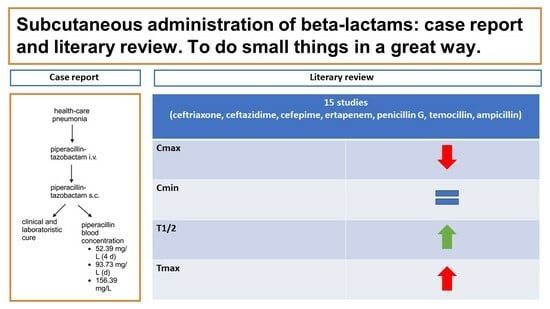
The Subcutaneous Administration of Beta-Lactams: A Case Report and Literary Review—To Do Small Things in a Great Way
Abstract
1. Introduction
2. Case Report
3. Methods
3.1. Search Strategy and Article Identification
3.2. Eligibility Criteria
3.3. Study Selection and Data Extraction
4. Results
4.1. Cephalosporins
4.2. Carbapenems
4.3. Penicillins
| Author, Publication Year | Study Design | Drug, Dosage | Patients (s.c. vs. Control Group) | Characteristics | PK Measures (s.c. vs. Control Group) | Clinical Outcome |
|---|---|---|---|---|---|---|
| Pilmis, 2020 [9] | Retrospective observational study | Cefepime, 3.1 g/day | 12 s.c. vs. 12 i.v. | 10 BJI, 2 BSI at urinary departure |
| no recurrence of infection at 6 months |
| Walker, 2005 [8] | PK study | Cefepime, 1 g | 10 (s.c. vs. i.v.) | Healthy volunteers |
| - |
| Borner, 1985 [5] | PK study | Ceftriaxone (0.5 g s.c. vs. 0.5 g i.v. vs. 2 g i.v) | 10 | Healthy volunteers |
| - |
| Harb, 2010 [7] | Phase I trial single-blind | Ceftriaxone, 1 g | 29 (ialuronidase-facilated s.c. infusion vs. standard s.c. infusion vs. i.v. infusion) | Healthy vounteers |
| - |
| Gauthier, 2014 [10] | Retrospective obseervational study | Ceftriaxone, | 38 s.c. vs. 110 i.m. | Patients with >75 years (15 pneumonia, 12 UTI, 3 AI, 3 colecistitis, 1 IE, 1 ABSSI) | Not specified | Clinical cure: 70.3% vs. 76.2% |
| Michelon, 2019 [19] | Case report | Ceftazidime, 1 g | 1 | Pseudomonas aerouginosa catheter related-UTI | Not specified | Clinical and laboratoristic cure |
| Duron, 2019 [20] | Case report | Ceftazidime, 6 g/day | 1 | VAP with BAL isolation of P. aerouginosa MSSA and Achrobacter xylosoxidans | Not specifiend | Clinical and laboratoristic cure |
| Ebihara, 2016 [6] | PK study | Ceftazidime, 0.5 g/10 mL in continuous infusion (without mentholate compression vs. mentholate compression) | 1 | Healthy volunteer |
| - |
| Pouderoux, 2019 [11] | Case series | Ceftriaxone, 1 g/24 h; ceftazidime 2 g/24 h; ertapenem 1 g/24 or 1 g/12 h | 10 (ceftriaxone 2, ceftazidime 1, ertapenem 6, 1 patient received ceftriaxone for 8 days then switched to ertapenem) | 7 PJI, 3 CO | For patients treated with ertapenem and ceftazidime Cmin was always higher than the targeted pathogen MIC | 6/10 had a clinical success, 2/10 presented a clinical failure after the s.c. antibiotic therapy, 1 presented a clinical failure before the s.c. antibiotic therapy, 1 lost to follow-up |
| Goutelle, 2021 [14] | Case series | Ceftazidime, ertapenem, ceftriaxone | 10 (ceftazidime 4, ertapenem 4, ceftriaxone 2) | BJI in chronic suppressive therapy | 2 cases with fT/MIC < 50% (1 patient treated with ceftazidime and 1 patient with ertapenem) | 9/10 have not developed a recurrence of infection |
| Frasca, 2009 [12] | PK study | Ertapenem, 1 g | 6 (s.c. vs. i.v.) | 5 VAP, 1 surgical wound infection |
| All survived |
| Goutelle, 2018 [13] | Retrospective PK study | Ertapenem (1 g/24 h, 2 g/24 h, 1 g/12 h) | 31 (s.c. vs. i.v.) | BJI | According to the Monte Carlo simulation, subcutaneous administration was associated with higher PTA values than the i.v. route | |
| Roubaud-Baudron, 2019 [15] | Prospective PK study | Ertapenem, 1 g/24 h | 26 (16 s.c. 10 i.v.) | Patients with >65 years (17 UTI, 5 pneumonia, 5) |
| Survival at 15 d: 75% vs. 70% |
| Matzneller, 2020 [16] | Prospective PK study | Temocillin, 2 g | 8 (s.c. vs. i.v.) | Healty volunteers |
| - |
| Kado, 2020 [17] | PK study | benzathine penicillin G, 1.2 MIU | 15 (7 s.c. vs. 8 i.m.) | Healty volunteers |
| - |
| Champoux, 1996 [18] | PK study | Ampicillin, 1 g | 24 (s.c. vs. i.v.) | Healty volunteer (12 young volunteers and 12 old volunteers) |
| - |
| Author, Publication Year | Drug, Dosage (s.c. vs. SoC) | Cmin (s.c. vs. SoC) | Cmax (s.c. vs. SoC) | AUC (s.c. vs. SoC or s.c./SoC) |
|---|---|---|---|---|
| Pilmis, 2020 [9] | Cefepime, 3.1 g/day (12 s.c. vs. 12 i.v.) | Cmin (median): 29.1 mg/L vs. 31.9 mg/L | // | // |
| Walker, 2005 [8] | Cefepime, 1 g (10 s.c. vs. i.v.) | // | // | AUC0−∞ (mean): 134.8 h∙mg/L vs. 137 h∙mg/L |
| Borner, 1985 [5] | Ceftriaxone (0.5 g s.c. vs. 0.5 g i.v. vs. 2 g i.v) | Cmin (mean) at 24 h: 6.6 mg/L vs. 6.5 mg/L | Cmax (mean): 37.1 mg/L vs. 83.8 mg/L | AUCsc/AUCev: 0.96 |
| Harb, 2010 [7] | Ceftriaxone, 1 g (29 ialuronidase-facilated s.c. infusion vs. standard s.c. infusion vs. i.v. infusion) | // | Cmax (mean): 92 μg/mL vs. 82.2 μg/mL vs. 150 μg/mL | AUC0−t (mean): 1139.3 μg∙h/L vs. 1115.6 μg∙h/L vs. 1065 μg∙h/L |
| Ebihara, 2016 [6] | Ceftazidime, 0.5 g/10 mL in continuous infusion (without mentholate compression vs. mentholate compression) | // | Cmax: 44.8 μg/mL vs. 57.4 μg/mL | // |
| Frasca, 2009 [12] | Ertapenem, 1 g/24 h (6 s.c. vs. i.v.) | Cmax (mean): 43 μg/mL vs. 115 μg/mL | AUC0−24/AUC0−24 (s.c./i.v.): 0.99 | |
| Roubaud-Baudron, 2019 [15] | Ertapenem, 1 g/24 h, (10 i.v. vs. 16 s.c.) | AUC (mean): 1126.92 mg∙h/L vs. 1005.27 mg∙h/L | ||
| Matzneller, 2020 [16] | Temocillin, 2 g (8 s.c. vs. i.v.) | C12 h (mean): 50.3 mg/L vs. 29.9 mg/L | Cmax (mean): 100 mg/L vs. 233 mg/L | AUC0−12 (mean): 818.1 mg∙h/L vs. 959.4 mg∙h/L |
| Kado, 2020 [17] | benzathine penicillin G, 1.2 MIU (7 s.c. vs. 8 i.m.) | Cmin: 10.5 ng/mL vs. 5.2 ng/mL | Cmax: 36.3 ng/mL vs. 56.8 ng/mL | // |
| Champoux, 1996 [18] | Ampicillin, 1 g (24 young s.c. vs. i.v.) |
| // |
|
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Tom-Revzon, C. Erratic absorption of intramuscular antimicrobial delivery in infants and children. Expert. Opin. Drug Metab. Toxicol. 2007, 3, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Tozer, T.Z. Absorption. In Clinical Pharmacokinetics: Concepts and Applications, 3rd ed.; Chapter 9; Lippincott Williams & Wilkins: New York, NY, USA, 1995; pp. 126–128. [Google Scholar]
- Ritschel, W.A.; Kearns, G.L. Handbook of Basic Pharmacokinetics. Including Clinical Applications, 6th ed.; American Pharmacists Association: Washington, DC, USA, 2004; pp. 228–229. [Google Scholar]
- Borner, K.; Lode, H.; Hampel, B.; Pfeuffer, M.; Koeppe, P. Comparative pharmacokinetics of ceftriaxone after subcutaneous and intravenous administration. Chemotherapy 1985, 31, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Oshima, S.; Yasuda, Y.; Okita, M.; Ohara, K.; Negishi, A.; Ohshima, S.; Iwasaki, H.; Yoneyama, A.; Kitazumi, E.; et al. A survey of subcutaneous blood flow in patients with SMID and subcutaneous ceftazidime administration using mentholated warm compresses in healthy subjects. J. Int. Med. Res. 2016, 44, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Harb, G.; Lebel, F.; Battikha, J.; Thackara, J.W. Safety and pharmacokinetics of subcutaneous ceftriaxone administered with or without recombinant human hyaluronidase (rHuPH20) versus intravenous ceftriaxone administration in adult volunteers. Curr. Med. Res. Opin. 2010, 26, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Neuhauser, M.N.; Tam, V.H.; Willey, J.S.; Palmer, J.L.; Bruera, E.; Prince, R.A. Subcutaneous Administration of Cefepime. J. Pain Symptom Manag. 2005, 30, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Pilmis, B.; Mizrahi, A.; Petitjean, G.; Le Monnier, A.; El Helali, N. Clinical evaluation of subcutaneous administration of cefepime. Méd. Mal. Infect. 2020, 50, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.; Schambach, S.; Crouzet, J.; Sirvain, S.; Fraisse, T. Subcutaneous and intravenous ceftriaxone administration in patients more than 75 years of age. Méd. Mal. Infect. 2014, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Pouderoux, C.; Becker, A.; Goutelle, S.; Lustig, S.; Triffault-Fillit, C.; Daoud, F.; Fessy, M.H.; Cohen, S.; Laurent, F.; Chidiac, C.; et al. Subcutaneous suppressive antibiotic therapy for bone and joint infections: Safety and outcome in a cohort of 10 patients. J. Antimicrob. Chemother. 2019, 74, 2060–2064. [Google Scholar] [CrossRef]
- Frasca, D.; Marchand, S.; Petitpas, F.; Dahyot-Fizelier, C.; Couet, W.; Mimoz, O. Pharmacokinetics of Ertapenem following Intravenous and Subcutaneous Infusions in Patients. Antimicrob. Agents Chemother. 2010, 54, 924–926. [Google Scholar] [CrossRef]
- Goutelle, S.; Valour, F.; Gagnieu, M.-C.; Laurent, F.; Chidiac, C.; Ferry, T.; Lyon Bone and Joint Infection Study Group. Population pharmacokinetics and probability of target attainment of ertapenem administered by subcutaneous or intravenous route in patients with bone and joint infection. J. Antimicrob. Chemother. 2018, 73, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Goutelle, S.; Conrad, A.; Pouderoux, C.; Braun, E.; Laurent, F.; Gagnieu, M.-C.; Cohen, S.; Guitton, J.; Valour, F.; Ferry, T. Pharmacokinetic/Pharmacodynamic Dosage Individualization of Suppressive Beta-Lactam Therapy Administered by Subcutaneous Route in Patients With Prosthetic Joint Infection. Front. Med. 2021, 8, 583086. [Google Scholar] [CrossRef] [PubMed]
- Roubaud Baudron, C.; Legeron, R.; Ollivier, J.; Bonnet, F.; Greib, C.; Guerville, F.; Cazanave, C.; Kobeh, D.; Cressot, V.; Moneger, N.; et al. Is the subcutaneous route an alternative for administering ertapenem to older patients? PHACINERTA study. J. Antimicrob. Chemother. 2019, 74, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Matzneller, P.; Ngougni Pokem, P.; Capron, A.; Lackner, E.; Wulkersdorfer, B.; Nussbaumer-Pröll, A.; Österreicher, Z.; Duchek, M.; Van De Velde, S.; Wallemacq, P.E.; et al. Single-dose pharmacokinetics of temocillin in plasma and soft tissues of healthy volunteers after intravenous and subcutaneous administration: A randomized crossover microdialysis trial. J. Antimicrob. Chemother. 2020, 75, 2650–2656. [Google Scholar] [CrossRef]
- Kado, J.H.; Salman, S.; Henderson, R.; Hand, R.; Wyber, R.; Page-Sharp, M.; Batty, K.; Carapetis, J.; Manning, L. Subcutaneous administration of benzathine benzylpenicillin G has favourable pharmacokinetic characteristics for the prevention of rheumatic heart disease compared with intramuscular injection: A randomized, crossover, population pharmacokinetic study in healthy adult volunteers. J. Antimicrob. Chemother. 2020, 75, 2951–2959. [Google Scholar] [CrossRef]
- Champoux, N.; Du Souich, P.; Ravaoarinoro, M.; Phaneuf, D.; Latour, J.; Cusson, J.R. Single-dose pharmacokinetics of ampicillin and tobramycin administered by hypodermoclysis in young and older healthy volunteers. Br. J. Clin. Pharmacol. 1996, 42, 325–331. [Google Scholar] [CrossRef]
- Michelon, H.; Tardivel, M.; Dinh, A.; Alvarez, J.; Salomon, E.; Le Quintrec, J.; Hirt, D.; Davido, B. Efficacy and safety of subcutaneous administration of ceftazidime as a salvage therapy in geriatrics: A case report. Fundamemntal Clin. Pharma 2020, 34, 521–524. [Google Scholar] [CrossRef]
- Duron, D.; Chanoine, S.; Peron, M.; Lepelley, M.; Allenet, B.; Epaulard, O.; Camara, B.; Bedouch, P. A successful antibiotic treatment by a new administration route: A case report of a subcutaneous administration of ceftazidime and tobramycin. Fundamemntal Clin. Pharma 2019, 33, 703–706. [Google Scholar] [CrossRef]
- Schoenenberger-Arnaiz, J.A.; Ahmad-Diaz, F.; Miralbes-Torner, M.; Aragones-Eroles, A.; Cano-Marron, M.; Palomar-Martinez, M. Usefulness of therapeutic drug monitoring of piperacillin and meropenem in routine clinical practice: A prospective cohort study in critically ill patients. Eur. J. Hosp. Pharm. 2020, 27, e30–e35. [Google Scholar] [CrossRef] [PubMed]
- Novy, E.; Martinière, H.; Roger, C. The Current Status and Future Perspectives of Beta-Lactam Therapeutic Drug Monitoring in Critically Ill Patients. Antibiotics 2023, 12, 681. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC Distribution. Available online: http://www.eucast.org (accessed on 1 November 2023).
- Tilanus, A.; Drusano, G. Optimizing the Use of Beta-Lactam Antibiotics in Clinical Practice: A Test of Time. Open Forum Infect. Dis. 2023, 10, ofad305. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Alexander, K.M.; Manigaba, K.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; Peloquin, C.A. Beta-lactam target attainment and associated outcomes in patients with bloodstream infections. Int. J. Antimicrob. Agents 2023, 61, 106727. [Google Scholar] [CrossRef]
- Kobayashi, D.; Cho, M.; Yokota, K.; Shimbo, T. Safety of Subcutaneous Piperacillin/Tazobactam Administration Compared to Intravenous Administration: Propensity Score–Matched Cohort Study. J. Am. Med. Dir. Assoc. 2020, 21, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, V.; Forestier, E.; Gavazzi, G.; Ferry, T.; Grégoire, N.; Breilh, D.; Paccalin, M.; Goutelle, S.; Roubaud-Baudron, C. Subcutaneous Antibiotic Therapy: The Why, How, Which Drugs and When. J. Am. Med. Dir. Assoc. 2021, 22, 50–55.e6. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, M.; Guilhaumou, R.; Million, M.; Parola, P.; Lagier, J.-C.; Brouqui, P.; Cassir, N. Subcutaneously administered antibiotics: A review. J. Antimicrob. Chemother. 2023, 78, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roubaud-Baudron, C.; Forestier, E.; Fraisse, T.; Gaillat, J.; De Wazières, B.; Pagani, L.; Ingrand, I.; Bernard, L.; Gavazzi, G.; Paccalin, M. Tolerance of subcutaneously administered antibiotics: A French national prospective study. Age Ageing 2016, 46, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Enkel, S.L.; Kado, J.; Hla, T.K.; Salman, S.; Bennett, J.; Anderson, A.; Carapetis, J.R.; Manning, L. Qualitative assessment of healthy volunteer experience receiving subcutaneous infusions of high-dose benzathine penicillin G (SCIP) provides insights into design of late phase clinical studies. PLoS ONE 2023, 18, e0285037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanza, G.M.; Liguoro, B.; Giuliano, S.; Moreal, C.; Montanari, L.; Angelini, J.; Cai, T.; Murri, R.; Tascini, C. The Subcutaneous Administration of Beta-Lactams: A Case Report and Literary Review—To Do Small Things in a Great Way. Infect. Dis. Rep. 2024, 16, 93-104. https://doi.org/10.3390/idr16010007
Leanza GM, Liguoro B, Giuliano S, Moreal C, Montanari L, Angelini J, Cai T, Murri R, Tascini C. The Subcutaneous Administration of Beta-Lactams: A Case Report and Literary Review—To Do Small Things in a Great Way. Infectious Disease Reports. 2024; 16(1):93-104. https://doi.org/10.3390/idr16010007
Chicago/Turabian StyleLeanza, Gabriele Maria, Beatrice Liguoro, Simone Giuliano, Chiara Moreal, Luca Montanari, Jacopo Angelini, Tommaso Cai, Rita Murri, and Carlo Tascini. 2024. "The Subcutaneous Administration of Beta-Lactams: A Case Report and Literary Review—To Do Small Things in a Great Way" Infectious Disease Reports 16, no. 1: 93-104. https://doi.org/10.3390/idr16010007
APA StyleLeanza, G. M., Liguoro, B., Giuliano, S., Moreal, C., Montanari, L., Angelini, J., Cai, T., Murri, R., & Tascini, C. (2024). The Subcutaneous Administration of Beta-Lactams: A Case Report and Literary Review—To Do Small Things in a Great Way. Infectious Disease Reports, 16(1), 93-104. https://doi.org/10.3390/idr16010007