COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Date at Which a Death Is Reported Versus Date of Occurrence
2.3. Estimation of the Year-of-Age Population in 2021
2.4. Estimation of the Year-of-Age Baseline for Deaths in England and Wales in 2019
2.5. Calculation of the Proportion of Deaths ‘With’ or ‘Due to’ COVID-19 Variants
3. Results
3.1. Validation of Deaths ‘Due to’ COVID-19 and Methods for Adjusting Deaths to a Common Year
- Monthly deaths for all-cause and ‘due to’ COVID-19 mortality by year-of-age [30]—not split by male and female, but does cover 2022.
- Adjusting deaths to the 2019 equivalent using mid-year population estimates contains hidden assumptions regarding the monthly profile of the influx of births during the World War I and II baby booms, and an alternative adjustment using annual births can be utilized.
- The matching between population/births and deaths also contains assumptions around the pattern of seasonal deaths. This is not an issue in the southern hemisphere, where winter occurs in the middle of a calendar year, but it creates inconsistencies in the northern hemisphere since winter spans two calendar years.
- The age profiles for excess mortality (with various adjustments) versus COVID-19 ‘due to’ deaths are roughly similar; however, deaths ‘due to’ COVID-19 appear to have been over-estimated, especially in 2021 among elderly females (with vaccination)—reflecting the well-known difficulty of attributing cause of death in elderly persons with multi-morbidities along with other factors [16]. See Supplementary Material S5.
- No discernible impact of COVID-19 upon all-cause deaths can be observed for ages 100+ or ages 1–30 either with or without adjustment—partly due to small number variation as in Supplementary Material S2.
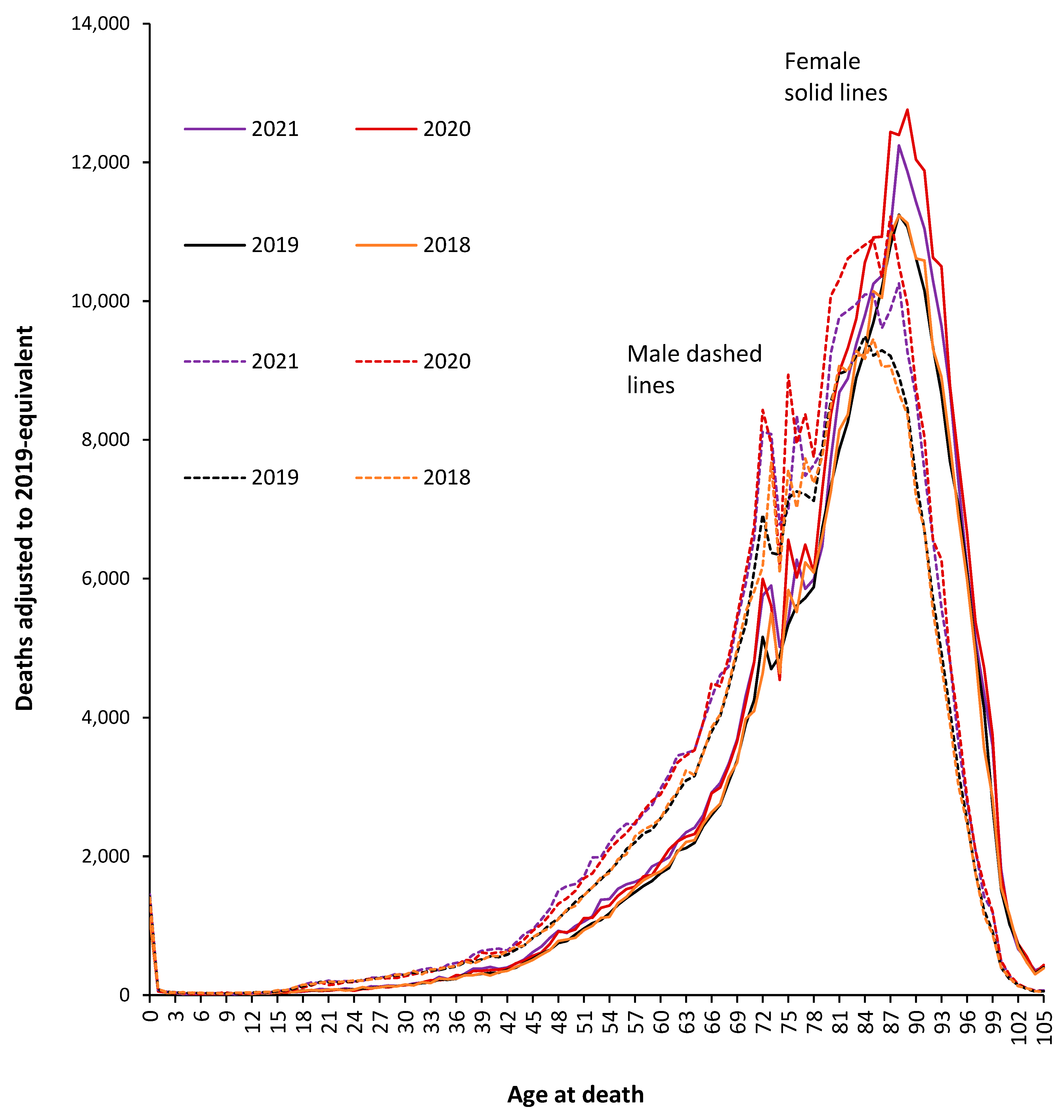
3.2. Population-Adjusted Deaths in 2020 and 2021 versus 2019
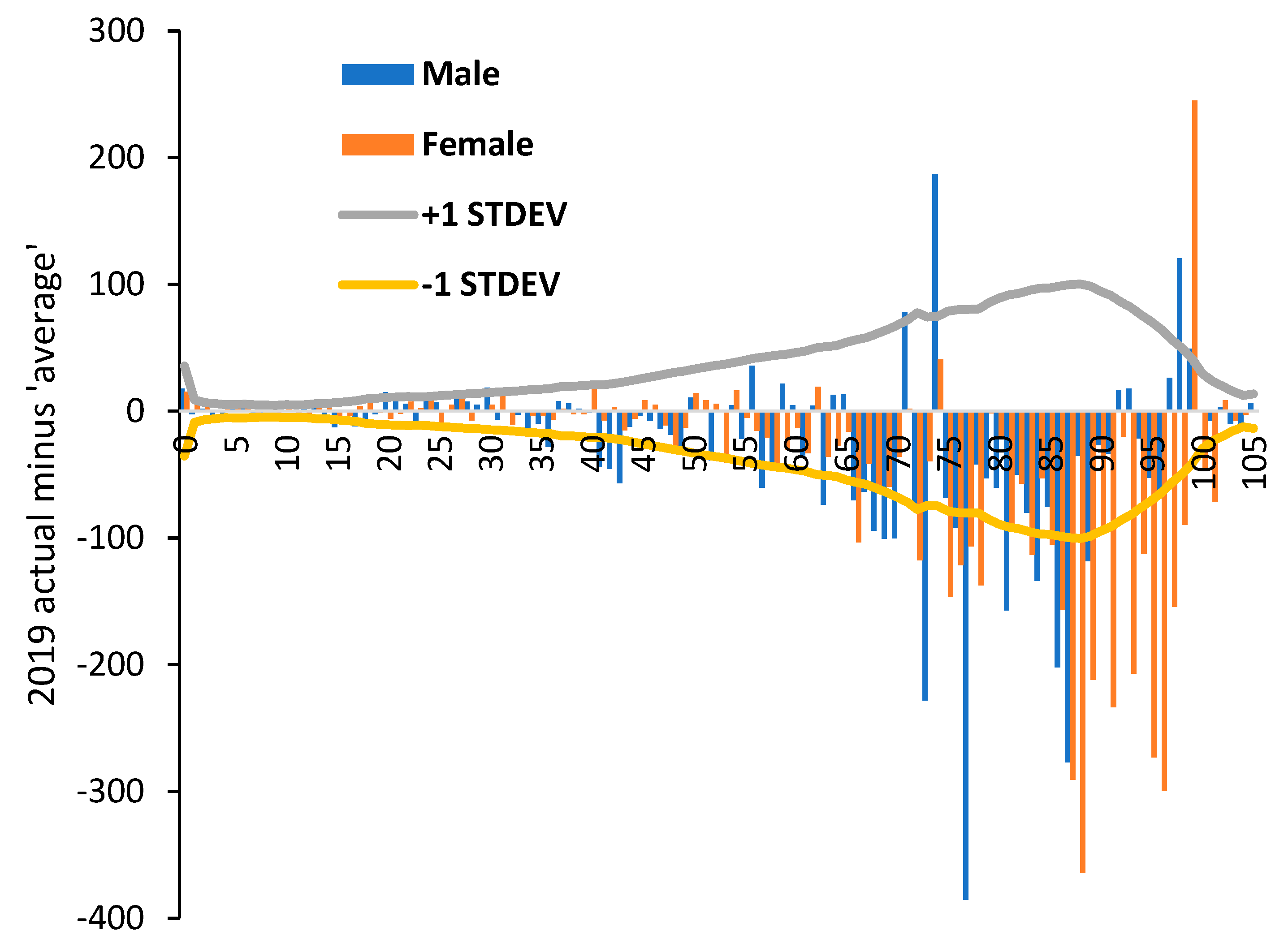
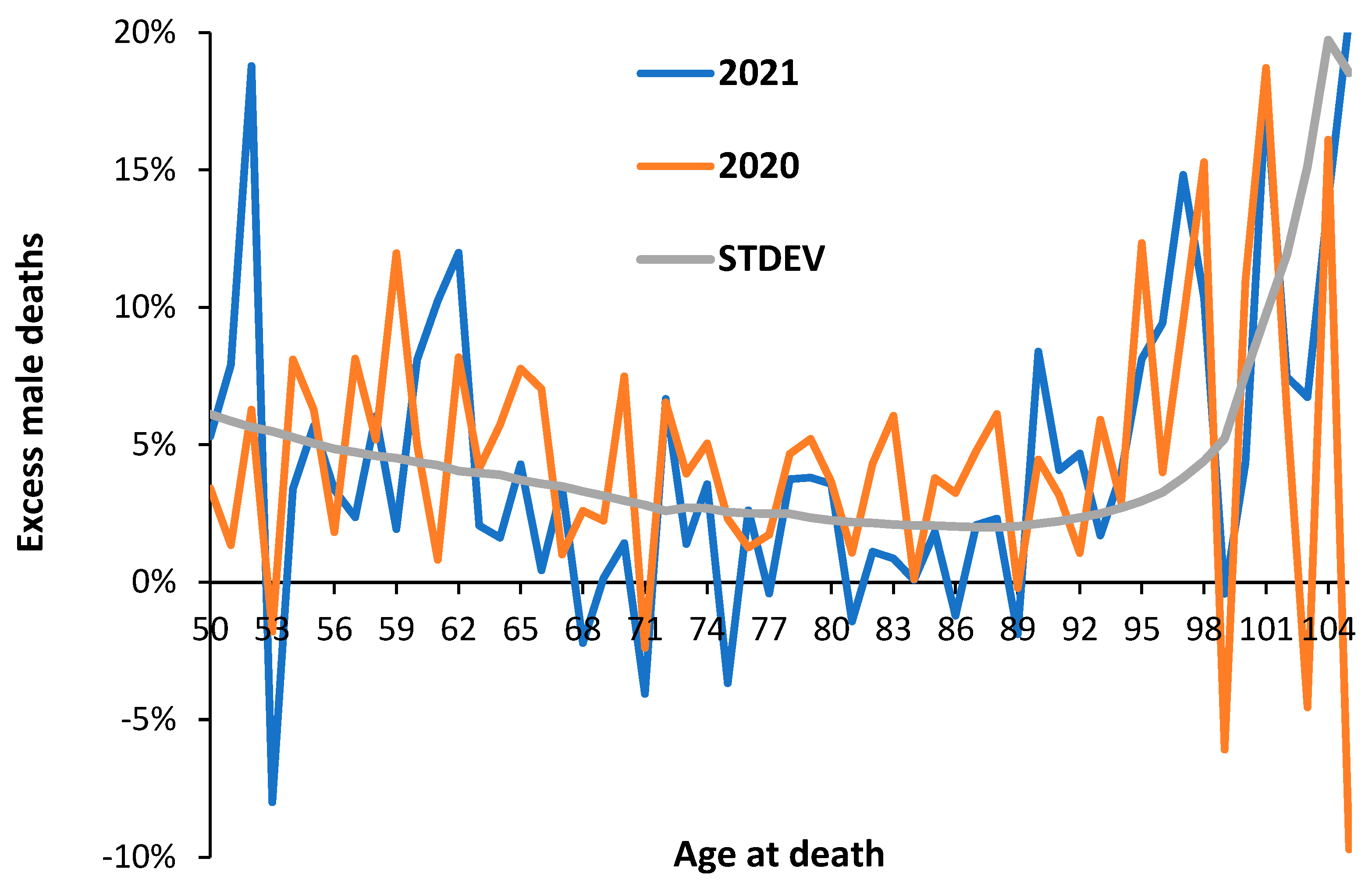
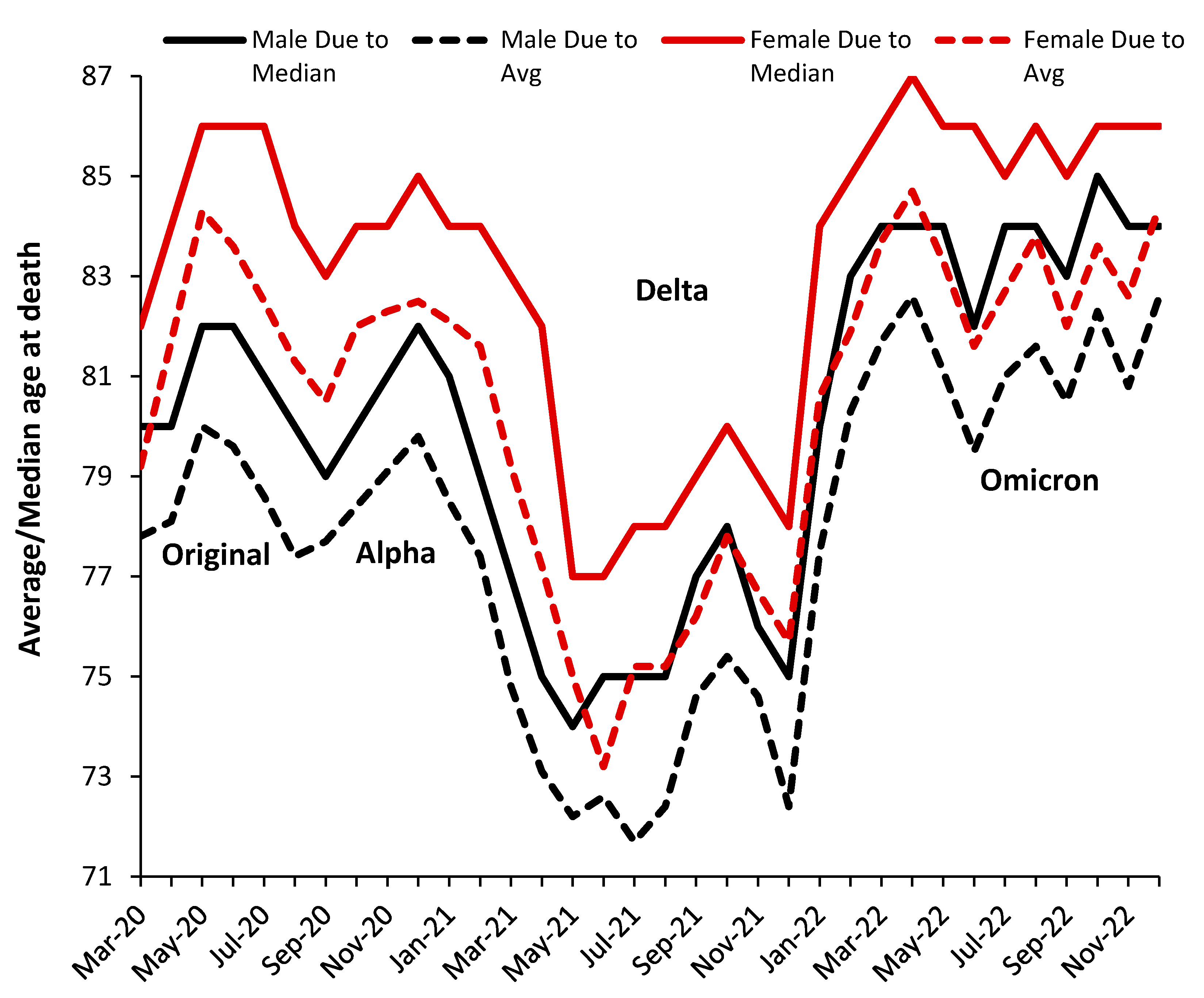
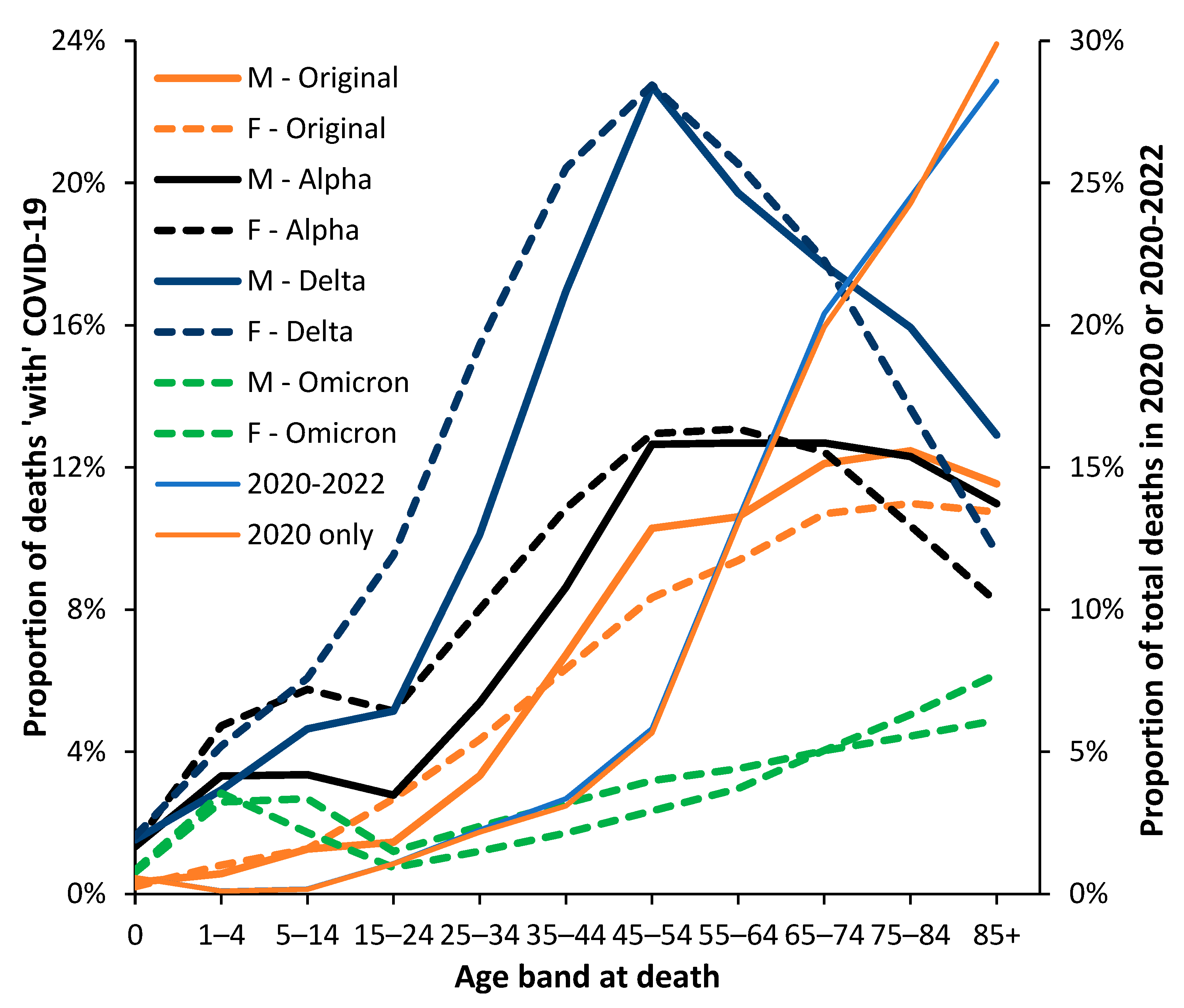
3.3. Male versus Female Deaths in England and Wales
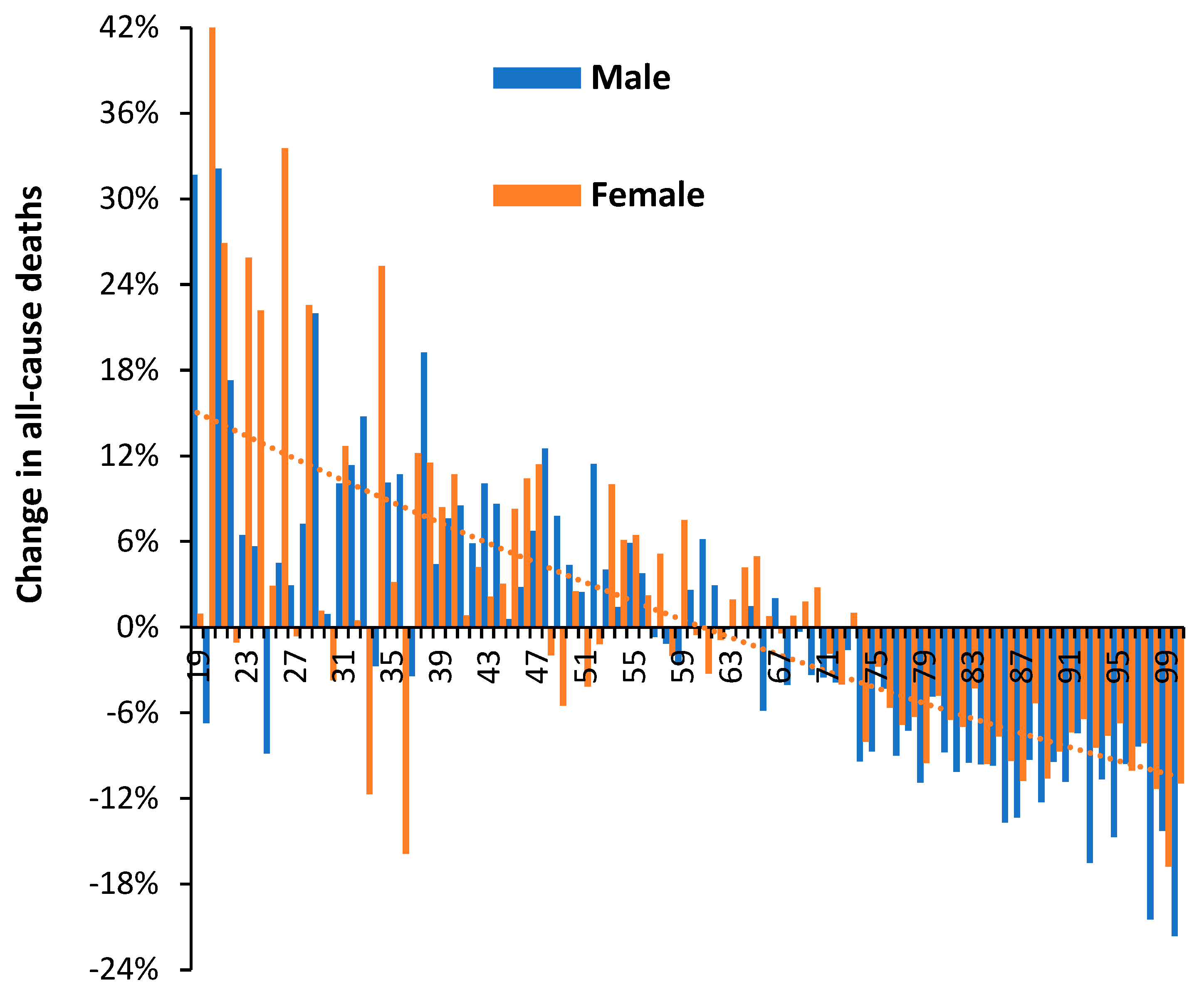
3.4. Net ‘Real World’ Change in All-Cause Deaths by Age and Gender
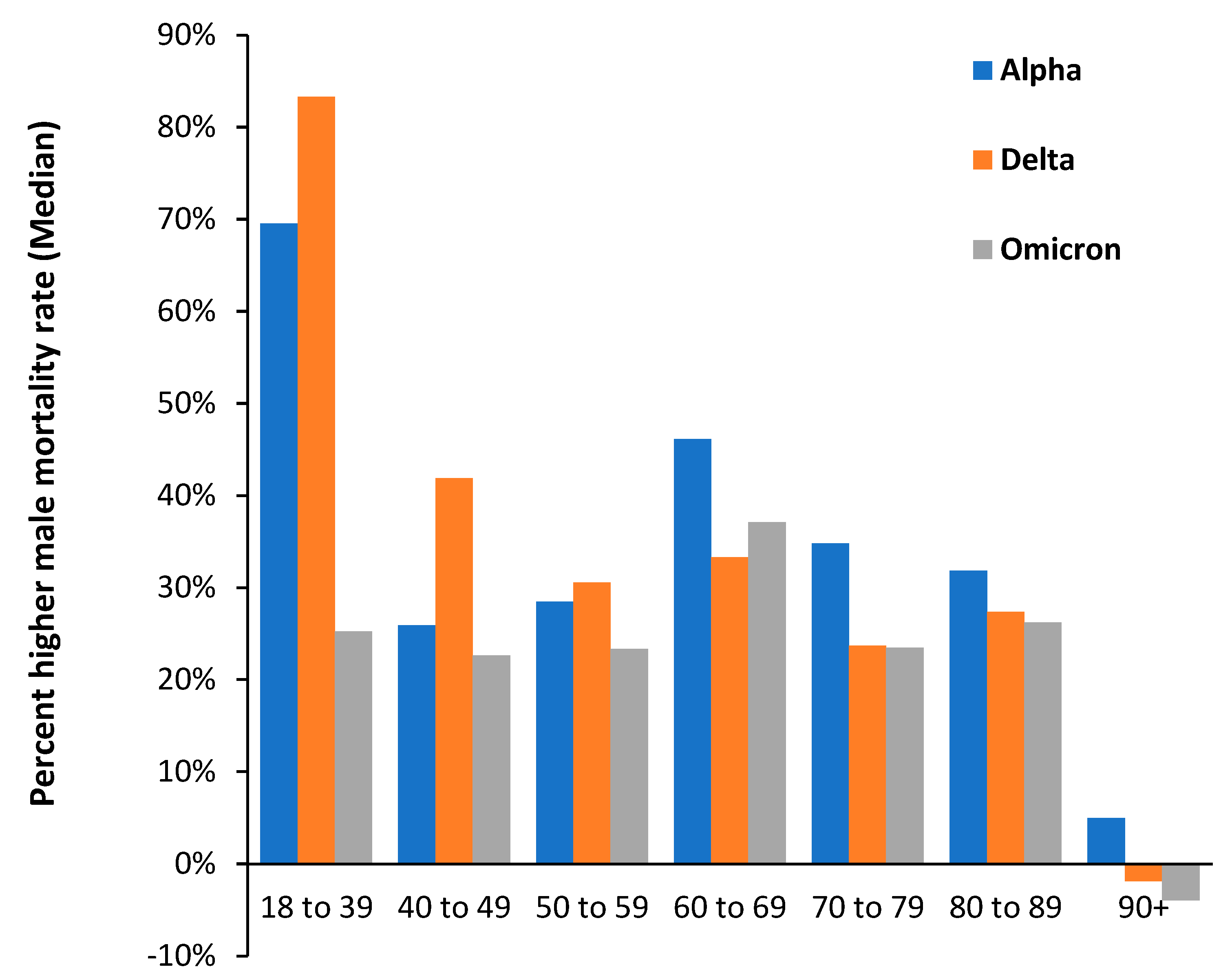
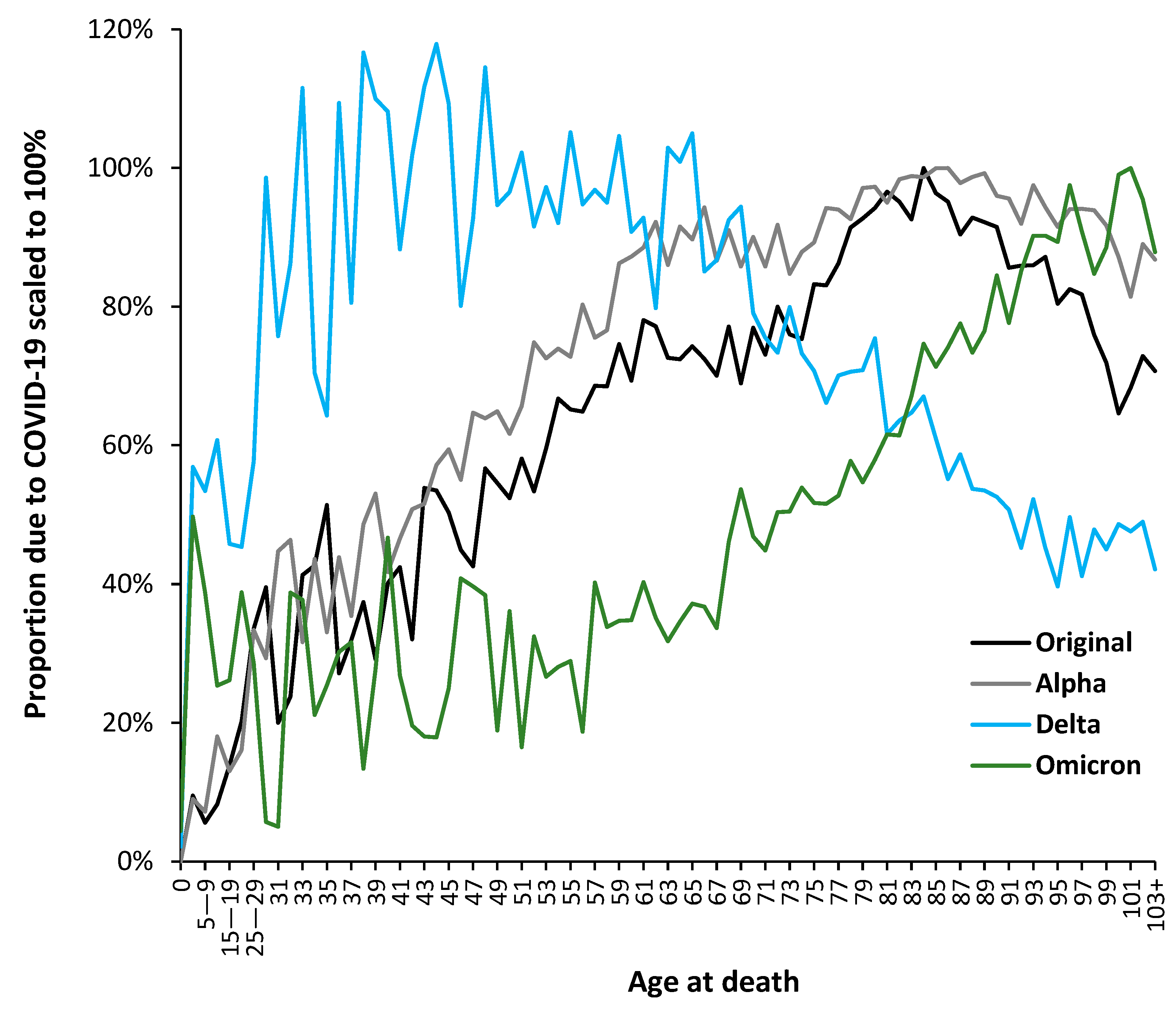
3.5. The Delta Variant, Second Half of 2021, Targeted at Younger Ages
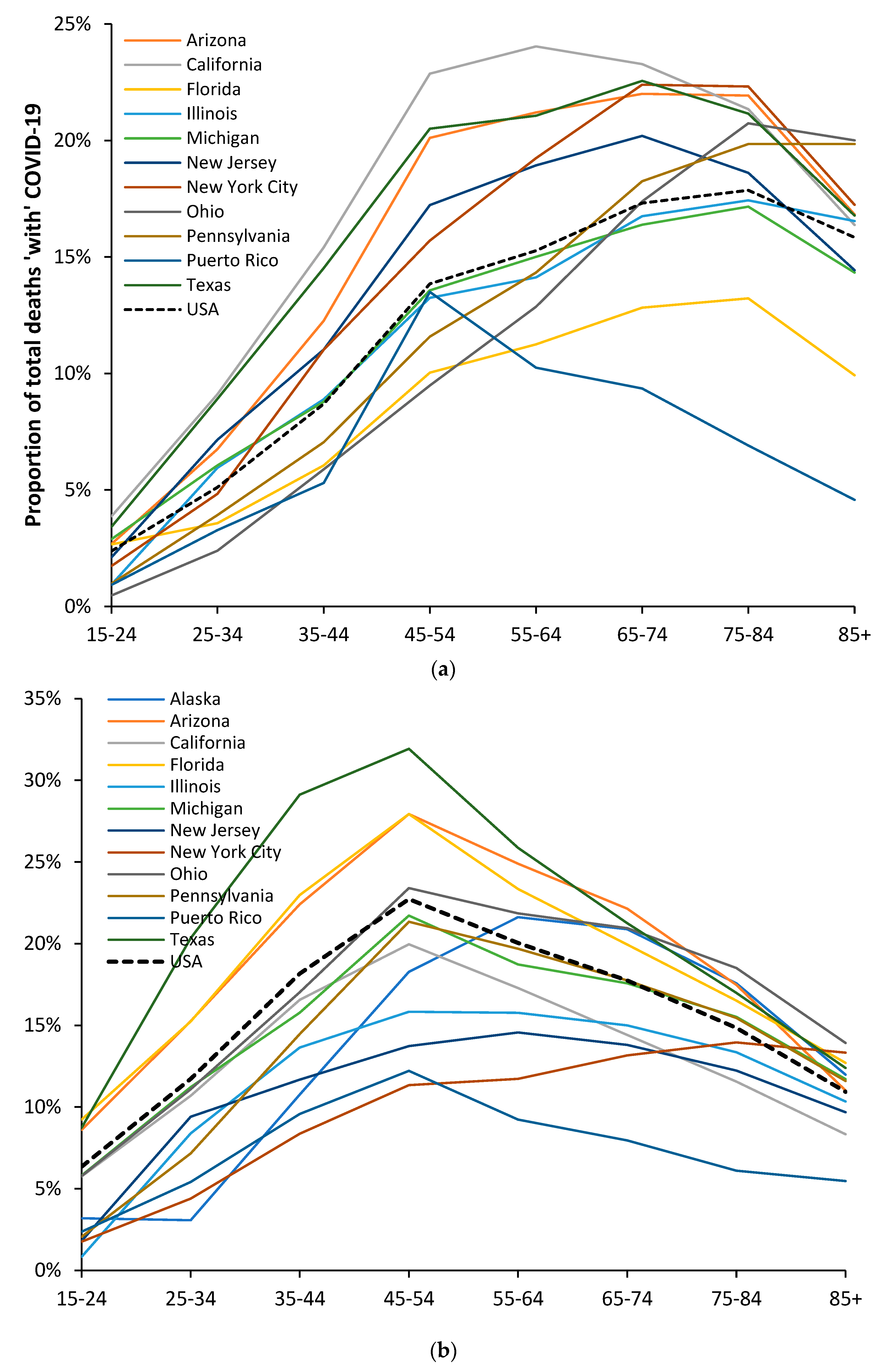
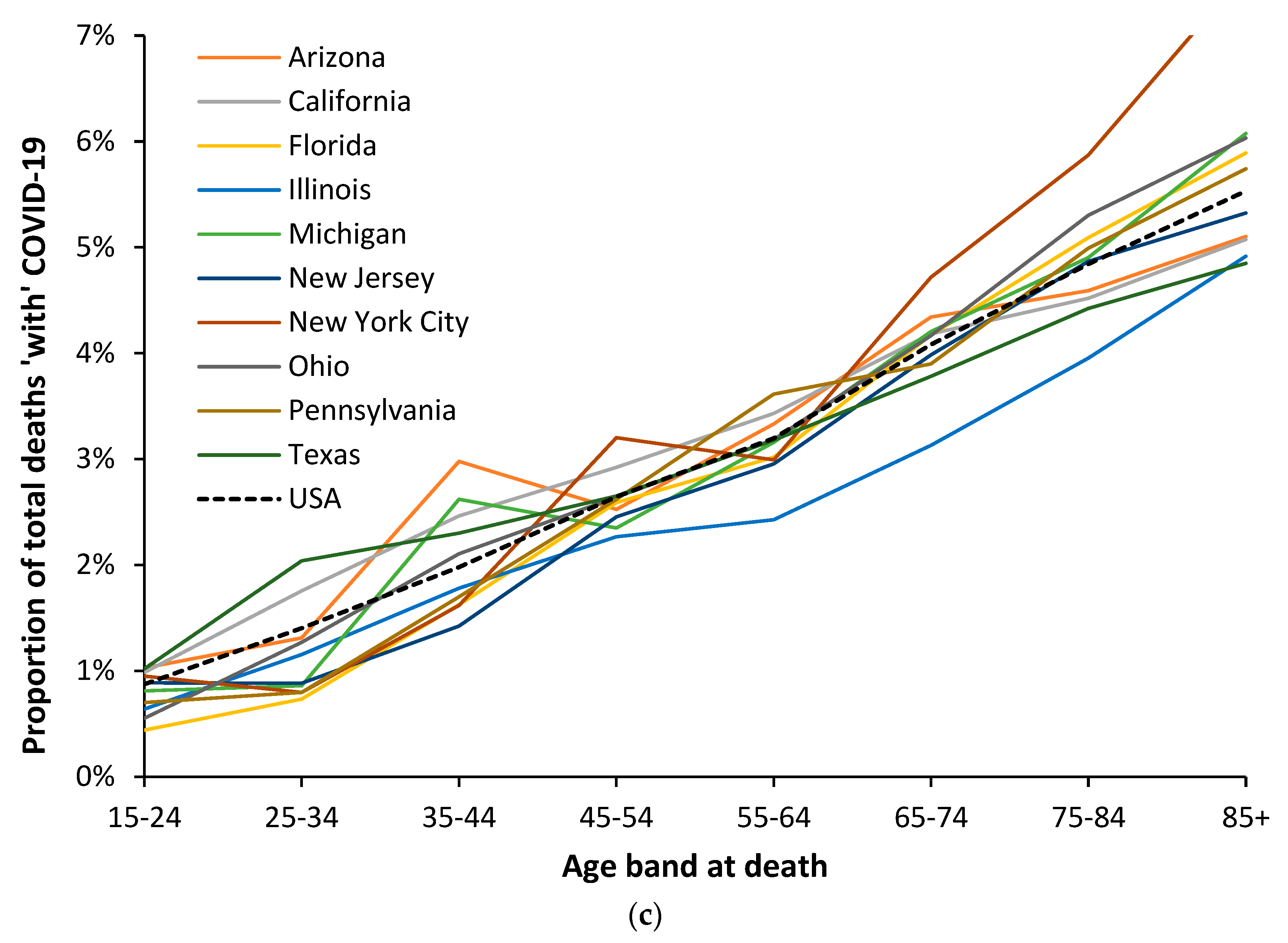
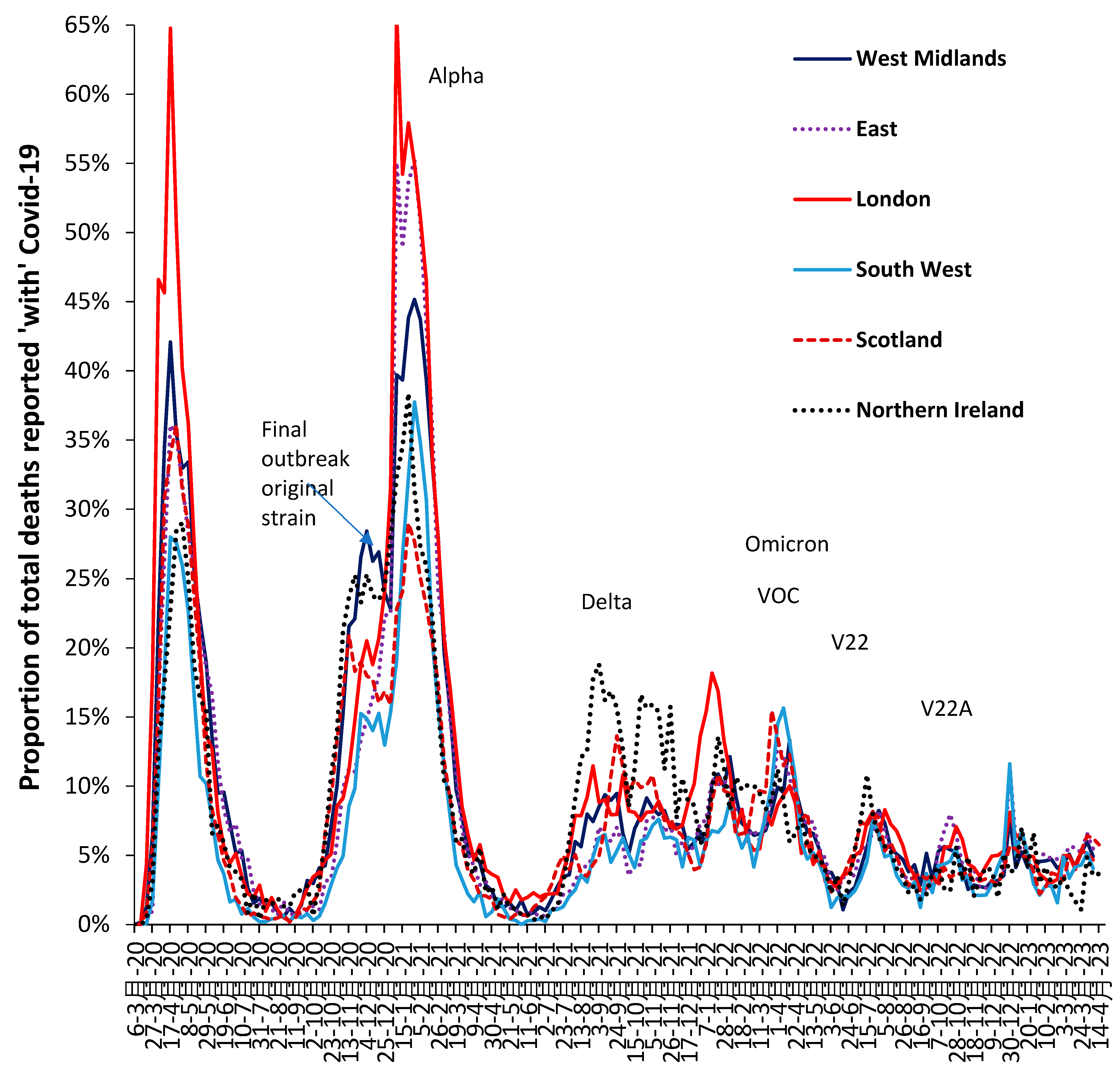
3.6. Modification of the Age Profile in Different Countries/States/Territories
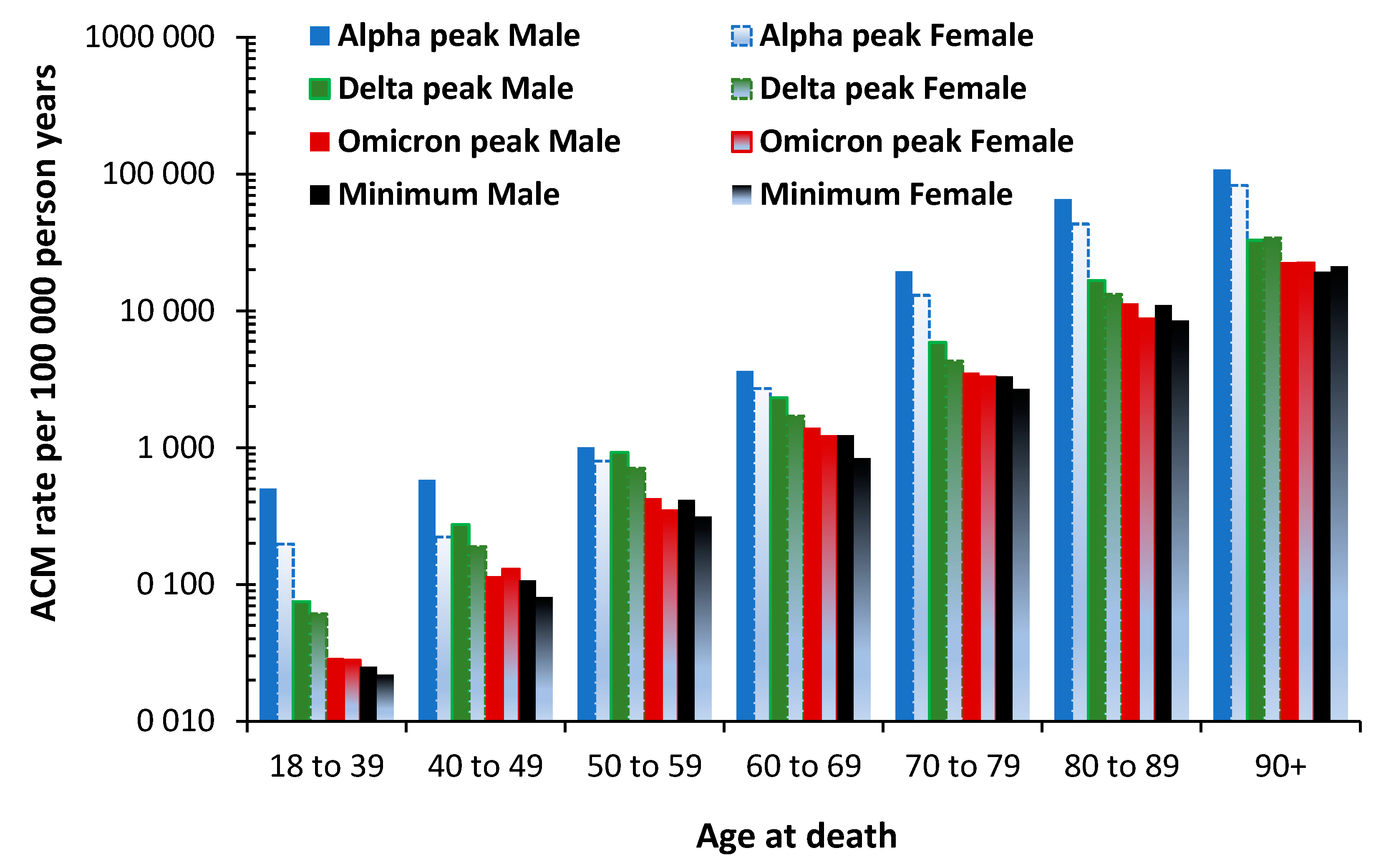
3.7. The All-Cause Mortality Peak for Each Variant
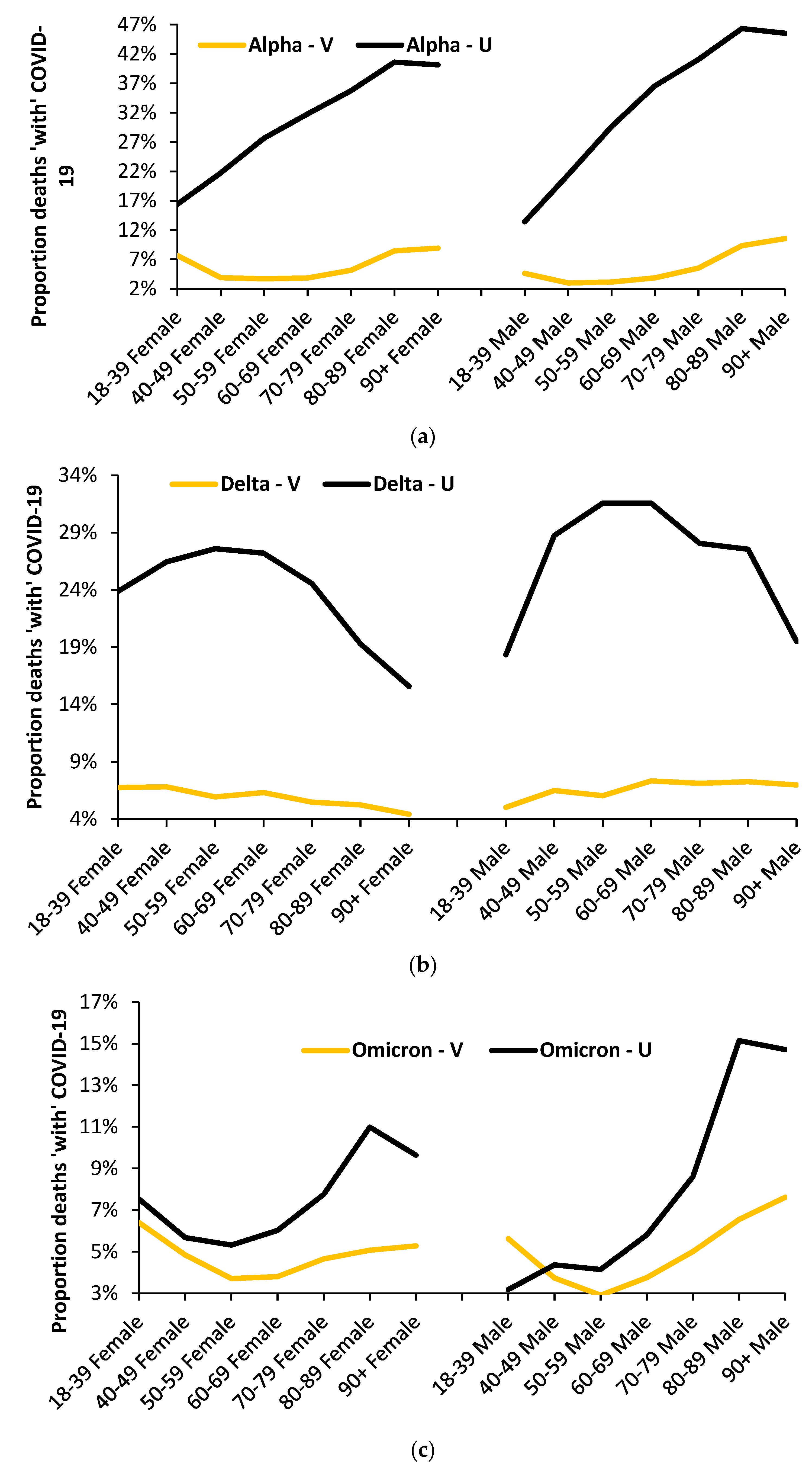
3.8. The Age Profile of the Unvaccinated versus the Vaccinated
4. Discussion
4.1. Impact of SARS-CoV-2 Variants on Disease Severity and Mortality
4.2. Local Risk Factors
- Age profiles for infection by the virus due to social networks or low risk aversion, i.e., nightclub and other large event attendance [65].
- Proportion of persons in each group in high-exposure work categories, i.e., healthcare staff, taxi drivers, teachers, police, bar and restaurant staff, etc. [66].
- Family transmission risk factors such as household crowding [67].
- Level of vaccination and the mix of vaccines employed.
4.3. The Shift in Mortality to Younger Children Subsequent to the Wuhan Strain
4.4. Year-of-Birth Cohort Effects
- Which birth cohorts show enhanced resistance to COVID-19 infection and death?
- Which birth cohorts show an enhanced response to COVID-19 vaccinations?
4.5. Puberty and Excess Female Deaths
4.6. Structure–Function and Other Aspects of Variant and Age Dependence
- Biological properties and peculiarities of the virus species.
- Biological properties and peculiarities of a potential host.
- Strength of innate and adaptive immunity against specific pathogens
- Presence and distribution (localization) of appropriate cell receptors for the initial adsorption of virus particles
- Presence and activity of cellular components necessary for virus’ cell entry and virus genome release, i.e., function of pinocytosis, the presence and activity of specific proteolytic enzymes in the cytoplasm, etc.
- Presence and activity of specific cellular RNA- or DNA-replication pathways
- Environmental conditions.
4.6.1. Roles for Immune Priming in Age Specificity
4.6.2. Roles for Pathogen Interference
4.6.3. Viral Entry as a Molecular Signal Event
4.7. Personal Risk versus Population Risk
4.8. Limitations of the Study
4.9. Wider Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Basu, D.; Chavda, V.; Mehta, A. Therapeutics for COVID-19 and post COVID-19 complications: An update. Curr. Res. Pharm. Drug Discov. 2022, 3, 100086. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Bhola, S.; Thakur, P.; Patel, S.K.S.; Kulshrestha, S.; Ratho, R.K.; Kumar, P. Waves and variants of SARS-CoV-2: Understanding the causes and effect of the COVID-19 catastrophe. Infection 2022, 50, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Demombynes, G. COVID-19 Age-Mortality Curves Are Flatter in Developing Countries. World Bank Group, July 2020. Available online: https://documents1.worldbank.org/curated/en/718461634217653573/pdf/COVID-19-Age-Mortality-Curves-for-2020-Are-Flatter-in-Developing-Countries-Using-Both-Official-Death-Counts-and-Excess-Deaths.pdf#:~:text=Both%20COVID-19%20and%20excess%20death%20age-mortality%20curves%20are,patterns%20of%20infection%20rates%20and%20infection%20fatality%20rates (accessed on 29 January 2023).
- Sasson, I. Age and COVID-19 mortality. Demogr. Res. 2021, 44, 379–396. [Google Scholar] [CrossRef]
- Adjei, S.; Hong, K.; Molinari, N.-A.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods—United States, April 2020–June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef]
- Stepanova, M.; Lam, B.; Younossi, E.; Felix, S.; Ziayee, M.; Price, J.; Pham, H.; de Avila, L.; Terra, K.; Austin, P.; et al. The impact of variants and vaccination on the mortality and resource utilization of hospitalized patients with COVID-19. BMC Infect. Dis. 2022, 22, 702. [Google Scholar] [CrossRef]
- Leiner, J.; Pellissier, V.; Hohenstein, S.; König, S.; Schuler, E.; Möller, R.; Nachtigall, I.; Bonsignore, M.; Hindricks, G.; Kuhlen, R.; et al. Characteristics and outcomes of COVID-19 patients during B.1.1.529 (Omicron) dominance compared to B.1.617.2 (Delta) in 89 German hospitals. BMC Infect. Dis. 2022, 22, 802. [Google Scholar] [CrossRef]
- Sanjuan, R.; Nebot, M.; Chirico, N.; Mansky, L.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef]
- Nobusawa, E.; Sato, K. Comparison of the mutation rates of human influenza A and B viruses. J. Virol. 2006, 80, 3675–3678. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Wu, X.; Zhong, Y.; Zhang, K.; Zhang, Y.; Boerwinkle, E.; Fu, Y. Moderate mutation rate in the SARS coronavirus genome and its implications. BMC Evol. Biol. 2004, 4, 21. [Google Scholar] [CrossRef]
- Pathan, R.; Biswas, M.; Khandaker, M. Time series prediction of COVID-19 by mutation rate analysis using recurrent neural network-based LSTM model. Chaos Solitons Fractals 2020, 138, 110018. [Google Scholar] [CrossRef]
- Cosar, B.; Karagulleoglu, Z.; Unal, S.; Ince, A.; Uncuoglu, D.; Tuncer, G.; Kilinc, B.; Ozkan, Y.; Ozkoc, H.; Demir, I.; et al. COVID–19 mutations and their viral variants. Cytokine Growth Factor Rev. 2022, 63, 10–22. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. COVID–19 Confirmed Deaths in England (to 31 December 2020)—Report. Available online: https://www.gov.uk/government/publications/covid-19-reported-sars-cov-2-deaths-in-england/covid-19-confirmed-deaths-in-england-to-31-december-2020-report (accessed on 29 August 2022).
- GOV.UK. Daily Tests Processed and Testing Capacity (UK): 20 March to 22 September 2020. Available online: https://www.gov.uk/government/publications/daily-tests-processed-and-testing-capacity-uk-20-march-to-22-september-2020 (accessed on 28 December 2022).
- Mueller, B. When Was the First U.S. COVID Death? C.D.C. Investigates 4 Early Cases. The New York Times, 9 September 2021. Available online: https://www.nytimes.com/2021/09/09/health/first-covid-deaths.html (accessed on 2 September 2022).
- Ioannidis, J.; Zonta, F.; Levitt, M. Flaws and uncertainties in pandemic global excess death calculations. Eur. J. Clin. Investig. 2023, 53, e014008. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. Investigation of COVID–19 Variants: Technical Briefings. Available online: https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-technical-briefings (accessed on 26 August 2022).
- Centers for Disease Control and Prevention. COVID–19 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 2 August 2022).
- Jin, R. The lag between daily reported Covid-19 cases and deaths and its relationship to age. J. Public Health Res. 2021, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Watson, A.; O’Regan, P.; Wysocki, O.; Burke, H.; Freitas, A.; Livingstone, R.; Dushianthan, A.; Celinski, M.; Batchelor, J.; et al. Wave comparisons of clinical characteristics and outcomes of COVID–19 admissions—Exploring the impact of treatment and strain dynamics. J. Clin. Virol. 2022, 146, 105031. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Cho, K.; Song, J.; Rahmati, M.; Koyanagi, A.; Lee, S.; Yon, D.; Il Shin, J.; Smith, L. The case fatality rate of COVID-19 during the Delta and the Omicron epidemic phase: A meta-analysis. J Med Virol. 2023, 95, e28522. [Google Scholar] [CrossRef]
- Webster, H.; Nyberg, T.; Sinnathamby, M.; Abdul Aziz, N.; Ferguson, N.; Seghezzo, G.; Blomquist, P.; Bridgen, J.; Chand, M.; Groves, N.; et al. Hospitalisation and mortality risk of SARS-CoV-2 variant Omicron sub-lineage BA.2 compared to BA.1 in England. Nat. Commun. 2022, 13, 6053. [Google Scholar] [CrossRef]
- Wikipedia. COVID–19 Vaccination in the United Kingdom. Available online: https://en.wikipedia.org/wiki/COVID-19_vaccination_in_the_United_Kingdom (accessed on 26 August 2022).
- GOV.UK. COVID Vaccinations in United Kingdom. Available online: https://coronavirus.data.gov.uk/details/vaccinations (accessed on 26 August 2022).
- Majeed, A.; Pollock, K.; Hodes, S.; Papaluca, M. Implementation of COVID–19 vaccination in the United Kingdom. BMJ 2022, 378, e070344378. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. JCVI Advises on COVID–19 Vaccine for People Aged under 40. 7 May 2021. Available online: https://www.gov.uk/government/news/jcvi-advises-on-covid-19-vaccine-for-people-aged-under-40 (accessed on 4 September 2022).
- Gagnon, A.; Miller, M.; Hallman, S.; Bourbeau, R.; Herring, D.; Earn, D.; Madrenas, J. Age-Specific Mortality During the 1918 Influenza Pandemic: Unravelling the Mystery of High Young Adult Mortality. PLoS ONE 2013, 8, e69586. [Google Scholar] [CrossRef]
- Office for National Statistics. Deaths Registered by Single Year of Age, UK. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesdeathsbysingleyearofagetables (accessed on 28 August 2022).
- Office for National Statistics. Deaths Registered in England and Wales, 2021 Edition. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables (accessed on 28 August 2022).
- Office for National Statistics. Single Year of Age and Average age of Death of People Whose Death Was Due to or Involved COVID–19. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/singleyearofageandaverageageofdeathofpeoplewhosedeathwasduetoorinvolvedcovid19 (accessed on 5 September 2022).
- Office for National Statistics. Deaths Registered in England and Wales: 2021 (Refreshed Populations), January 2023. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2021refreshedpopulations (accessed on 28 January 2023).
- Office for National Statistics. Deaths Registered Monthly in England and Wales. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/monthlyfiguresondeathsregisteredbyareaofusualresidence/ (accessed on 5 September 2022).
- Office for National Statistics. Analysis of Population Estimates Tool for UK. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/analysisofpopulationestimatestoolforuk (accessed on 28 August 2022).
- Office for National Statistics. Deaths Registered Weekly in England and Wales, Provisional. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/weeklyprovisionalfiguresondeathsregisteredinenglandandwales (accessed on 28 January 2023).
- Office for National Statistics. Deaths by Vaccination Status, England. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsbyvaccinationstatusengland (accessed on 28 August 2022).
- Office for National Statistics. Births in England and Wales: Summary Tables. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsummarytables (accessed on 10 February 2023).
- US Centers for Disease Control and Prevention. Provisional COVID-19 Deaths by Sex and Age, Updated 8 February 2023. Available online: https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-by-Sex-and-Age/9bhg-hcku (accessed on 10 February 2023).
- Office for National Statistics. Principal Projection—England Summary. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/tablea14principalprojectionenglandsummary (accessed on 27 April 2023).
- Office for National Statistics. Mortality Statistics in England and Wales QMI, July 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/methodologies/mortalitystatisticsinenglandandwalesqmi (accessed on 28 January 2023).
- Jones, R. Excess winter mortality (EWM) as a dynamic forensic tool: Where, when, which conditions, gender, ethnicity, and age. Int. J. Environ. Res. Public Health 2021, 18, 2161. [Google Scholar] [CrossRef]
- Jones, R. End of life and financial risk in GP commissioning. Br. J. Healthc. Manag. 2012, 18, 374–381. [Google Scholar] [CrossRef]
- Xu, Z.; FitzGerald, G.; Guo, Y.; Jalaludin, B.; Tong, S. Impact of heatwave on mortality under different heatwave definitions: A systematic review and meta-analysis. Environ. Intern. 2016, 89–90, 193–203. [Google Scholar] [CrossRef]
- Dear, K.; Wang, Z. Climate and health: Mortality attributable to heat and cold. Lancet 2015, 386, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Djeundje, V.B.; Haberman, S.; Bajekal, M.; Lu, J. The slowdown in mortality improvement rates 2011–2017: A multi-country analysis. Eur. Actuar. J. 2022, 12, 839–878. [Google Scholar] [CrossRef]
- Jones, R.; Ponomarenko, A. Trends in excess winter mortality (EWM) from 1900/01 to 2019/20—evidence for a complex system of multiple long-term trends. Int. J. Environ. Res. Public Health 2022, 19, 3407. [Google Scholar] [CrossRef] [PubMed]
- Ely, J. Chris Whitty Warns Britain Faces ‘Prolonged Period’ of Excess Deaths Not Caused by COVID Due to Collateral Effects of Lockdown. Mail Online, 9 February 2023. Available online: https://www.dailymail.co.uk/health/article-11491871/Chris-Whitty-warns-Britain-faces-prolonged-period-excess-deaths.html (accessed on 17 February 2023).
- Office for National Statistics. Deaths Due to COVID-19, Registered in England and Wales. Published on 1 July 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsduetocovid19registeredinenglandandwales2020 (accessed on 17 February 2023).
- Stokel-Walker, C. What do we know about covid vaccines and preventing transmission? BMJ 2022, 376, o298. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.; Walker, A.; Peto, T. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl. J. Med. 2022, 386, 744–756. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef]
- National Records Scotland. Table 9: Weekly Provisional and Finalised Figures on Death Registrations Involving COVID-19 and All Cause Deaths in Scotland. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.nrscotland.gov.uk%2Ffiles%2F%2Fstatistics%2Fvital-events%2Fweekly-deaths%2Fweekly-deaths-23.xlsx&wdOrigin=BROWSELINK (accessed on 28 April 2023).
- Luo, O.J.; Lei, W.; Zhu, G.; Ren, Z.; Xu, Y.; Xiao, C.; Zhang, H.; Cai, J.; Luo, Z.; Gao, L.; et al. Multidimensional single-cell analysis of human peripheral blood reveals characteristic features of the immune system landscape in aging and frailty. Nat. Aging 2022, 2, 348–364. [Google Scholar] [CrossRef]
- Ramasubramanian, R.; Meier, H.C.S.; Vivek, S.; Klopack, E.; Crimmins, E.; Faul, J.; Nikolich-Zugich, J.; Thyagarajan, B. Evaluation of T-cell aging-related immune phenotypes in the context of biological aging and multimorbidity in the Health and Retirement Study. Immun. Ageing 2022, 19, 33. [Google Scholar] [CrossRef]
- Vasa, A.; Kini, M.; Neugarten, J.; Bellin, E.; Golestaneh, L. Does comorbidity burden explain the higher COVID–19 mortality risk among men? A retrospective cross-sectional analysis of a well-defined cohort of patients in Bronx, New York. BMJ Open 2022, 12, e063862. [Google Scholar] [CrossRef]
- Nguyen, N.; Chinn, J.; De Ferrante, M.; Kirby, K.; Hohmann, S.; Amin, A. Male gender is a predictor of higher mortality in hospitalized adults with COVID–19. PLoS ONE 2022, 16, e0254066. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Ponomarenko, A. Roles for pathogen interference in influenza vaccination, with implications to vaccine effectiveness (VE) and attribution of influenza deaths. Infect. Dis. Rep. 2022, 14, 710–758. [Google Scholar] [CrossRef]
- Goldinger, A.; Shakhbazov, K.; Henders, A.; McRae, A.; Montgomery, G.; Powell, J. Seasonal effects on gene expression. PLoS ONE 2015, 10, e0126995. [Google Scholar] [CrossRef]
- Dopico, X.; Evangelou, M.; Ferreira, R.; Guo, H.; Pekalski, M.; Smyth, D.; Cooper, N.; Burren, O.; Fulford, A.; Hennig, B.; et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 2015, 6, 7000. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.; Daly, M.; Ganna, A. The human genetic epidemiology of COVID-19. Nat. Rev. Genet. 2022, 23, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Cao, H.; Teng, S.; Zhang, F. A phenome-wide investigation of risk factors for severe COVID-19. J. Med. Virol. 2022, 95, e28264. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Ojha, V. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Ann. Vasc. Surg. 2022, 84, 12–20.e1. [Google Scholar] [CrossRef]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.; Uzzo, M.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef]
- Office for National Statistics. Comparing the Risk of Death Involving Coronavirus (COVID-19) by Variant, England: December 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/articles/comparingtheriskofdeathinvolvingcoronaviruscovid19byvariantengland/december2021 (accessed on 28 January 2023).
- Karaivanov, A. A social network model of COVID-19. PLoS ONE 2020, 15, e0240878. [Google Scholar] [CrossRef]
- Office for National Statistics. Which Occupations Have the Highest Potential Exposure to the Coronavirus (COVID-19)? Published 11 May 2020. Available online: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/articles/whichoccupationshavethehighestpotentialexposuretothecoronaviruscovid19/2020-05-11 (accessed on 25 February 2023).
- Villela, D. Household crowding hampers mitigating the transmission of SARS-CoV-2. Rev. Soc. Bras. Med. Trop. 2021, 54, e08212020. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y. The Clinical Characteristics and Risk Factors of Severe COVID-19. Gerontology 2021, 67, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, X.; Wang, C.; Zhang, S.; Yu, R.; Xie, Y.; Yuan, S.; Cheng, L.; Shi, L.; Zhang, X. An integrated strategy to identify COVID-19 causal genes and characteristics represented by LRRC37A2. J. Med. Virol. 2023, 95, e28585. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Hernandez, K.; Aronow, B.; Wurtele, E. African Americans and European Americans exhibit distinct gene expression patterns across tissues and tumors associated with immunologic functions and environmental exposures. Sci. Rep. 2021, 11, 9905. [Google Scholar] [CrossRef]
- Benetti, E.; Tita, R.; Spiga, O.; Ciolfi, A.; Birolo, G.; Bruselles, A.; Doddato, G.; Giliberti, A.; Marconi, C.; Musacchia, F.; et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet. 2020, 28, 1602–1614. [Google Scholar] [CrossRef]
- Jones, R. Multidisciplinary insights into health care financial risk and hospital surge capacity, Part 1: Nearness to death, infectious outbreaks, and COVID-19. J. Health Care Financ. 2021, 47. [Google Scholar]
- Jones, R.P.; Ponomarenko, A. Effect of Age, Sex, and COVID-19 Vaccination History on All-Cause Mortality: Unexpected Outcomes in a Complex Biological and Social System. Preprints 2023, 2023040248. [Google Scholar] [CrossRef]
- Lawlor, D.; Andersen, A.-M.; Batty, G. Birth cohort studies: Past, present and future. Int. J. Epidemiol. 2009, 38, 897–902. [Google Scholar] [CrossRef]
- Tai, Y.; Chiang, C.; Chiang, Y.; Wu, C.; Lee, W.; Cheng, W. Age-specific trend and birth cohort effect on different histologic types of uterine corpus cancers. Sci. Rep. 2023, 13, 1019. [Google Scholar] [CrossRef]
- Ajdacic-Gross, V.; Schmid, M.; Tschopp, A.; Gutzwiller, F. Birth Cohort Effects in Neurological Diseases: Amyotrophic Lateral Sclerosis, Parkinson’s Disease and Multiple Sclerosis. Neuroepidemiology 2012, 38, 56–63. [Google Scholar] [CrossRef]
- Ohuma, E.; Okiro, E.; Ochola, R.; Sande, C.; Cane, P.; Medley, G.; Bottomley, C.; Nokes, D. The Natural History of Respiratory Syncytial Virus in a Birth Cohort: The Influence of Age and Previous Infection on Reinfection and Disease. Am. J. Epidemiol. 2012, 176, 794–802. [Google Scholar] [CrossRef]
- Eshima, N.; Tokumaru, O.; Hara, S.; Bacal, K.; Korematsu, S.; Karukaya, S.; Uruma, K.; Okabe, N.; Matsuishi, T. Age-specific sex-related differences in infections: A statistical analysis of National Surveillance Data in Japan. PLoS ONE 2012, 7, e42261. [Google Scholar] [CrossRef]
- Glynn, J.; Moss, P. Systematic analysis of infectious disease outcomes by age shows lowest severity in school-age children. Sci. Data 2020, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Dunn, S. Effect of puberty on the immune system: Relevance to multiple sclerosis. Front. Pediatr. 2022, 10, 1059083. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.; Ismail, N. Puberty as a vulnerable period to the effects of immune challenges: Focus on sex differences. Behav. Brain Res. 2017, 320, 374–382. [Google Scholar] [CrossRef]
- Shepherd, R.; Cheung, A.; Pang, K.; Saffery, R.; Novakovic, B. Sexual dimorphism in innate immunity: The role of sex hormones and epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef]
- Thompson, E.; Nicodemus-Johnson, J.; Kim, K.; Gern, J.; Jackson, D.; Lemanske, R.; Ober, C. Global DNA methylation changes spanning puberty are near predicted estrogen-responsive genes and enriched for genes involved in endocrine and immune processes. Clin. Epigenetics 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Sanjuán, R.; Domingo-Calap, P. Mechanisms of viral mutation. Cell Mol. Life Sci. 2016, 73, 4433–4448. [Google Scholar] [CrossRef]
- Khan, M.; Nazli, A.; Al-Furas, H.; Asad, M.; Ajmal, I.; Khan, D.; Shah, J.; Farooq, M.; Jiang, W. An overview of viral mutagenesis and the impact on pathogenesis of SARS-CoV-2 variants. Front. Immunol. 2022, 13, 1034444. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Basu, R.; Bashyam, M. Assessing the evolution of SARS-CoV-2 lineages and the dynamic associations between nucleotide variations. Access Microbiol. 2023; in press. [Google Scholar] [CrossRef]
- Yamamoto, R.; Chung, R.; Vazquez, J.; Sheng, H.; Steinberg, P.; Ioannidis, N.; Sudmant, P. Tissue-specific impacts of aging and genetics on gene expression patterns in humans. Nat. Commun. 2022, 13, 5803. [Google Scholar] [CrossRef]
- Frenk, S.; Houseley, J. Gene expression hallmarks of cellular ageing. Biogerontology 2018, 19, 547–566. [Google Scholar] [CrossRef]
- Cao, J.; Gollapudi, S.; Sharman, E.; Jia, Z.; Gupta, S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell 2010, 9, 19–31, Erratum in: Aging Cell 2010, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Batra, G.; Kumar, M.; Mishra, A.; Singla, R.; Singh, A.; Singh, R.; Medhi, B. BCG as a game-changer to prevent the infection and severity of COVID-19 pandemic? Allergol. Immunopathol. 2020, 48, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Coerdt, K.; Khachemoune, A. Corona viruses: Reaching far beyond the common cold. Afr. Health Sci. 2021, 21, 207–213. [Google Scholar] [CrossRef]
- Francis, T. On the doctrine of original antigenic sin. Proc. Am. Phil. Soc. 1960, 104, 572–578. [Google Scholar]
- Rijkers, G.; van Overveld, F. The “original antigenic sin” and its relevance for COVID–19 (COVID–19) vaccination. Clin. Immunol. Comm. 2021, 1, 13–16. [Google Scholar] [CrossRef]
- Vatti, A.; Monsalve, D.; Pacheco, Y.; Chang, C.; Anaya, J.; Gershwin, M. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017, 83, 12–21. [Google Scholar] [CrossRef]
- Lyons-Weiler, J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID–19 via autoimmunity. J. Transl. Autoimmun. 2020, 3, 100051. [Google Scholar] [CrossRef]
- Adalja, A.A.; Henderson, D. Original Antigenic Sin and Pandemic (H1N1) 2009. Emerg. Infect. Dis. 2010, 16, 1028–1029. [Google Scholar] [CrossRef]
- Xu, R.; Ekiert, D.; Krause, J.; Hai, R.; Crowe, J.; Wilson, I. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 2010, 328, 357–360. [Google Scholar] [CrossRef]
- Nguyen, A.; Noymer, A. Influenza Mortality in the United States, 2009 Pandemic: Burden, Timing and Age Distribution. PLoS ONE 2013, 8, e64198. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Spreeuwenberg, P.; Lustig, R.; Taylor, R.; Fleming, D.; Kroneman, M.; Van Kerkhove, M.; Mounts, A.; Paget, W.; GLaMOR Collaborating Teams. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: A modeling study. PLoS Med. 2013, 10, e1001558. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, D.; Anderson, T.K.; Kimble, J.B.; Chang, J.; Lopes, S.; Souza, C.K.; Pekosz, A.; Shaw-Saliba, K.; Rothman, R.E.; Chen, K.-F.; et al. Antigenic Characterization and Pandemic Risk Assessment of North American H1 Influenza A Viruses Circulating in Swine. Microbiol. Spectr. 2022, 10, e0178122. [Google Scholar] [CrossRef] [PubMed]
- Mehfoud, N.; Kimmerly, V.; Shipe, M. Mapping the 1889–1890 Russian Flu. August 2014. Available online: https://circulatingnow.nlm.nih.gov/2014/08/11/mapping-the-1889-1890-russian-flu/ (accessed on 26 January 2023).
- Fonville, J.M.; Wilks, S.H.; James, S.L.; Fox, A.; Ventresca, M.; Aban, M.; Xue, L.; Jones, T.C.; Len, M.H.; Pham, Q.T.; et al. Antibody landscapes after influenza virus infection or vaccination. Science 2014, 346, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Dugan, H.L.; Guthmiller, J.J.; Arevalo, P.; Huang, M.; Chen, Y.-Q.; Neu, K.E.; Henry, C.; Zheng, N.-Y.; Lan, L.Y.-L.; Tepora, M.E.; et al. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci. Transl. Med. 2020, 12, eabd3601. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Zhong, D.; Xiao, S.; Debes, A.; Egbert, E.; Caturegli, P.; Colantuoni, E.; Milstone, A. Durability of Antibody Levels After Vaccination With mRNA SARS-CoV-2 Vaccine in Individuals With or Without Prior Infection. JAMA 2021, 326, 2524–2526. [Google Scholar] [CrossRef]
- Deng, L.; Li, P.; Zhang, X.; Jiang, Q.; Turner, D.; Zhou, C.; Gao, Y.; Qian, F.; Zhang, C.; Lu, H.; et al. Risk of SARS-CoV-2 reinfection: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 20763. [Google Scholar] [CrossRef]
- Diani, S.; Leonardi, E.; Cavezzi, A.; Ferrari, S.; Iacono, O.; Limoli, A.; Bouslenko, Z.; Natalini, D.; Conti, S.; Mantovani, M.; et al. SARS-CoV-2-The Role of Natural Immunity: A Narrative Review. J. Clin. Med. 2022, 11, 6272. [Google Scholar] [CrossRef]
- Shenai, M.; Rahme, R.; Noorchashm, H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: A systematic review and pooled analysis. Cureus 2021, 13, e19102. [Google Scholar] [CrossRef]
- Aghbash, P.; Eslami, N.; Shirvaliloo, M.; Baghi, H. Viral coinfections in COVID-19. J. Med. Virol. 2021, 93, 5310–5322. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Creti, R.; Palma, C.; Pantosti, A. Bacterial coinfections in COVID-19: An underestimated adversary. Ann. Ist. Super Sanita 2020, 56, 359–364. [Google Scholar] [CrossRef]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Bravo Vázquez, L.; Reyes-Pérez, P.; Estrada-Meza, C.; Aponte Alburquerque, R.; Pathak, S.; Banerjee, A.; Bandyopadhyay, A.; Chakraborty, S.; Srivastava, A. The role of microRNAs in solving COVID–19 puzzle from infection to therapeutics: A mini-review. Virus Res. 2022, 308, 198631. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; AlHajri, N.; Ibrahim, A.; Javed, M.; Salem, K.; Pottoo, F.; Kamal, M. Micro-RNAs in the regulation of immune response against SARS CoV-2 and other viral infections. J. Adv. Res. 2021, 30, 133–145. [Google Scholar] [CrossRef]
- Moatar, A.; Chis, A.; Marian, C.; Sirbu, I. Gene Network Analysis of the Transcriptome Impact of COVID–19 Interacting MicroRNAs in COVID–19 Disease. Int. J. Mol. Sci. 2022, 23, 9239. [Google Scholar] [CrossRef]
- Grehl, C.; Schultheiß, C.; Hoffmann, K.; Binder, M.; Altmann, T.; Grosse, I.; Kuhlmann, M. Detection of COVID–19 derived small RNAs and changes in circulating small RNAs associated with COVID–19. Viruses 2021, 13, 1593. [Google Scholar] [CrossRef]
- Marchi, R.; Sugita, B.; Centa, A.; Fonseca, A.; Bortoletto, S.; Fiorentin, K.; Ferreira, S.; Cavalli, L. The role of microRNAs in modulating COVID–19 infection in human cells: A systematic review. Infect. Genet. Evol. 2021, 91, 104832. [Google Scholar] [CrossRef]
- Meng, F.; Siu, G.; Mok, B.; Sun, J.; Fung, K.; Lam, J.; Wong, N.; Gedefaw, L.; Luo, S.; Lee, T.; et al. Viral microRNAs encoded by nucleocapsid gene of COVID–19 are detected during infection, and targeting metabolic pathways in host cells. Cells 2021, 10, 1762. [Google Scholar] [CrossRef]
- Singh, M.; Chazal, M.; Quarato, P.; Bourdon, L.; Malabat, C.; Vallet, T.; Vignuzzi, M.; van der Werf, S.; Behillil, S.; Donati, F.; et al. A virus-derived microRNA targets immune response genes during COVID–19 infection. EMBO Rep. 2022, 23, e54341. [Google Scholar] [CrossRef]
- Sica, A.; Colombo, M.; Trama, A.; Horn, L.; Garassino, M.; Torri, V. Immunometabolic Status of COVID-19 Cancer Patients. Physiol. Rev. 2020, 100, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Sassen, S.; Miska, E.A.; Caldas, C. MicroRNA: Implications for cancer. Virchows Arch. 2008, 452, 1–10. [Google Scholar] [CrossRef]
- Heyn, G.S.; Corrêa, L.H.; Magalhães, K.G. The Impact of Adipose Tissue-Derived miRNAs in Metabolic Syndrome, Obesity, and Cancer. Front. Endocrinol. 2020, 11, 563816. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Zhang, X. The Profiling and Role of miRNAs in Diabetes Mellitus. J. Diabetes Clin. Res. 2019, 1, 5–23. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Wang, S.Q.; Chen, Y.; Fu, L.Y.; Xu, Y.N.; Li, L.; Tao, L.; Shen, X.C. MicroRNAs Regulating Mitochondrial Function in Cardiac Diseases. Front. Pharmacol. 2021, 12, 663322. [Google Scholar] [CrossRef] [PubMed]
- Uzuner, E.; Ulu, G.T.; Gürler, S.B.; Baran, Y. The Role of MiRNA in Cancer: Pathogenesis, Diagnosis, and Treatment. Methods Mol. Biol. 2022, 2257, 375–422. [Google Scholar] [CrossRef]
- Boon, R.; Vickers, K. Intercellular transport of microRNAs. Arter. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Shin, J.; Bayry, J. An age-related decline of CD62L and vaccine response: A role of microRNA 92a? Hum. Vaccin. Immunother. 2014, 10, 1404–1405. [Google Scholar] [CrossRef][Green Version]
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.; Potenza, N. What microRNAs could tell us about the human X chromosome. Cell. Mol. Life Sci. 2020, 77, 4069–4080. [Google Scholar] [CrossRef]
- Nakanishi, T.; Pigazzini, S.; Degenhardt, F.; Cordioli, M.; Butler-Laporte, G.; Maya-Miles, D.; Bujanda, L.; Bouysran, Y.; Niemi, M.; Palom, A.; et al. Age-dependent impact of the major common genetic risk factor for COVID–19 on severity and mortality. J. Clin. Investig. 2021, 131, e152386. [Google Scholar] [CrossRef] [PubMed]
- Zeberg, H.; Pääbo, S. The major genetic risk factor for severe COVID–19 is inherited from Neanderthals. Nature 2020, 587, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Zeberg, H. The major genetic risk factor for severe COVID–19 is associated with protection against HIV. Proc. Natl. Acad. Sci. USA 2022, 119, e2116435119. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Tong, B.; Sun, L.; Shi, S.; Zheng, B.; Wang, Z.; Dong, X.; Zheng, P. N501Y mutation of spike protein in SARS-CoV-2 strengthens its binding to receptor ACE2. eLife 2021, 10, e69091. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2022, 602, 294–299. [Google Scholar] [CrossRef]
- The Animation Lab. Building a Visual Consensus Model of the SARS-CoV-2 Life Cycle. Available online: https://animationlab.utah.edu/cova (accessed on 26 February 2023).
- Dubrow, A. Hidden states of the COVID-19 Spike Protein. Available online: https://phys.org/news/2020-10-hidden-states-covid-spike-protein.html (accessed on 26 February 2023).
- Luhur, A.; Chawla, G.; Sokol, N. MicroRNAs as components of systemic signaling pathways in Drosophila melanogaster. Curr. Top. Dev. Biol. 2013, 105, 97–123. [Google Scholar] [CrossRef]
- Leroy, H.; Han, M.; Woottum, M.; Bracq, L.; Bouchet, J.; Xie, M.; Benichou, S. Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci. 2020, 21, 9644. [Google Scholar] [CrossRef]
- Hernández, J.; Podbilewicz, B. The hallmarks of cell-cell fusion. Development 2017, 144, 4481–4495. [Google Scholar] [CrossRef]
- Sedger, L. microRNA control of interferons and interferon induced anti-viral activity. Mol. Immunol. 2013, 56, 781–793. [Google Scholar] [CrossRef]
- Bouvet, M.; Voigt, S.; Tagawa, T.; Albanese, M.; Chen, Y.; Chen, Y.; Fachko, D.; Pich, D.; Göbel, C.; Skalsky, R.; et al. Multiple Viral microRNAs Regulate Interferon Release and Signaling Early during Infection with Epstein-Barr Virus. mBio 2021, 12, e03440-20. [Google Scholar] [CrossRef]
- The Severe COVID–19 GWAS Group. Genomewide association study of severe COVID–19 with respiratory failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Pereira, A.; Bes, T.; Velho, M.; Marques, E.; Jannes, C.; Valino, K.; Dinardo, C.; Costa, S.; Duarte, A.; Santos, A.; et al. Genetic risk factors and COVID–19 severity in Brazil: Results from BRACOVID study. Hum. Mol. Genet. 2022, 31, 3021–3031. [Google Scholar] [CrossRef] [PubMed]
- Downes, D.J.; Cross, A.R.; Hua, P.; Roberts, N.; Schwessinger, R.; Cutler, A.J.; Munis, A.M.; Brown, J.; Mielczarek, O.; de Andrea, C.E.; et al. Identification of LZTFL1 as a candidate effector gene at a COVID–19 risk locus. Nat. Genet. 2021, 53, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Kousathanas, A.; Pairo-Castineira, E.; Rawlik, K.; Stuckey, A.; Odhams, C.; Walker, S.; Russell, C.; Malinauskas, T.; Wu, Y.; Millar, J.; et al. Whole-genome sequencing reveals host factors underlying critical COVID–19. Nature 2022, 607, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, P.; Nicoletto, A.; Sheward, D.; Das, H.; Castro Dopico, X.; Perez Vidakovics, L.; Hanke, L.; Chernyshev, M.; Narang, S.; Kim, S.; et al. Immunoglobulin germline gene polymorphisms influence the function of SARS-CoV-2 neutralizing antibodies. Immunity 2023, 56, 193–206.e7. [Google Scholar] [CrossRef]
- Joung, S.; Weber, B.; Wu, M.; Liu, Y.; Tang, A.; Driver, M.; Sternbach, S.; Wynter, T.; Hoang, A.; Barajas, D.; et al. Serological response to vaccination in post-acute sequelae of COVID. BMC Infect. Dis. 2023, 23, 97. [Google Scholar] [CrossRef]
- Kay, C.; Paqndya, D. How Errors, Inaction Sent a Deadly COVID Variant Around the World. Bloomberg 29 December 2021. Available online: https://www.bloomberg.com/news/features/2021-12-29/how-delta-variant-spread-in-india-deadly-errors-inaction-co (accessed on 10 August 2023).
- Oaten, J. ABC News, 9 June 2021. How the Coronavirus Delta Variant Rampaged through India before Spreading across the Globe. Available online: https://www.abc.net.au/news/2021-06-09/how-delta-coronavirus-variant-fuelled-indias-devastation/100195300 (accessed on 10 August 2023).
- Griette, Q.; Magal, P.; Seydi, O. Unreported Cases for Age Dependent COVID-19 Outbreak in Japan. Biology 2020, 9, 132. [Google Scholar] [CrossRef]
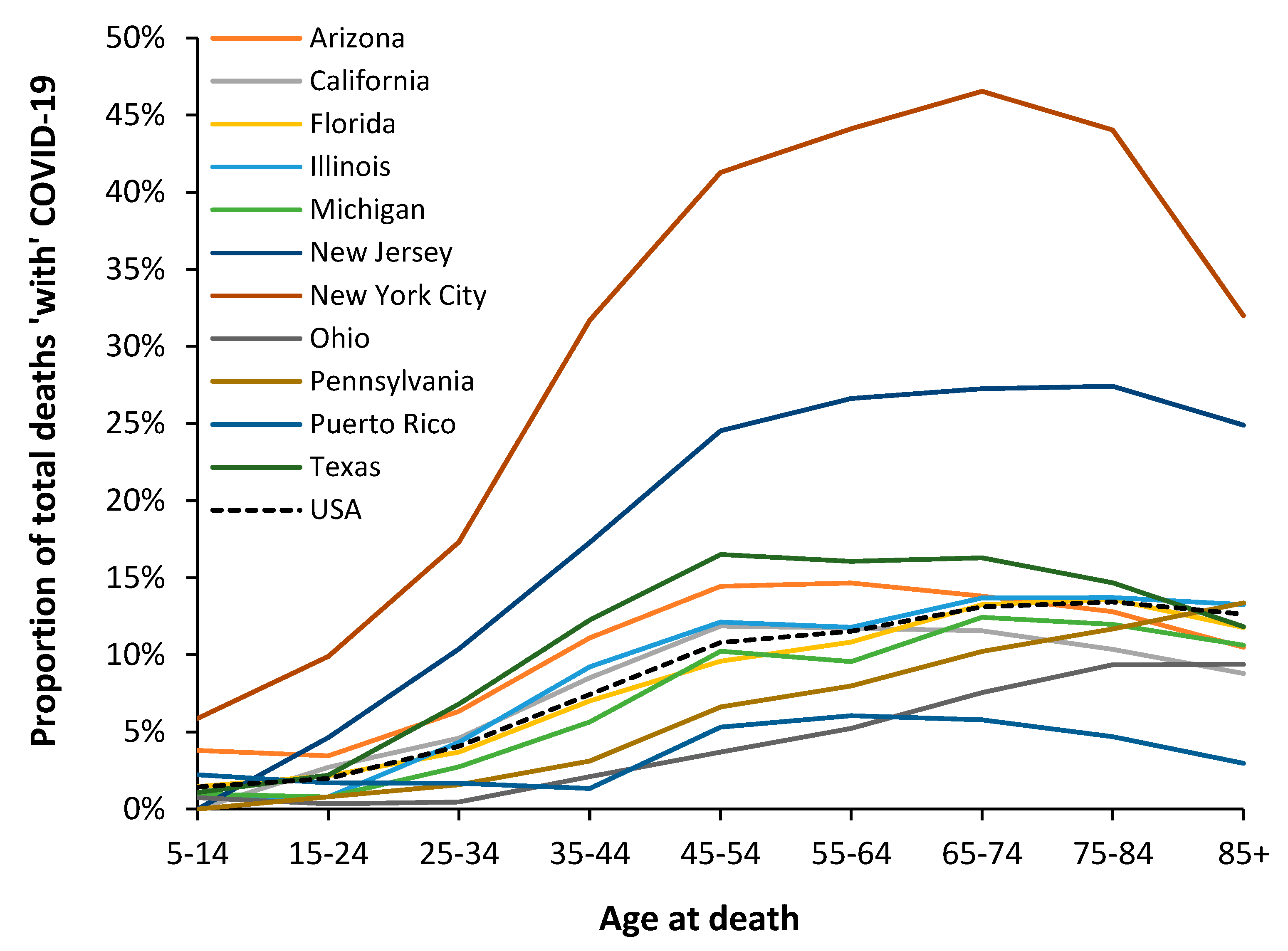
| Month | Proportion of Total Deaths Due to COVID-19: Average Age 65+ | Variant Arrives (Infections) | Variant Dominates Deaths |
|---|---|---|---|
| October 2022 | 4% | Omicron | |
| September 2022 | 2% | Omicron | |
| August 2022 | 4% | Omicron | |
| July 2022 | 4% | Omicron | |
| June 2022 | 2% | Omicron | |
| May 2022 | 4% | Omicron | |
| April 2022 | 7% | Omicron | |
| March 2022 | 5% | Omicron | |
| February 2022 | 6% | Mixed Delta/Omicron | |
| January 2022 | 8% | Omicron starts | Mixed Delta/Omicron |
| December 2021 | 5% | Delta | |
| November 2021 | 7% | Delta | |
| October 2021 | 6% | Delta | |
| September 2021 | 6% | Delta | |
| August 2021 | 5% | Delta | |
| July 2021 | 2% | Mixed Alpha/Delta | |
| June 2021 | 1% | Delta starts | Mixed Alpha/Delta |
| May 2021 | 1% | Alpha | |
| April 2021 | 2% | Alpha | |
| March 2021 | 9% | Alpha | |
| February 2021 | 30% | Alpha | |
| January 2021 | 38% | Alpha | |
| December 2020 | 22% | Alpha | |
| November 2020 | 19% | Alpha | |
| October 2020 | 7% | Mixed Alpha/Original | |
| September 2020 | 1% | Alpha starts | Mixed Alpha/Original |
| August 2020 | 1% | Original | |
| July 2020 | 3% | Original | |
| June 2020 | 9% | Original | |
| May 2020 (β) | 23% | Original | |
| April 2020 (β) | 32% | Original | |
| March 2020 (β) | 3% | Original |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.P.; Ponomarenko, A. COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality. Infect. Dis. Rep. 2023, 15, 600-634. https://doi.org/10.3390/idr15050058
Jones RP, Ponomarenko A. COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality. Infectious Disease Reports. 2023; 15(5):600-634. https://doi.org/10.3390/idr15050058
Chicago/Turabian StyleJones, Rodney P., and Andrey Ponomarenko. 2023. "COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality" Infectious Disease Reports 15, no. 5: 600-634. https://doi.org/10.3390/idr15050058
APA StyleJones, R. P., & Ponomarenko, A. (2023). COVID-19-Related Age Profiles for SARS-CoV-2 Variants in England and Wales and States of the USA (2020 to 2022): Impact on All-Cause Mortality. Infectious Disease Reports, 15(5), 600-634. https://doi.org/10.3390/idr15050058








