1. Introduction
During the COVID(Corona Virus Disease-)-19 pandemic, strict infection control measures were globally enforced in an attempt to keep the estimated numbers of pandemic-associated excess deaths, which were observed even in resource-rich industrialized countries [
1], as low as possible. Military facilities were not exempt from this rule. Recently, several military medical researchers provided reports on how COVID-19 infection control was implemented at military units on deployment, in the home country as well as on armed Navy vessels [
2,
3,
4,
5]. As reported for pandemic management on a NATO (North Atlantic Treaty Organization) airbase in the Afghan deployment setting, the standard procedures comprised the isolation of infected individuals, quarantine for contacts, mask-based airway protection, and so-called “social distancing”, symptom screening, active contract tracing, as well as extensive (or, as called by the authors, “aggressive”) and obligatory molecular diagnostic on-site screening to identify asymptomatic or hypothetically dissimulating virus carries [
6]. As reported by Israeli military medical researchers, quarantine orders resulting from contact tracing especially had a considerable impact on the operational readiness of their forces. Balanced against the relatively mild medical impact of SARS-CoV-2 on their predominantly young and healthy soldiers, they advocated for more liberal strategies in similar situations [
7]. US (United States-) American military medical researchers calculated differences in quarantine costs, with quarantining based on geographic movement being more costly than quarantining based on close contact with infected individuals. The lost time on active duty per contained infection was nevertheless considerable [
8].
Irrespective of any normative interpretations on the appropriateness of chosen anti-pandemic measures, the experience reported by the Israeli and US American colleagues above [
7,
8] shows that strict infection control measures necessarily come with a price defined by undesired effects apart from infectious disease transmission prevention. Partly controversially discussed undesired effects among military personnel comprised overweight and diabetic diathesis due to reduced physical activity [
9,
10,
11], age-dependent and predisposition-dependent increases in suicide rates [
12,
13,
14], compensatory increases in risky sexual activity [
15,
16], affective disorders [
17], higher rates of gambling problems [
18] and considerable burn-out rates in military medical health staff [
19]. Typical for such multi-factorially influenced medical conditions, it is difficult to define which effects have been in response to the medical impact of COVID-19 itself or to side effects of the anti-pandemic response on the individuals’ social life and well-being.
Indirect support of the latter option is provided by assessments of the beliefs and attitudes of military personnel towards the COVID-19 pandemic, which showed that the spectrum of attitudes was considerable and, by far, not all soldiers considered the disease-associated threat as serious [
20,
21]. It is likely that negative attitudes towards restrictive infection control measures might have been facilitated by the fact that soldiers were much less endangered than civilians by COVID-19 in terms of both the need for intensive care treatment and death [
22], which is a typical “healthy worker effect”. Preventive strategies, including COVID-19 testing of military personnel with partly insufficiently reliable assays as described elsewhere [
23] may also have negatively influenced the soldiers’ trust in enforced infection control strategies. Insofar, it is not surprising that in spite of the fact that soldiers are in general both particularly affected by various infectious disease transmission risks [
24] and particularly predisposed to participating in infectious disease spread [
25], US American military medical researchers recently identified poor adherence to infection control procedures as a major risk of infectious disease spread among and by military personnel [
26].
Apart from soldiers, police officers were shown to be at a particularly high risk of acquiring SARS-CoV-2 infections in studies from Europe, South America and the Indian subcontinent [
27,
28,
29,
30]. High occupational exposure risks were considered as a likely explanation [
28]. Nevertheless, investigators from Poland were able to demonstrate that consequent adherence to infection control procedures like prolongated hand washing, wearing face masks and physical distancing reduced SARS-CoV-2 (Severe Acute Respiratory Syndrome Corona Virus 2) infection rates in police officers as well [
30].
Systematic assessments on the effects of the pandemic-associated infection control procedures of both deployed military personnel and deployed police officers are, unfortunately, scarce. This also applies to effects on infectious diseases apart from COVID-19. From civilian assessments, it is known that other infectious diseases were also considerably less frequently recorded due to the pandemic-associated enforcement of stricter infection control procedures, a phenomenon which was even believed to affect immunity at the subpopulation level [
31].
In the study presented here, we aimed to investigate hypothetical differences in the results of post-deployment assessments for German soldiers and police officers returning from predominantly tropical deployments regarding diagnosed infectious diseases in the pre-pandemic period and in the pandemic period. Applying this holistic approach, the hypothesis was tested whether or not increased infection control procedures during the pandemic period have led to an overall reduced infectious disease load in the deployed forces.
4. Discussion
The study was conducted to assess the potential impact of pandemic management strategies as enforced in Germany between 2020 and 2022 in response to the COVID-19 pandemic on the results of post-deployment screening approaches for German soldiers and police officers returning from predominantly tropical deployments. The hypothesis was that the increased infection control focus during the pandemic period might have affected the occurrence of non-respiratory infections as well. Civilian assessments had pointed in this direction before [
36]. The assessment led to a number of results.
First, there was a minor to moderate decline in the number of post-deployment medical assessments both for German military and police officers during the pandemic period. Within this period, medically assessed personnel were older and more likely to be male than female. During the whole study period, assessed returnees had predominantly been deployed to Sub-Saharan African deployment sites prior to their post-deployment assessments. It remains unclear how far pandemic-associated conditions affected the soldiers’ or police officers’ readiness to participate in the voluntary post-deployment assessments, potentially being associated with fewer consultations of returnees with personal perception of low infection risks on deployment.
Second, the calculated odds ratios did not indicate relevant changes in the proportions of febrile illness on deployment, with trends pointing in different directions for soldiers and police officers. Interestingly, there was a tendency for a decrease in fever on deployment in the assessed German soldiers in contrast to an also non-significant increase in the assessed deployed German police officers during the pandemic period. It might be speculated that strict enforcement of infection control protocols within military field camps could have ensured a protection level against febrile illness, which could not be provided to police officers operating in dislocated settings apart from standardized field camp infrastructure. This assumption is in line with previous observations pointing towards more infections in deployed personnel operating without much infrastructural background [
37].
Third, other than for febrile illness, there was a significance in deployed soldiers and a homologous trend in deployed police officers towards less gastroenteritis in the pandemic period. This is in striking contrast to the finding that there were no changed odds ratios for the diagnostic detection of microorganisms in post-deployment stool samples. This applied both for microbes with likely etiological relevance and for etiologically harmless protozoan colonizers, indicating the consumption of food and beverages under poor hygiene conditions. While this finding might be a mathematical phenomenon associated with the applied statistics for pathogens observed with low case numbers only, enteric protozoan parasites were so frequently recorded in stool samples of deployed soldiers that relevantly changed infection or colonization rates would most likely not have gone undetected. Therefore, we conclude that gastroenteric colonization or infection risks for deployed German soldiers and police officers did not relevantly change during the pandemic period in comparison to the pre-pandemic era.
Fourth, some pandemic-associated changes were recorded for indirect pathogen detection approaches, which were only available for post-deployment assessments of German soldiers. The recorded decline in
Mycobacterium tuberculosis-specific immuno-conversion during military deployments in the pandemic period might well be explained by the pandemic-associated application of mechanical airway protection. The detection of less positive anti-malarial antibody titers during the pandemic period is less easy to explain. A generally increased awareness of infection risks alone seems to be a poor explanation, considering the lack of significance for declines in many other pathogen detections. In any case, even these two significant findings need to be interpreted with care. First, both diagnostic assessments of
M. tuberculosis-specific immune-conversion and of anti-malarial antibodies to confirm previous malaria on deployment were not routine screening parameters, and so, their choice depended on individual medical decisions. Accordingly, varying judgments of presented medical conditions by different physicians are a likely source of bias. In addition, the recorded significances would not have passed the Bonferroni correction [
38], an approach which was not applied in this explorative hypothesis-forming assessment.
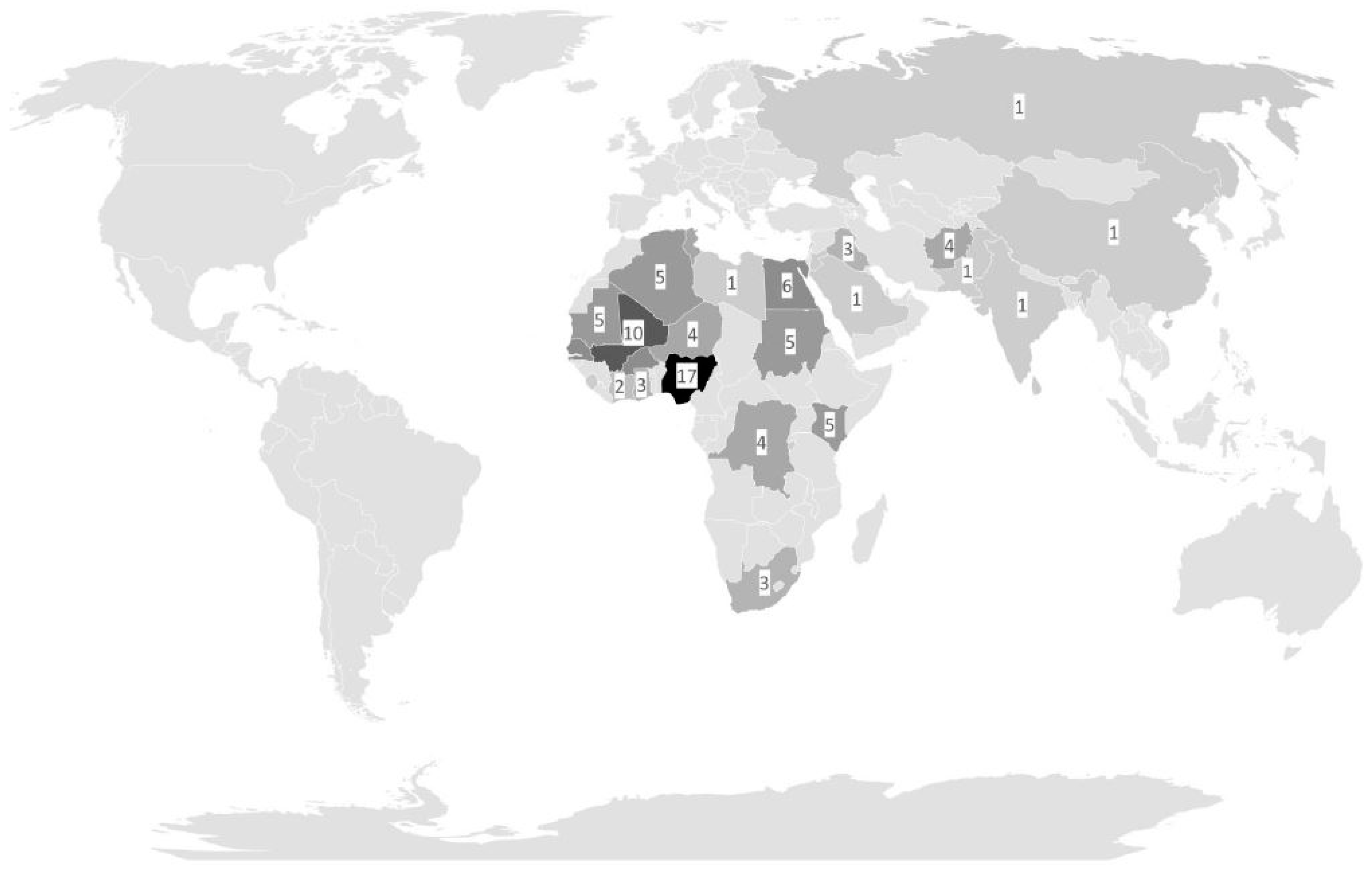
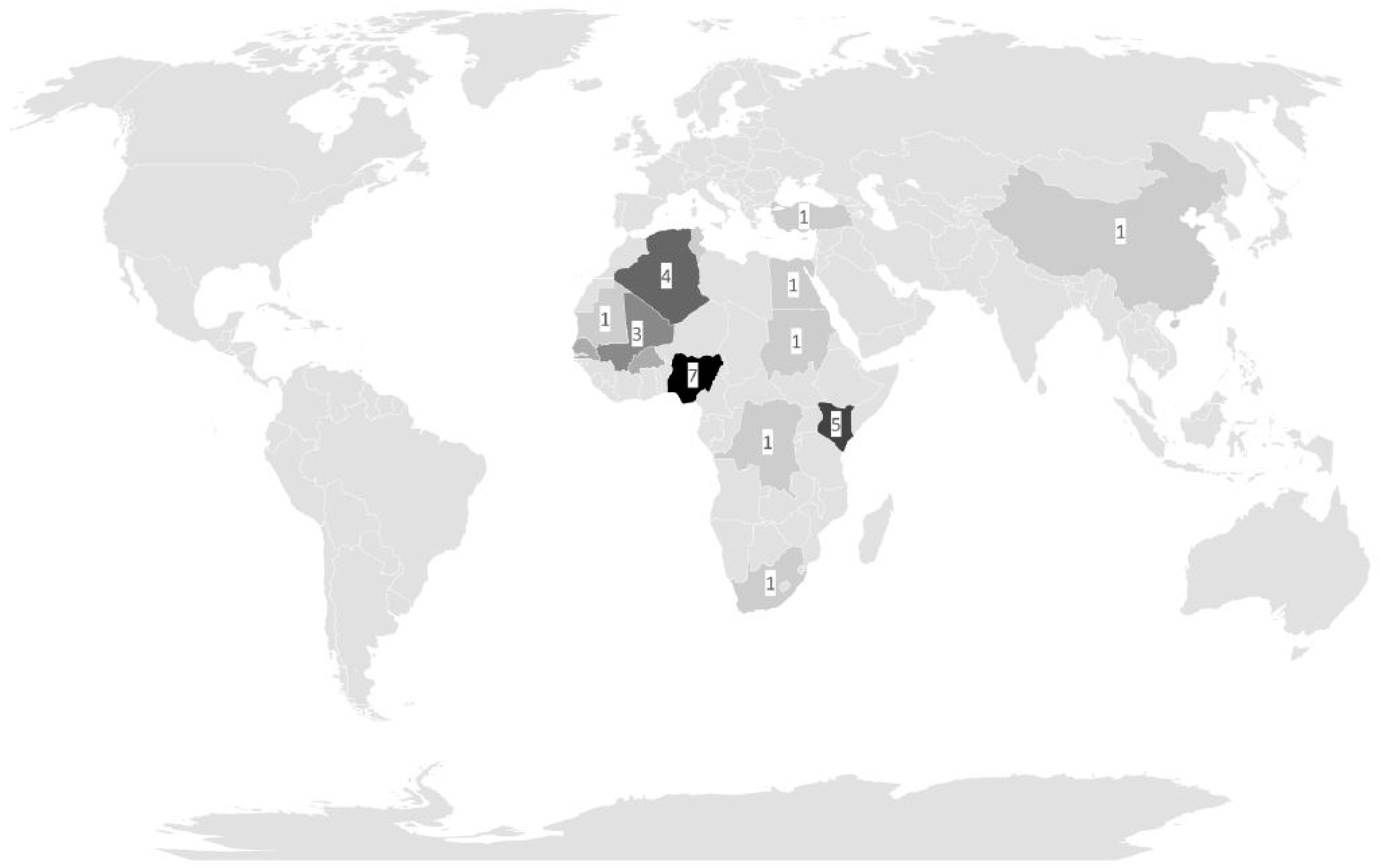
As expected, because of the distribution of the deployment sites, most infections were associated with deployments to the WHO African region and the WHO Eastern Mediterranean region. Observed symptoms in patients colonized with apathogenic microorganisms matched the general distribution within the assessed populations and, insofar, did not point towards unexpected vulnerability. The recorded side findings of declined proportions of mefloquine-based antimalarial chemoprophylaxis with an associated increase in atovaquone–proguanil-based antimalarial chemoprophylaxis on military deployments reflected the impact of a German pharmaceutical authority warning (“Rote Hand Brief”) on neuropsychiatric side effects of mefloquine and the waving of timely limitations of prophylactic atovaquone-proguanil-use in the manufacturer’s recommendations within the study period [
33]. Insofar, these findings were expected.
The study has a number of limitations. First, low overall infection rates limited the detectability of minor changes in infection risks for methodical reasons. Second, the screening approaches for soldiers and police officers were not identical for organizational reasons and so direct comparisons between these two populations were avoided. Third, considering the long total study period of more than 15 years, adaptations of diagnostic methods might have affected the comparability of individual test results, which is a common problem in retrospective long-term assessments. The same applies to adaptions of hygiene regimens for the deployment setting. Fourth, bias associated with individual diagnostic decisions by varying physicians over the assessment period cannot be denied. Fifth, the study design did not allow stratification by individual deployment-adapted infection control procedures during the COVID-19 pandemic but just provided a general overview. The same applies to the lack of information on individual adherence of the returnees with enforced infection control procedures, which is a likely source of bias, as suggested recently [
26]. Sixth, no reliable deployment-associated denominators can be provided, because the count of deployed forces varied over time or was partly not available for confidentiality reasons. Accordingly, the calculations were just based on absolute numbers of medically assessed individuals. For the same reasons, no comparison with the local infectious disease situation at the deployment site is feasible. Seventh, the definition of the pandemic period focused on the years 2020–2022 might be an issue of debate. The choice was based on the period in which the strictest infection control procedures were enforced in Germany, which rapidly waned starting at the beginning of 2023 [
32]. Eighth, no reliable data on deployment-related SARS-CoV-2 infections in the assessed soldiers and police officers were available as potential indicators of the general effectiveness of enforced infection control procedures on deployment. Ninth, varying proportions of soldiers and police officers returning from specific deployment sites in the pre-pandemic period and in the pandemic period are a likely source of bias.