Examining the Efficacy of Antimicrobial Therapy in Preventing the Development of Postinfectious Glomerulonephritis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Data Collection
2.3. Statistical Analysis
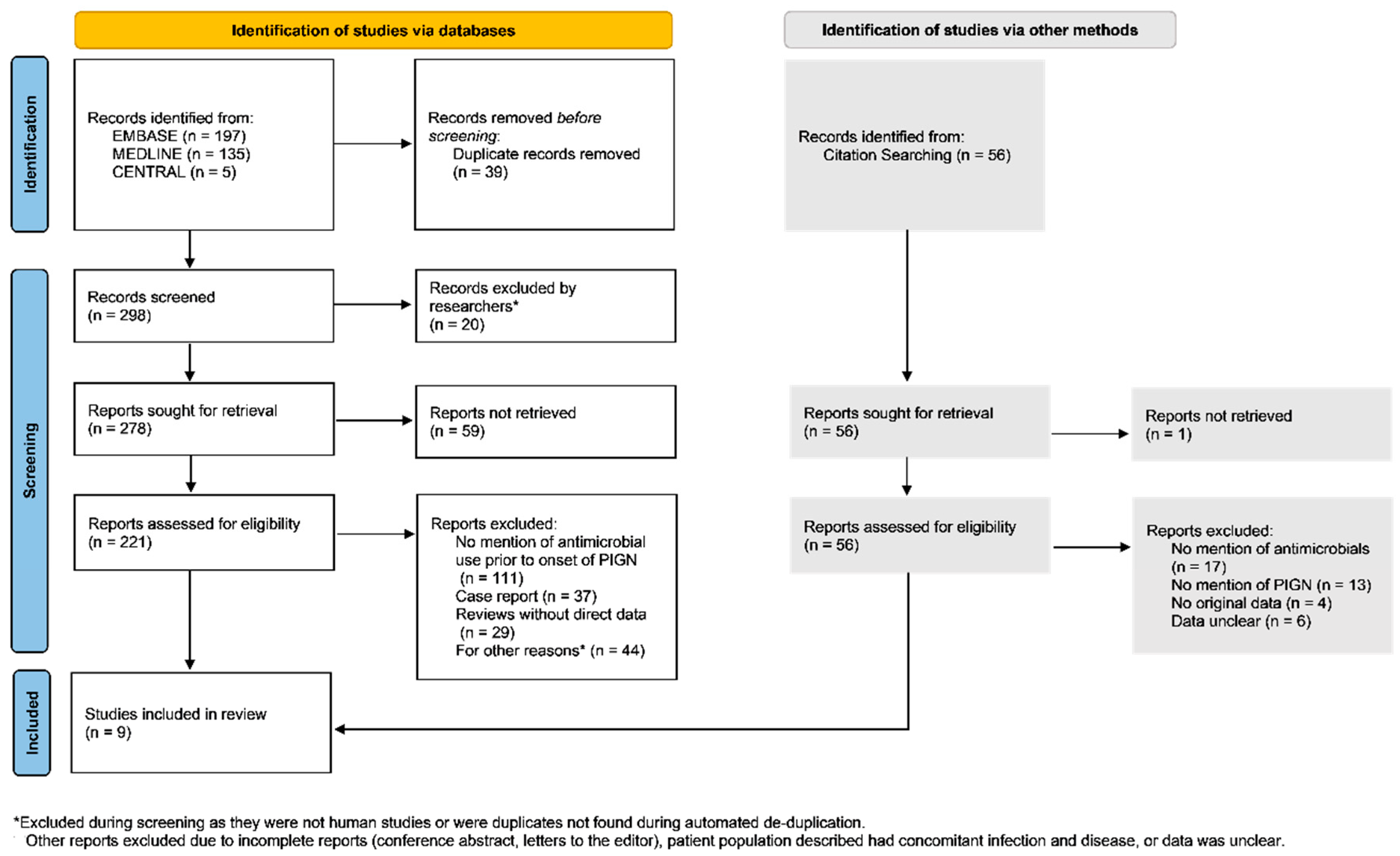
3. Results
3.1. Antimicrobial Use and Postinfectious Glomerulonephritis
3.2. Glomerulonephritis in Patients with Initial Use of Antimicrobial Therapy
3.3. Glomerulonephritis in Patients without Initial Use of Antimicrobial Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Padala, S.A.; Ludhwani, D. Poststreptococcal Glomerulonephritis; StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kılıc, B.D.; Kara, M.A.; Buyukcelik, M.; Balat, A. Pediatric post-streptococcal glomerulonephritis: Clinical and laboratory data. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2018, 60, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, A.A.; Parikh, S.V.; Nadasdy, T. Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nat. Rev. Nephrol. 2020, 16, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Usman, N.; Annamaraju, P. Type III Hypersensitivity Reaction; StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Eison, T.M.; Ault, B.H.; Jones, D.P.; Chesney, R.W.; Wyatt, R.J. Post-streptococcal acute glomerulonephritis in children: Clinical features and pathogenesis. Pediatric Nephrol. 2011, 26, 165–180. [Google Scholar] [CrossRef]
- Couser, W.G. Pathogenesis and treatment of glomerulonephritis-an update. J. Bras. De Nefrol. 2016, 38, 107–122. [Google Scholar] [CrossRef]
- Rodríguez-Iturbe, B.; Batsford, S. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney Int. 2007, 71, 1094–1104. [Google Scholar] [CrossRef][Green Version]
- Luo, Y.-H.; Chuang, W.-J.; Wu, J.-J.; Lin, M.T.; Liu, C.-C.; Lin, P.-Y.; Roan, J.-N.; Wong, T.-W.; Chen, Y.-L.; Lin, Y.-S. Molecular mimicry between streptococcal pyrogenic exotoxin B and endothelial cells. Lab Investig. 2010, 90, 1492–1506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez-Iturbe, B.; Musser, J.M. The Current State of Poststreptococcal Glomerulonephritis. J. Am. Soc. Nephrol. 2008, 19, 1855–1864. [Google Scholar] [CrossRef]
- Kanjanabuch, T.; Kittikowit, W.; Eiam-Ong, S. An update on acute postinfectious glomerulonephritis worldwide. Nat. Rev. Nephrol. 2009, 5, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Tolaymat, A. Changing epidemiology of acute post-streptococcal glomerulonephritis in Northeast Florida: A comparative study. Pediatr Nephrol. 2008, 23, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Facklam, R.R.; Breiman, R.F. Changing epidemiology of group A streptococcal infection in the USA. Lancet 1990, 336, 1167–1171. [Google Scholar] [CrossRef]
- Glassock, R.J.; Alvarado, A.; Prosek, J.; Hebert, C.; Parikh, S.; Satoskar, A.; Nadasdy, T.; Forman, J.; Rovin, B.; Hebert, L.A. Staphylococcus-related glomerulonephritis and poststreptococcal glomerulonephritis: Why defining “post” is important in understanding and treating infection-related glomerulonephritis. Am. J. Kidney Dis. 2015, 65, 826–832. [Google Scholar] [CrossRef]
- Shulman, S.T.; Bisno, A.L. 200-Nonsuppurative Poststreptococcal Sequelae: Rheumatic Fever and Glomerulonephritis. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 2300–2309. [Google Scholar]
- Sainato, R.J.; Weisse, M.E. Poststreptococcal Glomerulonephritis and Antibiotics: A Fresh Look at Old Data. Clin. Pediatrics 2019, 58, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Musser, J.M.; Beres, S.B.; Zhu, L.; Olsen, R.J.; Vuopio, J.; Hyyryläinen, H.-L.; Gröndahl-Yli-Hannuksela, K.; Kristinsson, K.G.; Darenberg, J.; Henriques-Normark, B.; et al. Reduced In Vitro Susceptibility of Streptococcus pyogenes to β-Lactam Antibiotics Associated with Mutations in the pbp2x Gene Is Geographically Widespread. J. Clin. Microbiol. 2020, 58, e01993-19. [Google Scholar] [CrossRef]
- Kaplan, S.L.; Mason, E.O. Management of Infections Due to Antibiotic-Resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 1998, 11, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V. Mechanisms of Antibiotic Resistance. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- National Institute of Health. Study Quality Assessment Tools|NHLBI; NIH: Bethesda, MD, USA, 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 31 January 2022).
- Farrah, K.; Young, K.; Tunis, M.C.; Zhao, L. Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Syst. Rev. 2019, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.; Scholz, H.; Helmerking, M. Short-course antibiotic treatment of 4782 culture-proven cases of group A streptococcal tonsillopharyngitis and incidence of poststreptococcal sequelae. J. Infect. Dis. 2000, 182, 509–516. [Google Scholar] [CrossRef][Green Version]
- Hovelius, B.; Bygren, P.; Christensen, P.; A Mårdh, P. Respiratory tract infections at a community care centre—with emphasis on group A streptococci. Scand. J. Infect. Dis. Suppl. 1983, 39, 59–67. [Google Scholar]
- Scrace, M.; Koko, K. An outbreak of acute post-streptococcal glomerulonephritis in remote Far North Queensland. Aust. J. Rural. Health 2006, 14, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Streeton, C.L.; Hanna, J.N.; Messer, R.D.; Merianos, A. An epidemic of acute post-streptococcal glomerulonephritis among Aboriginal children. J. Paediatr. Child Health 1995, 31, 245–248. [Google Scholar] [CrossRef]
- Chamovitz, R.; Catanzaro, F.J.; Stetson, C.A.; Rammelkamp, C.H. Prevention of rheumatic fever by treatment of previous streptococcal infections. I. Evaluation of benzathine penicillin G. N. Engl. J. Med. 1954, 251, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Stetson, C.A.; Rammelkamp, C.H.; Krause, R.M.; Kohen, R.J.; Perry, W.D. Epidemic acute nephritis: Studies on etiology, natural history and prevention. Medicine 1955, 34, 431–450. [Google Scholar] [CrossRef]
- Weinstein, L.; Bachrach, L.; Boyer, N.H. Observations on the Development of Rheumatic Fever and Glomerulonephritis in Cases of Scarlet Fever Treated with Penicillin. N. Engl. J. Med. 1950, 242, 1002–1010. [Google Scholar] [CrossRef]
- Stillerman, M.; Bernstein, S.H. Streptococcal Pharyngitis Therapy. Am. J. Dis. Child. 1964, 107, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Schaad, U.B.; Kellerhals, P.; Altwegg, M. Azithromycin versus penicillin V for treatment of acute group A streptococcal pharyngitis. Pediatric Infect. Dis. J. 2002, 21, 304–308. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Zaffanello, M.; Cataldi, L.; Franchini, M.; Fanos, V. Evidence-based treatment limitations prevent any therapeutic recommendation for acute poststreptococcal glomerulonephritis in children. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, RA79–RA84. [Google Scholar]
- Adam, D.; Scholz, H.; Helmerking, M. Comparison of short-course (5 day) cefuroxime axetil with a standard 10 day oral penicillin V regimen in the treatment of tonsillopharyngitis. J. Antimicrob. Chemother. 2000, 45, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Scholz, H. Streptococcal-A tonsillopharyngitis: A 5-day course of cefuroxime axetil versus a 10-day course of penicillin V. results depending on the children’s age. Chemotherapy 2004, 50, 51–54. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Marks, S.D. Post-infectious glomerulonephritis. Paediatr. Int. Child Health 2017, 37, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Van De Voorde, R.G. Acute poststreptococcal glomerulonephritis: The most common acute glomerulonephritis. Pediatrics Rev. 2015, 36, 3–12. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Park, H.; Kim, S. Predominance of emm4 and antibiotic resistance of Streptococcus pyogenes in acute pharyngitis in a southern region of Korea. J. Med. Microbiol. 2019, 68, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Tyrstrup, M.; Melander, E.; Hedin, K.; Beckman, A.; Mölstad, S. Children with respiratory tract infections in Swedish primary care; prevalence of antibiotic resistance in common respiratory tract pathogens and relation to antibiotic consumption. BMC Infect. Dis. 2017, 17, 603. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J. Antimicrobial Resistance: An Antimicrobial/Diagnostic Stewardship and Infection Prevention Approach. Med. Clin. N. Am. 2018, 102, 819–829. [Google Scholar] [CrossRef]


| Study | Year | Study Location | Type of Study | Date of Study | Population Studied | Type of Sample | Prior Use of Antimicrobials | No Prior Use of Antimicrobials | Total No. of Patients | Antimicrobial Agent Utilized | Infectious Etiology Discussed | Major Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (n = ) | Cases (n = ) | No treatment (n = ) | Cases (n = ) | |||||||||||
| Adam et al. | 2000 | Germany | RCT | 1995–1998 | Ages 1–18 | Throat Culture | 4482 | 2 | 0 | 0 | 4482 | Oral Penicillin V vs oral macrolides, cephalosporin | GAS | Efficacy of 5-day antibiotic regimen was equivalent to 10 days of penicillin V |
| Chamovitz et al. | 1954 | Wyoming, USA | RCT | 1953 | Not stated (Air Force Base) | No samples taken | 257 | 0 | 109 | 1 | 366 | Intramuscular DBED Penicillin | Exudative tonsillitis or pharyngitis | Post-streptococcal sequelae, including glomerulonephritis and rheumatic fever, occurred in control patients whereas none occurred in penicillin treated patients |
| Hovelius et al. | 1983 | Sweden | Cohort | 1976–1977 | All Ages | Throat Culture | 220 | 9 | 0 | 0 | 220 | Penicillin V (47%), Erythromycin (9%), amoxicillin or doxycycline (4%) | GAS | PSGN was diagnosed in 9 out of 220 patients, supporting the assumption that early penicillin treatment reduces incidence of PSGN |
| Schaad et al. | 2002 | Switzerland | RCT | 1996–1999 | Ages 2–12 | Throat Culture | 269 | 8 | 0 | 0 | 269 | Oral Penicillin V vs Oral Azithromycin | GAS | Clinical efficacy of 3-day azithromycin and 10-day penicillin treatments were similar, although had lower levels of bacteriologic eradication |
| Scrace & Koko et al. | 2006 | Australia | Cohort | 2005 | Ages 2–12 | No samples taken | 56 | 3 | 60 | 8 | 116 | Intramuscular Penicillin | Screening for infected scabies | Screening of children with subsequent treatment using IM penicillin may be an effective community management strategy for APSGN outbreaks |
| Stetson et al. | 1955 | Maine, USA | RCT | 1952 | Not stated (Naval Base) | No samples taken | 44 | 1 | 140 | 10 | 184 | Intramuscular Penicillin | Pharyngitis | The incidence of acute nephritis was higher among untreated patients, compared to those receiving early penicillin therapy |
| Stillerman & Bernstein | 1963 | New York, USA | RCT | 1956–1960 | Not stated (pediatric offices) | Throat Culture | 442 | 1 | 0 | 0 | 442 | Oral Phenoxymethyl Penicillin | GAS | There was a significantly higher cure rate for nontypeable Streptococcus strains when treated with larger-dose penicillin compaired to smaller-dose treatment. |
| Streeton et al. | 1995 | Austrialia | Cohort | 1995 | Ages 2–14 | No samples taken | 583 | 58 | 0 | 0 | 583 | Intramuscular Penicillin | Screening for skin infection | Epidemic of APSGN was associated with GAS skin infections. Use of penicillin may have reduced community transmission. |
| Weinstein et al. | 1968 | Massachusetts, USA | Cohort | 1948 | All Ages | Throat and Nose Cultures | 167 | 6 | 0 | 0 | 167 | Parenteral Penicillin | GAS | The treatment of scarlet fever with penicillin therapy does not prevent "late" streptococcal sequelae, including rheumatic fever or glomerulonephritis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bateman, E.; Mansour, S.; Okafor, E.; Arrington, K.; Hong, B.-Y.; Cervantes, J. Examining the Efficacy of Antimicrobial Therapy in Preventing the Development of Postinfectious Glomerulonephritis: A Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2022, 14, 176-183. https://doi.org/10.3390/idr14020022
Bateman E, Mansour S, Okafor E, Arrington K, Hong B-Y, Cervantes J. Examining the Efficacy of Antimicrobial Therapy in Preventing the Development of Postinfectious Glomerulonephritis: A Systematic Review and Meta-Analysis. Infectious Disease Reports. 2022; 14(2):176-183. https://doi.org/10.3390/idr14020022
Chicago/Turabian StyleBateman, Emily, Sara Mansour, Euchariachristy Okafor, Kedzie Arrington, Bo-Young Hong, and Jorge Cervantes. 2022. "Examining the Efficacy of Antimicrobial Therapy in Preventing the Development of Postinfectious Glomerulonephritis: A Systematic Review and Meta-Analysis" Infectious Disease Reports 14, no. 2: 176-183. https://doi.org/10.3390/idr14020022
APA StyleBateman, E., Mansour, S., Okafor, E., Arrington, K., Hong, B.-Y., & Cervantes, J. (2022). Examining the Efficacy of Antimicrobial Therapy in Preventing the Development of Postinfectious Glomerulonephritis: A Systematic Review and Meta-Analysis. Infectious Disease Reports, 14(2), 176-183. https://doi.org/10.3390/idr14020022






