Etiology and Outcomes of Healthcare-Associated Meningitis and Ventriculitis—A Single Center Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van de Beek, D.; Drake, J.M.; Tunkel, A.R. Nosocomial bacterial meningitis. N. Engl. J. Med. 2010, 362, 146–154. [Google Scholar] [CrossRef]
- Korinek, A.M.; Golmard, J.L.; Elcheick, A.; Bismuth, R.; van Effenterre, R.; Coriat, P.; Puybasset, L. Risk factors for neurosurgical site infections after craniotomy: A critical reappraisal of antibiotic prophylaxis on 4578 patients. Br. J. Neurosurg. 2005, 19, 155–162. [Google Scholar] [CrossRef] [PubMed]
- McClelland, S., 3rd; Hall, W.A. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin. Infect. Dis. 2007, 45, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, B.; Yu, S.; Sun, F.; Ruan, Q.; Zhang, W.; Shao, L.; Chen, S. The incidence and risk factors of meningitis after major craniotomy in China: A retrospective cohort study. PLoS ONE 2014, 9, e101961. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, O.R.; Nazli Zeka, A.; Tasbakan, M.; Pullukcu, H.; Arda, B.; Yamazhan, T.; Sipahi, H.; Ulusoy, S. Pooled analysis of 899 nosocomial meningitis episodes from Turkey. Turk. J. Med. Sci. 2017, 47, 29–33. [Google Scholar] [CrossRef]
- Zhan, R.; Zhu, Y.; Shen, Y.; Shen, J.; Tong, Y.; Yu, H.; Wen, L. Post-operative central nervous system infections after cranial surgery in China: Incidence, causative agents, and risk factors in 1470 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 861–866. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Busl, K.M. Nosocomial Infections in the Neurointensive Care Unit. Neurosurg. Clin. N. Am. 2018, 29, 299–314. [Google Scholar] [CrossRef]
- Schade, R.P.; Schinkel, J.; Roelandse, F.W.; Geskus, R.B.; Visser, L.G.; van Dijk, J.M.; Voormolen, J.H.; Van Pelt, H.; Kuijper, E.J. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J. Neurosurg. 2006, 104, 101–108. [Google Scholar] [CrossRef]
- Zarrouk, V.; Vassor, I.; Bert, F.; Bouccara, D.; Kalamarides, M.; Bendersky, N.; Redondo, A.; Sterkers, O.; Fantin, B. Evaluation of the management of postoperative aseptic meningitis. Clin. Infect. Dis. 2007, 44, 1555–1559. [Google Scholar] [CrossRef]
- Conen, A.; Walti, L.N.; Merlo, A.; Fluckiger, U.; Battegay, M.; Trampuz, A. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: A retrospective analysis over an 11-year period. Clin. Infect. Dis. 2008, 47, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Erdem, I.; Hakan, T.; Ceran, N.; Metin, F.; Akcay, S.S.; Kucukercan, M.; Berkman, M.Z.; Goktas, P. Clinical features, laboratory data, management and the risk factors that affect the mortality in patients with postoperative meningitis. Neurol. India 2008, 56, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Reichert, M.C.; Medeiros, E.A.; Ferraz, F.A. Hospital-acquired meningitis in patients undergoing craniotomy: Incidence, evolution, and risk factors. Am. J. Infect. Control. 2002, 30, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.T.; Bharara, S.; Tubbs, R.S.; Wolff, C.L.; Gillespie, G.Y.; Markert, J.M.; Blount, J.P. Polymerase chain reaction for the rapid detection of cerebrospinal fluid shunt or ventriculostomy infections. Neurosurgery 2005, 57, 1237–1243; discussion 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.; Bitterman, R.; Shofty, B.; Paul, M.; Neuberger, A. Management of post-neurosurgical meningitis: Narrative review. Clin. Microbiol. Infect. 2017, 23, 621–628. [Google Scholar] [CrossRef]
- Ramakrishna, J.M.; Libertin, C.R.; Yang, J.N.; Diaz, M.A.; Nengue, A.L.; Patel, R. 16S rRNA Gene PCR/Sequencing of Cerebrospinal Fluid in the Diagnosis of Post-operative Meningitis. Access Microbiol. 2020, 2, acmi000100. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.; Kim, N.; Son, H.; Ha, E.J.; Koh, E.J.; Phi, J.H.; Park, C.K.; Kim, J.E.; Kim, S.K.; et al. Nanopore 16S sequencing enhances the detection of bacterial meningitis after neurosurgery. Ann. Clin. Transl. Neurol. 2022, 9, 312–325. [Google Scholar] [CrossRef]
- Perdigao Neto, L.V.; Medeiros, M.; Ferreira, S.C.; Nishiya, A.S.; de Assis, D.B.; Boszczowski, I.; Costa, S.F.; Levin, A.S. Polymerase chain reaction targeting 16S ribosomal RNA for the diagnosis of bacterial meningitis after neurosurgery. Clinics 2021, 76, e2284. [Google Scholar] [CrossRef]
- Schuurman, T.; de Boer, R.F.; Kooistra-Smid, A.M.; van Zwet, A.A. Prospective study of use of PCR amplification and sequencing of 16S ribosomal DNA from cerebrospinal fluid for diagnosis of bacterial meningitis in a clinical setting. J. Clin. Microbiol. 2004, 42, 734–740. [Google Scholar] [CrossRef]
- Welinder-Olsson, C.; Dotevall, L.; Hogevik, H.; Jungnelius, R.; Trollfors, B.; Wahl, M.; Larsson, P. Comparison of broad-range bacterial PCR and culture of cerebrospinal fluid for diagnosis of community-acquired bacterial meningitis. Clin. Microbiol. Infect. 2007, 13, 879–886. [Google Scholar] [CrossRef]
- Zarrouk, V.; Leflon-Guibout, V.; Robineaux, S.; Kalamarides, M.; Nicolas-Chanoine, M.H.; Sterkers, O.; Fantin, B. Broad-range 16S rRNA PCR with cerebrospinal fluid may be unreliable for management of postoperative aseptic meningitis. J. Clin. Microbiol. 2010, 48, 3331–3333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rath, P.M.; Schoch, B.; Adamzik, M.; Steinmann, E.; Buer, J.; Steinmann, J. Value of multiplex PCR using cerebrospinal fluid for the diagnosis of ventriculostomy-related meningitis in neurosurgery patients. Infection 2014, 42, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.L.; Tokarz, R.; Briese, T.; Lipkin, W.I.; Jain, K.; Whittier, S.; Shah, J.; Connolly, E.S.; Yin, M.T. Evaluation of a multiplex polymerase chain reaction for early diagnosis of ventriculostomy-related infections. J. Neurosurg. 2015, 123, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Senturk, G.C.; Ozay, R.; Kul, G.; Altay, F.A.; Kuzi, S.; Gurbuz, Y.; Tutuncu, E.; Eser, T. Evaluation of Post-operative Meningitis: Comparison of Meningitis Caused by Acinetobacter spp. and Other Possible Causes. Turk. Neurosurg. 2019, 29, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Valdoleiros, S.R.; Torrao, C.; Freitas, L.S.; Mano, D.; Goncalves, C.; Teixeira, C. Nosocomial meningitis in intensive care: A 10-year retrospective study and literature review. Acute Crit. Care 2022, 37, 61–70. [Google Scholar] [CrossRef]
- Khan, F.Y.; Abukhattab, M.; Baager, K. Nosocomial postneurosurgical Acinetobacter baumannii meningitis: A retrospective study of six cases admitted to Hamad General Hospital, Qatar. J. Hosp. Infect. 2012, 80, 176–179. [Google Scholar] [CrossRef]
- Kurtaran, B.; Kuscu, F.; Ulu, A.; Inal, A.S.; Komur, S.; Kibar, F.; Cetinalp, N.E.; Ozsoy, K.M.; Arslan, Y.K.; Yilmaz, D.M.; et al. The Causes of Postoperative Meningitis: The Comparison of Gram-Negative and Gram-Positive Pathogens. Turk. Neurosurg. 2018, 28, 589–596. [Google Scholar] [CrossRef]
- Tuon, F.F.; Penteado-Filho, S.R.; Amarante, D.; Andrade, M.A.; Borba, L.A. Mortality rate in patients with nosocomial Acinetobacter meningitis from a Brazilian hospital. Braz. J. Infect. Dis. 2010, 14, 437–440. [Google Scholar] [CrossRef]
- Couffin, S.; Lobo, D.; Cook, F.; Jost, P.H.; Bitot, V.; Birnbaum, R.; Nebbad, B.; Ait-Mamar, B.; Lahiani, W.; Martin, M.; et al. Coagulase-negative staphylococci are associated to the mild inflammatory pattern of healthcare-associated meningitis: A retrospective study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 755–763. [Google Scholar] [CrossRef]
- Markantonis, S.L.; Markou, N.; Fousteri, M.; Sakellaridis, N.; Karatzas, S.; Alamanos, I.; Dimopoulou, E.; Baltopoulos, G. Penetration of colistin into cerebrospinal fluid. Antimicrob. Agents Chemother. 2009, 53, 4907–4910. [Google Scholar] [CrossRef]
- Srihawan, C.; Castelblanco, R.L.; Salazar, L.; Wootton, S.H.; Aguilera, E.; Ostrosky-Zeichner, L.; Sandberg, D.I.; Choi, H.A.; Lee, K.; Kitigawa, R.; et al. Clinical Characteristics and Predictors of Adverse Outcome in Adult and Pediatric Patients with Healthcare-Associated Ventriculitis and Meningitis. Open Forum. Infect. Dis. 2016, 3, ofw077. [Google Scholar] [CrossRef] [PubMed]
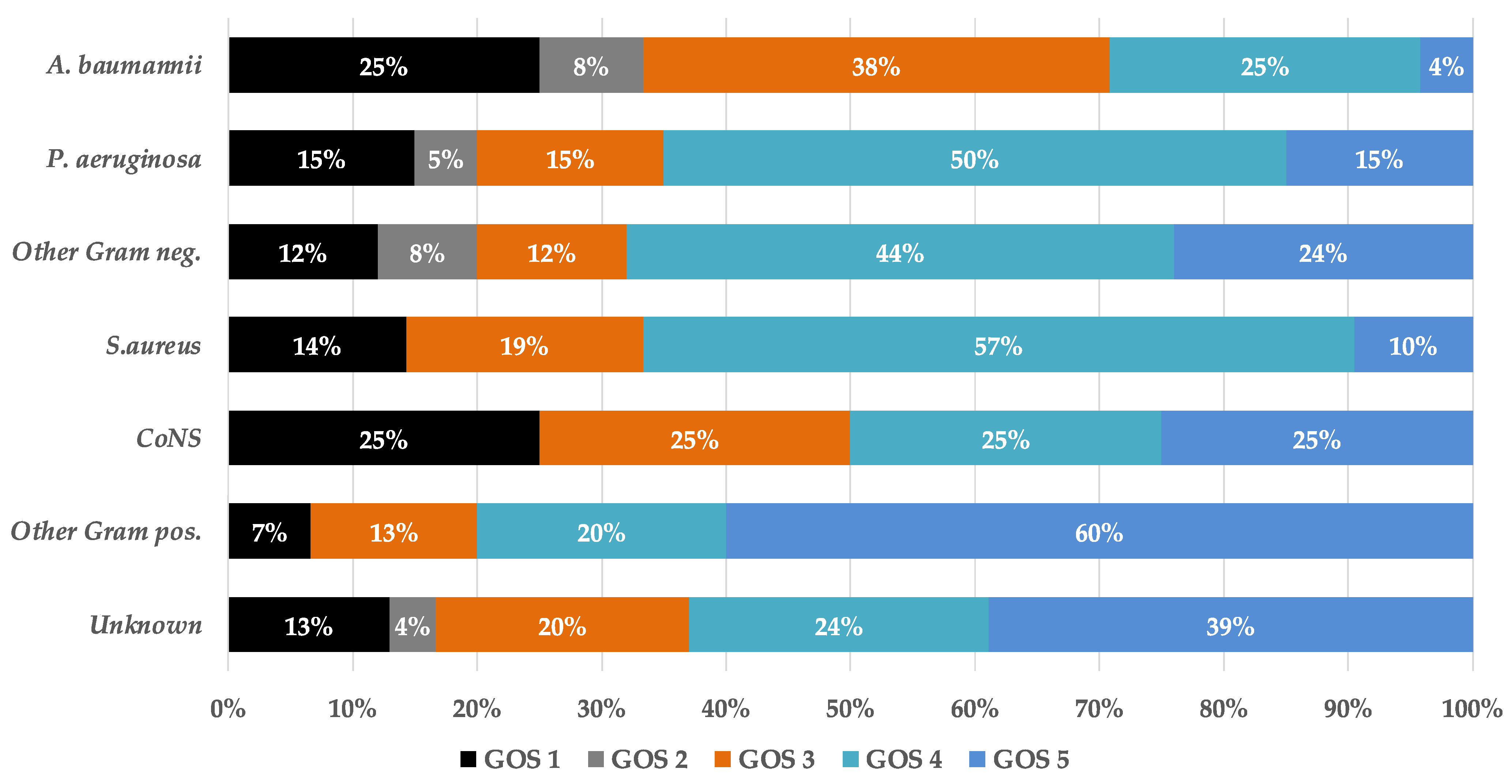
| Characteristics | Number of Patients (%) or Median (IQR) |
|---|---|
| Median age, years | 53 (35–66) |
| Male sex | 91 (63.19%) |
| Female sex | 53 (36.81%) |
| Immunocompromised | 16 (11.11%) |
| Indication for neurosurgical procedure | |
| Hemorrhage | 54 (37.50%) |
| Subarachnoid | 29 (20.14%) |
| Intraventricular | 10 (6.94%) |
| Intracerebral | 15 (10.42%) |
| Hydrocephalus | 42 (29.17%) |
| Trauma | 26 (18.06%) |
| Brain tumor | 72 (50.00%) |
| Other | 15 (10.42%) |
| Presence of ventriculoperitoneal (VP) shunt or external ventricular (EVD) before infection | 24 (16.67%) |
| Clinical presentation | |
| Time from neurosurgery, days | 7 (3–14) |
| Fever | 100 (69.44%) |
| Glasgow coma score ≤ 14 | 63 (43.73%) |
| Glasgow coma score ≤ 8 | 28 (19.44%) |
| Headache | 73 (50.69%) |
| Changes in mental status | 83 (57.64%) |
| Nausea/vomiting | 48 (33.33%) |
| Focal neurological deficit | 74 (51.39%) |
| Neck stiffness | 51 (35.42%) |
| Seizures | 20 (13.89%) |
| Photophobia | 15 (10.42%) |
| Cerebrospinal fluid leak | 27 (18.75%) |
| Etiology | Number of Episodes (%) |
|---|---|
| Etiology unknown | 61 (40.39%) |
| Gram-positive bacteria | |
| Staphylococcus aureus | 21 (13.91%) |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 16 (10.60%) |
| Methicillin-susceptible Staphylococcus aureus (MSSA) | 5 (3.31%) |
| Coagulase-negative Staphylococcus (CoNS) | 12 (7.95%) |
| Streptococcus spp. | 6 (3.97%) |
| Enterococcus spp. | 5 (3.31%) |
| Rothia mucilaginosa | 1 (0.66%) |
| Corynebacterium spp. | 2 (1.32%) |
| Bacillus spp. | 1 (0.66%) |
| Cutibacterium acnes | 2 (1.32%) |
| Gram-negative bacteria | |
| Pseudomonas spp. | 20 (13.25%) |
| Enterobacter spp. | 8 (5.30%) |
| Klebsiella pneumoniae | 7 (4.64%) |
| Acinetobacter baumannii | 24 (15.89%) |
| Escherichia coli | 4 (2.65%) |
| Citrobacter spp. | 3 (1.99%) |
| Serratia marcescens | 2 (1.32%) |
| Fusobacterium nucleatum | 1 (0.66%) |
| Other microorganisms | |
| Candida albicans | 3 (3.99%) |
| Mycobacterium tuberculosis | 2 (1.32%) |
| Mixed infection | 28 (18.54%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panic, H.; Gjurasin, B.; Santini, M.; Kutlesa, M.; Papic, N. Etiology and Outcomes of Healthcare-Associated Meningitis and Ventriculitis—A Single Center Cohort Study. Infect. Dis. Rep. 2022, 14, 420-427. https://doi.org/10.3390/idr14030045
Panic H, Gjurasin B, Santini M, Kutlesa M, Papic N. Etiology and Outcomes of Healthcare-Associated Meningitis and Ventriculitis—A Single Center Cohort Study. Infectious Disease Reports. 2022; 14(3):420-427. https://doi.org/10.3390/idr14030045
Chicago/Turabian StylePanic, Hana, Branimir Gjurasin, Marija Santini, Marko Kutlesa, and Neven Papic. 2022. "Etiology and Outcomes of Healthcare-Associated Meningitis and Ventriculitis—A Single Center Cohort Study" Infectious Disease Reports 14, no. 3: 420-427. https://doi.org/10.3390/idr14030045
APA StylePanic, H., Gjurasin, B., Santini, M., Kutlesa, M., & Papic, N. (2022). Etiology and Outcomes of Healthcare-Associated Meningitis and Ventriculitis—A Single Center Cohort Study. Infectious Disease Reports, 14(3), 420-427. https://doi.org/10.3390/idr14030045






