better viability, promotion of osteogenic differentiation and osteogenesis (increase in ALP, BSP, OC, and Runx2 mRNA expression, increased mineralization) |
| Honda et al. [8], 2013 * | Ag (1–20 mol%)/HAp powders | Yes (high within 2 d) | S. aureus | Bactericidal effect, adhesion and biofilm formation (>5 mol%) inhibition | MC3T3-E1 | No effect on viability (only 5 mol% was tested) |
| Svensson et al. [11], 2013 * | Ag/Pd/Au-coated Ti screws | - | S. aureus | Adhesion inhibition | - | - |
| Devlin-Mullin et al. [30], 2017 * | AgNPs-coated Ti solid and foam implants | - | MRSA, S. epidermidis | Adhesion and biofilm formation inhibition (on S. epidermidis, no effect on MRSA) | Saos-2, HMVEC | No effect on morphology, viability and adhesion |
| Xie et al. [31], 2019 * | AgNPs/HAp/CS/PDA-coated Ti nails | - | E. coli, S. aureus, S. epidermidis | Adhesion and biofilm formation inhibition, regulation of biofilm-related genes (icaA, icaR) | MC3T3-E1 | No effect on viability (but cytotoxicity on AgNTs/HAp), enhanced osteogenic differentiation (increased ALP activity and mineralization) |
| Shevtsov et al. [32], 2019 * | Ag-coated Ti tablets and SBIP | - | P. aeruginosa, S. aureus, S. epidermidis | Adhesion and biofilm formation inhibition (including of planktonic bacteria) | MG-63, dermal fibroblasts, MSCs | No effect on morphology and adhesion |
| Funao et al. [33], 2016 * | Ag+ (0.1–10)/HAp/IP6 Ti pins | Yes (plateau by d1 + d3 depending on [Ag+], through d7) | S. aureus | Growth inhibition (1–10 mMAg+) | L-929 fibroblasts | No effect on viability (<20% at 5 mM Ag+, >50% at 10 mM) |
| Tran et al. [34], 2013 * | Ag (1.8–11.36 wt%)/TiO/siloxane-coated stainless steel IM nails | - | S. aureus | Bactericidal effect, adhesion inhibition (>1.8%) | Osteoblasts | No effect on viability (cytotoxicity for >11.36%) |
| Kuo et al. [35], 2022 * | SrMBG (10 wt% Sr) and AgSrMBG (10 wt% Sr and 1.64 wt%) Ag powders/ PEM films | Yes (at d8, PEM >57% of weight lost, PEM/ SrMBG 43%, PEM/AgSrMBG 37%) | E. coli | Growth inhibition | - | - |
| Hu et al. [36], 2020 * | TaN-Ag, TaN-(Ag, Cu), TaON-Ag, and TaN-coated Ti needles | - | CoNS, E. coli, MRSA, MSSA, P. aeruginosa | Growth inhibition (of TaON-Ag coating) | MSCs | No effect on osteogenesis |
| Inoue et al. [37], 2017 * | Iodine-coated (on oxidation film) Ti6Al4V metallic washers and K-wires | - | S. aureus | Adhesion and biofilm formation inhibition | - | - |
| Author Year | Coating Technology and Type of Implant a | Release Profile Burst Release | Antimicrobial Activity | Biocompatibility | ||
|---|---|---|---|---|---|---|
| Pathogens | Outcome | Cells | Outcome | |||
| Bergemann et al. [9], 2017 ** | TiCuN and TiCuN + BONIT® films | Yes (high within 24 h for TiCuN, low for TiCuN + BONIT®) | S. epidermidis | Biofilm formation inhibition (including planktonic bacteria) for TiCuN | MG-63 | Reduction in initial adhesion (for TiCuN; enhanced for TiCuN + BONIT®), no effect on morphology (less spreading on TiCuN + BONIT®), inhibition of viability (for both implants and with 2 different culturing approaches) |
| Tran et al. [38], 2019 * | Se (0.25–128 ppm) NPs on Ti plates and screws | - | MRSA, S. epidermidis | Growth inhibition (as low as 0.5 ppm Se; for >32 ppm, no difference) | hOBs | No effect on morphology, viability, and adhesion |
| Tan et al. [39], 2018 * | RP–IR780–RGDC Ti implants and rods | - | S. aureus | Growth and biofilm formation inhibition (upon irradiation and at 50 °C) | MC3T3-E1 | Improved viability, adhesion, and promotion of osteogenic differentiation (increased ALP activity and ALP, OC, and Runx2 mRNA expression) |
| Gomez-Barrena et al. [40], 2011 ** | VE (0.4, 3 wt% doped) or (0.1% blended) UHMWPE disks and squares, respectively | - | S. aureus, S. epidermidis | Adhesion inhibition (of S. epidermidis for both 0.4 and 3%, intra-species differences for 0.1% blended (inhibition of a collection strain of S.aureus, but not of clinical strains, while inhibition of 2 clinical strains of S.epidermidis, but not of the collection strain)] | - | - |
| Heidenau et al. [41], 2005 ** | Cu-TiO2 and 4xCu-TiO2-coated Ti6Al4V round metal plates | - | S. aureus | Adhesion inhibition (slight for Cu-TiO2; pronounced for 4xCu-TiO2, including of planktonic bacteria for 4xCu-TiO2) | MC3T3-E1 | No effect on viability (for Cu-TiO2 compared to TiO2; increased compared to Ti6Al4V), decreased viability (for 4xCu-TiO2), slight effect on morphology (“injured”, dead cells) |
| Li et al. [42], 2014 * | Zn/TiO2-NTs-coated Ti substrates | Yes (max. during d1, through d30 especially for NT-Zn3h) | S. aureus | Adhesion inhibition (including of planktonic bacteria; more pronounced for NT-Zn3h) | MC3T3-E1 | No effect on morphology (improved spreading), no effect on viability (decreased for NT-Zn3h on d4), no effect on initial adhesion, promotion of osteogenic differentiation (increased ALP activity, ALP, Col-1, OC, and OPG mRNA expression, and matrix mineralization) |
| Yuan et al. [43], 2019 * | MBD-14 (2, 5, 10 μg/mL)-loaded PEEK (SP) rectangular and cylindrical samples | - | P. aeruginosa, S. aureus | Growth inhibition (especially for 5, 10 μg/mL) | MSCs | Enhanced viability, adhesion, and osteogenic differentiation (increased ALP activity, increased ALP, Col-1, and OC mRNA and protein expression) |
| Kazemzadeh-Narbat et al. [44], 2012 * | HHC36 AMPep-loaded CaP-coated Ti plates and cylindrical implants | Yes (approx. 70% within 30 min, 90% within d1, through d7) | P. aeruginosa, S. aureus | Bactericidal effect | MG-63 | No effect on viability (cytotoxicity observed for >200 μg/mL), increased adhesion |
| Author Year | Animal Model | Coating Technology and Type of Implant a | Antimicrobial Activity and Biocompatibility | |
|---|---|---|---|---|
| Pathogens | Outcomes | |||
| Stavrakis et al., 2016 [1] * | Mice | VA and Tigecyclin PEG-PPS Ti K-wires | S. aureus | Reduction in bacterial forming colonies and of infection osteolysis |
| Kucharikova et al., 2016 [5] * | Mice | VA and Caspofungin in 3 aminopropyl-triethoxy silane Ti round disks | C. albicans, S. aureus | Biofilm formation reduction, no effect on osseointegration |
| Vester et al., 2010 [7] * | Rats | Gentamicin PDLLA Ti IM nails and K-wires | S. aureus, S. epidermidis | Prevention of bacterial adhesion and resistance, no effect on osseointegration |
| Gerits et al., 2016 [10] * | Mice | SPI031 Ti disks | P. aeruginosa, S. aureus | Growth and adhesion inhibition, no effect on osseointegration |
| Harris et al., 2017 [20] * | Mice | Amikacin and C2DA PC-coated stainless steel K-wires | P. aeruginosa, S. aureus | Biofilm formation reduction |
| Metsemakers et al., 2015 [21] * | Mice, Rabbits | Doxy-loaded PLEX-coated TAN rectangular implants or IM nails | MRSA, MSSA | Complete protection and infection reduction against implant-associated MSSA and MRSA osteomyelitis, respectively |
| Kaur et al., 2016 [22] # | Mice | Phage and Linezolid HPMC-coated Ti K-wires | S. aureus | Reduced bacterial adherence and inflammation and faster resumption of limb motor function |
| Riool et al. 2017 [23] * | Mice | CHX/ dopamine/epoxy-based Al sheets and Ti implants | S. aureus | Bactericidal effect, reduction in colony forming units, well-tolerated with no-toxicity |
| Yu et al., 2021 [26] * | Rats | (MMT/PLL-VA)8 Ti K-wires | S. aureus | Bactericidal effect |
| Aguilera-Correa et al., 2019 [27] * | Mice | Moxifloxacin-loaded organic–inorganic sol-gel K-wires | E. coli, S. aureus | Prevention of prosthetic joint infection |
| Bai et al., 2023 [28] * | Rats | (MMT/HA-RFP)10 Ti K-wires | S. aureus | Analysis of biofilm formation revealed antibacterial activity, good biocompatibility |
| Liao et al., 2021 [29] * | Rats | (MMT/PLL-CHX)10 Ti K-wires | S. aureus | Antibacterial activity, good biocompatibility |
| Yuan et al., 2019 [43] * | Rats | MBD-14-loaded PEEK (SP) rectangular and cylindrical samples | P. aeruginosa, S. aureus | Antibacterial activity, good osseointegration |
| Williams et al., 2019 [45] # | Sheep | CZ-01127 compound on silicone polymer Ti cylindrical plugs | MRSA | Local bacteria eradication of normal bone ingrowth |
| Peeters et al., 2019 [49] * | Rats | 5-aryl-2-aminoimidazole compound covalently attached to open porous Ti implants | S. aureus | Biofilm formation reduction, no effect on osseointegration |
| Shiels et al., 2018 [50] * | Rats | CHX polymer layer Ti K-wires | N/A (contaminated wound) | Reduced bacteria colonization and osteolysis, increased fracture union |
| Liu et al., 2017 [51] # | Rabbits | NTATi-G | S. aureus | Bacterial growth inhibition, increased bone volume |
| Song et al., 2013 [53] # | Rats | Doxy coaxial PCL/PVA electrospinning nanofiber Ti pins | S. aureus | Bacterial growth inhibition, enhanced osseointegration |
| Jennings et al., 2016 [54] # | Rabbits | VA-loaded PC Ti wires | S. aureus | Reduction in colony forming units, infiltration of inflammatory cells, increased bone growth |
| Gulcu et al., 2016 [57] # | Rats | Gentamicin and Fosfomycin PDLLA stainless steel K-wires | S. aureus | Fosfomycin is not effective in bacterial prophylaxis |
| Alt et al., 2014 [59] # | Rabbits | RFP-fosfomycin-coated Ti K-wires | MRSA, MSSA | Reduction in infection susceptibility |
| Giavaresi et al., 2014 [60] # | Rabbits | VA-loaded DAC Ti sand-blasted IM nails | MRSA | Reduction in bacterial colonization, increased histocompatibility |
| Moojen et al., 2009 [64] # | Rabbits | Tobramycin perapatite Ti cylindrical implants | S. aureus | Reduction in infection susceptibility increased osseointegration |
| Author Year | Animal Model | Coating Technology and Type of Implant a | Antimicrobial Activity and Biocompatibility | |
|---|---|---|---|---|
| Pathogens | Outcome | |||
| Xu et al., 2018 [4] * | Rats | AgNPs/PDA-coated PEGda hydrogel | E. coli, S. aureus | Bacteriostatic activity, maxillary bone defects healing |
| Xie et al., 2019 [31] * | Rats | AgNPs/HAp/CS/PDA-coated Ti nails | E. coli, S. aureus, S. epidermidis | Bacterial adhesion and biofilm formation inhibition, enhanced osseointegration |
| Shevtsov et al., 2019 [32] * | Rabbits | Ag-coated Ti tablets and SBIP | P. aeruginosa, S. aureus, S. epidermidis | Biofilm formation reduction, good biocompatibility, no toxicity |
| Funao et al., 2016 [33] * | Mice | Ag+/HAp/IP6 Ti pins | S. aureus | Antimicrobial activity, reduced osteomyelitis markers, no toxicity |
| Tran et al., 2013 [34] * | Caprine | Ag+/ TiO/siloxane-coated stainless steel IM nails | S. aureus | Bacterial adhesion reduction, no effect on osteoblast function, reduced osteolysis and infection serum markers |
| Kuo et al., 2022 [35] * | Rats | SrMBG and AgSrMBG powders/ PEM films | E. coli | Long-term antibacterial, angiogenic, and osseointegration activities |
| Hu et al., 2020 [36] * | Rats | TaN-Ag, TaN-(Ag, Cu), TaON-Ag, and TaN-coated Ti needles | E. coli, MSSA | Antibacterial activity, no effect on osseointegration |
| Tan et al., 2018 [39] * | Rats | RP–IR780–RGDC Ti implants and rods | S. aureus | Antibacterial activity, biofilm formation inhibition, excellent biocompatibility |
| Croes et al., 2018 [46] * | Rats | Ag and VA CS-based Ti rods | S. aureus | Reduction in infection rate (by VA, not Ag), increased inflammation and osteoclast formation (by Ag) |
| Martin et al., 2018 [47] # | Rabbits | Carboxymethyl CS-Zn stainless steel pins | S. aureus | Prevention of pin-tract infections |
| Lovati et al., 2018 [48] # | Rats | VE phosphate Ti K-wires | S. aureus | Increased bone deposition |
| Mauerer et al., 2017 [52] # | Rabbits | 4x Cu-TiO2 Ti6Al4V bolts | MRSA | Reduction in infection rate and blood infection indices |
| Kose et al., 2016 [55] # | Rabbits | Ag doped HAp Ti nails | MRSA | Bacterial growth reduction, no toxicity on osteoblastic function |
| Tsukamoto et al., 2014 [61] # | Rats | Ag HAp Ti rods | N/A | No acute or subacute toxicity |
| Cheng et al., 2014 [62] * | Rats | Ag-TiO2-NT rods | MRSA | Increased antibacterial activity and bio-integration properties |
| Akiyama et al., 2013 [63] # | Rats | Ag-HAp Ti rods | MRSA | Increased antibacterial activity and infection rates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaspiris, A.; Vasiliadis, E.; Pantazaka, E.; Lianou, I.; Melissaridou, D.; Savvidis, M.; Panagopoulos, F.; Tsalimas, G.; Vavourakis, M.; Kolovos, I.; et al. Current Progress and Future Perspectives in Contact and Releasing-Type Antimicrobial Coatings of Orthopaedic Implants: A Systematic Review Analysis Emanated from In Vitro and In Vivo Models. Infect. Dis. Rep. 2024, 16, 298-316. https://doi.org/10.3390/idr16020025
Kaspiris A, Vasiliadis E, Pantazaka E, Lianou I, Melissaridou D, Savvidis M, Panagopoulos F, Tsalimas G, Vavourakis M, Kolovos I, et al. Current Progress and Future Perspectives in Contact and Releasing-Type Antimicrobial Coatings of Orthopaedic Implants: A Systematic Review Analysis Emanated from In Vitro and In Vivo Models. Infectious Disease Reports. 2024; 16(2):298-316. https://doi.org/10.3390/idr16020025
Chicago/Turabian StyleKaspiris, Angelos, Elias Vasiliadis, Evangelia Pantazaka, Ioanna Lianou, Dimitra Melissaridou, Matthaios Savvidis, Fotios Panagopoulos, Georgios Tsalimas, Michail Vavourakis, Ioannis Kolovos, and et al. 2024. "Current Progress and Future Perspectives in Contact and Releasing-Type Antimicrobial Coatings of Orthopaedic Implants: A Systematic Review Analysis Emanated from In Vitro and In Vivo Models" Infectious Disease Reports 16, no. 2: 298-316. https://doi.org/10.3390/idr16020025
APA StyleKaspiris, A., Vasiliadis, E., Pantazaka, E., Lianou, I., Melissaridou, D., Savvidis, M., Panagopoulos, F., Tsalimas, G., Vavourakis, M., Kolovos, I., Savvidou, O. D., & Pneumaticos, S. G. (2024). Current Progress and Future Perspectives in Contact and Releasing-Type Antimicrobial Coatings of Orthopaedic Implants: A Systematic Review Analysis Emanated from In Vitro and In Vivo Models. Infectious Disease Reports, 16(2), 298-316. https://doi.org/10.3390/idr16020025







