Bacterial Species and Antimicrobial Resistance of Clinical Isolates from Pediatric Patients in Yangon, Myanmar, 2020
Abstract
:1. Introduction
2. Materials and Methods
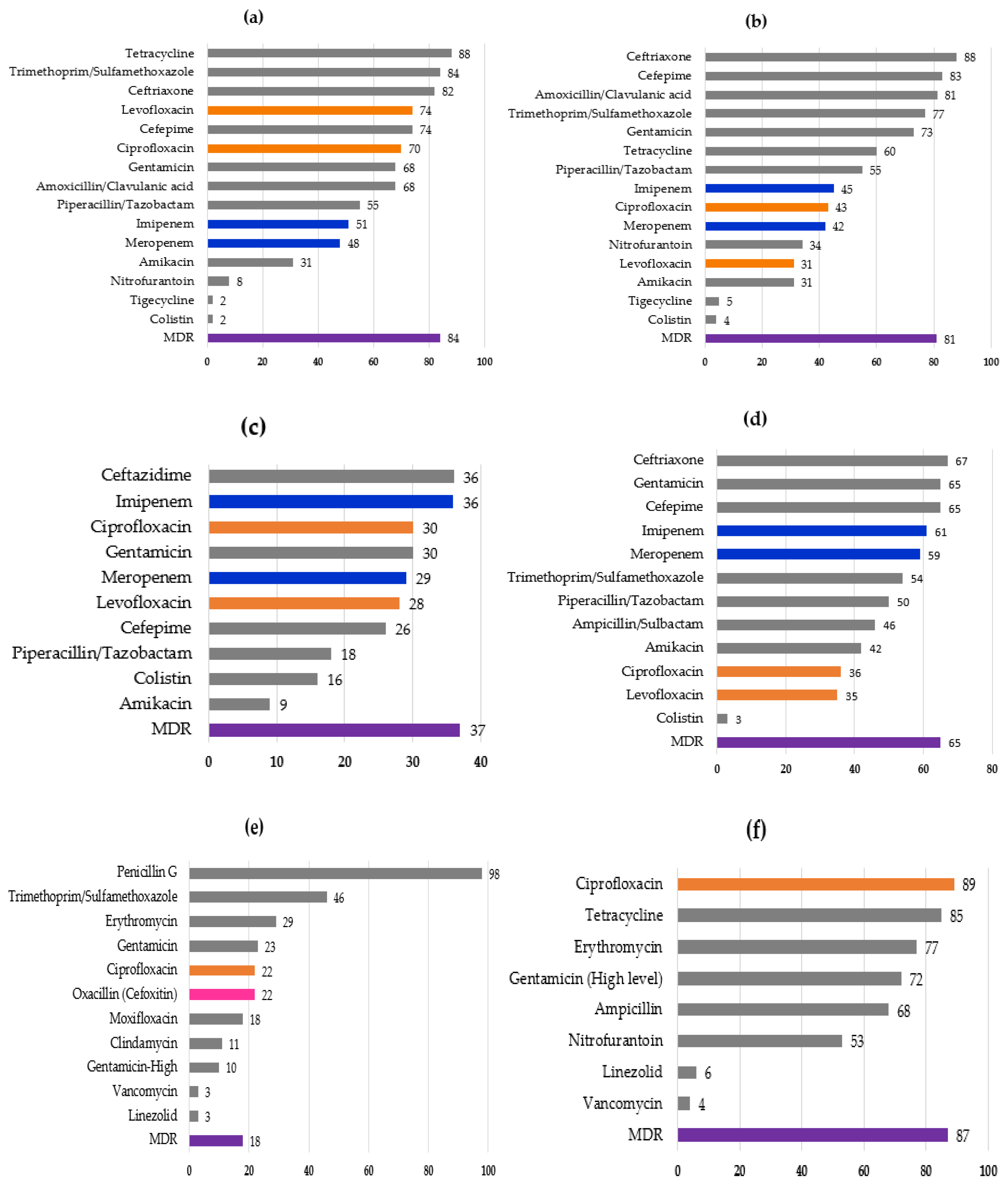
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zellweger, R.M.; Carrique-Mas, J.; Limmathurotsakul, D.; Day, N.P.J.; Thwaites, G.E.; Baker, S. Southeast Asia Antimicrobial Resistance Network. A current perspective on antimicrobial resistance in Southeast Asia. J. Antimicrob. Chemother. 2017, 72, 2963–2972. [Google Scholar] [CrossRef] [Green Version]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, e00048-19. [Google Scholar] [CrossRef]
- Kakkar, M.; Chatterjee, P.; Chauhan, A.S.; Grace, D.; Lindahl, J.; Beeche, A.; Jing, F.; Chotinan, S. Antimicrobial resistance in South East Asia: Time to ask the right questions. Glob. Health Action 2018, 11, 1483637. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 29 November 2021).
- Hawkey, P.M. Multidrug-resistant Gram-negative bacteria: A product of globalization. J. Hosp. Infect. 2015, 89, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Huang, Y.C. New epidemiology of Staphylococcus aureus infection in Asia. Clin. Microbiol. Infect. 2014, 20, 605–623. [Google Scholar] [CrossRef] [Green Version]
- Le Doare, K.; Bielicki, J.; Heath, P.T.; Sharland, M. Systematic Review of Antibiotic Resistance Rates Among Gram-Negative Bacteria in Children with Sepsis in Resource-Limited Countries. J. Pediatric Infect. Dis. Soc. 2015, 4, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H.; ARPEC project group. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, P.; Xu, H.; Jing, C.; Deng, J.; Wang, H.; Hua, C.; Chen, Y.; Chen, X.; Zhang, T.; Zhang, H.; et al. Bacterial Epidemiology and Antimicrobial Resistance Profiles in Children Reported by the ISPED Program in China, 2016 to 2020. Microbiol. Spectr. 2021, 9, e0028321. [Google Scholar] [CrossRef] [PubMed]
- Myat, T.O.; Hannaway, R.F.; Zin, K.N.; Htike, W.W.; Win, K.K.; Crump, J.A.; Murdoch, D.R.; Ussher, J.E. ESBL- and Carbapenemase-Producing Enterobacteriaceae in Patients with Bacteremia, Yangon, Myanmar, 2014. Emerg. Infect. Dis. 2017, 23, 857–859. [Google Scholar] [CrossRef]
- Aung, M.S.; San, N.; Maw, W.W.; San, T.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Kobayashi, N. Prevalence of Extended-Spectrum Beta-Lactamase and Carbapenemase Genes in Clinical Isolates of Escherichia coli in Myanmar: Dominance of blaNDM-5 and Emergence of blaOXA-181. Microb. Drug Resist. 2018, 24, 1333–1344. [Google Scholar] [CrossRef]
- Myat, T.O.; Oo, K.M.; Mone, H.K.; Htike, W.W.; Biswas, A.; Hannaway, R.F.; Murdoch, D.R.; Ussher, J.E.; Crump, J.A. A prospective study of bloodstream infections among febrile adolescents and adults attending Yangon General Hospital, Yangon, Myanmar. PLoS Negl. Trop. Dis. 2020, 14, e0008268. [Google Scholar] [CrossRef]
- Aung, M.S.; San, T.; Urushibara, N.; San, N.; Oo, W.M.; Soe, P.E.; Kyaw, Y.; Ko, P.M.; Thu, P.P.; Hlaing, M.S.; et al. Molecular Characterization of Methicillin-Susceptible and -Resistant Staphylococcus aureus Harboring Panton-Valentine Leukocidin-Encoding Bacteriophages in a Tertiary Care Hospital in Myanmar. Microb. Drug Resist. 2020, 26, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Win, N.C.; San, N.; Hlaing, M.S.; Myint, Y.Y.; Thu, P.P.; Aung, M.T.; Yaa, K.T.; Maw, W.W.; Urushibara, N.; et al. Prevalence of Extended-Spectrum Beta-Lactamase/Carbapenemase Genes and Quinolone-Resistance Determinants in Klebsiella pneumoniae Clinical Isolates from Respiratory Infections in Myanmar. Microb. Drug Resist. 2021, 27, 36–43. [Google Scholar] [CrossRef]
- Aung, M.S.; San, T.; Urushibara, N.; San, N.; Hlaing, M.S.; Soe, P.E.; Htut, W.H.W.; Moe, I.; Mon, W.L.Y.; Chan, Z.C.N.; et al. Clonal Diversity and Molecular Characteristics of Methicillin-Susceptible and -Resistant Staphylococcus aureus from Pediatric Patients in Myanmar. Microb. Drug Resist. 2021. [Google Scholar] [CrossRef] [PubMed]
- Oo, N.A.T.; Edwards, J.K.; Pyakurel, P.; Thekkur, P.; Maung, T.M.; Aye, N.S.S.; New, H.M. Neonatal Sepsis, Antibiotic Susceptibility Pattern, and Treatment Outcomes among Neonates Treated in Two Tertiary Care Hospitals of Yangon, Myanmar from 2017 to 2019. Trop. Med. Infect. Dis. 2021, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- San, T.; Moe, I.; Ashley, E.A.; San, N. High burden of infections caused by ESBL-producing MDR Escherichia coli in paediatric patients, Yangon, Myanmar. JAC Antimicrob. Resist. 2021, 3, dlab011. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th Informational Supplement, M100–S129; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- San, N.; Aung, M.S.; Thu, P.P.; Myint, Y.Y.; Aung, M.T.; San, T.; Mar, T.T.; Lwin, M.M.; Maw, W.W.; Hlaing, M.S.; et al. First detection of the mcr-1 colistin resistance gene in Escherichia coli from a patient with urinary tract infection in Myanmar. New Microbes New Infect. 2019, 30, 100550. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Zi, H.; New, K.M.; Maw, W.W.; Aung, M.T.; Min, W.W.; Nyein, N.; Kawaguchiya, M.; Urushibara, N.; Sumi, A.; et al. Drug resistance and genetic characteristics of clinical isolates of staphylococci in Myanmar: High prevalence of PVL among methicillin-susceptible Staphylococcus aureus belonging to various sequence types. New Microbes New Infect. 2016, 10, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soe, P.E.; Han, W.W.; Sagili, K.D.; Satyanarayana, S.; Shrestha, P.; Htoon, T.T.; Tin, H.H. High Prevalence of Methicillin-Resistant Staphylococcus aureus among Healthcare Facilities and Its Related Factors in Myanmar (2018–2019). Trop. Med. Infect. Dis. 2021, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Medernach, R.L.; Rispens, J.R.; Marshall, S.H.; Hujer, A.M.; Domitrovic, T.N.; Rudin, S.D.; Zheng, X.; Qureshi, N.K.; Konda, S.; et al. Community Origins and Regional Differences Highlight Risk of Plasmid-mediated Fluoroquinolone Resistant Enterobacteriaceae Infections in Children. Pediatr. Infect. Dis. J. 2019, 38, 595–599. [Google Scholar] [CrossRef]
- Akgoz, M.; Akman, I.; Ates, A.B.; Celik, C.; Keskin, B.; Ozmen Capin, B.B.; Karahan, Z.C. Plasmidic Fluoroquinolone Resistance Genes in Fluoroquinolone-Resistant and/or Extended Spectrum Beta-Lactamase-Producing Escherichia coli Strains Isolated from Pediatric and Adult Patients Diagnosed with Urinary Tract Infection. Microb. Drug Resist. 2020, 26, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Abelson Storb, K.; Osterlund, A.; Kahlmeter, G. Antimicrobial resistance in Escherichia coli in urine samples from children and adults: A 12 year analysis. Acta Paediatr. 2004, 93, 487–491. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Species | Number of Isolates in Each Specimen (%) | |||||
|---|---|---|---|---|---|---|
| Urine | Blood | Wound/Pus/Tissue | Respiratory Specimens | Ear/Eye Discharge | Total | |
| Escherichia coli | 100 (32) | 8 (3) | 29 (13) | 137 (15) | ||
| Klebsiella sp. | 50 (16) | 44 (16) | 29 (13) | 11 (13) | 134 (14) | |
| Enterobacter cloacae | 6 (3) | 4 (5) | 3 (8) | 13 (1) | ||
| other Enterobacter sp. | 21 (7) | 21 (2) | ||||
| Proteus mirabilis | 3 (8) | 3 (0.3) | ||||
| Serratia marcescens | 11 (4) | 3 (4) | 2 (5) | 16 (2) | ||
| Salmonella sp. | 11 (4) | 11 (1) | ||||
| Other Enterobacterales | 30 (10) | 7 (2) | 9 (4) | 3 (8) | 49 (6) | |
| Pseudomonas aeruginosa | 25 (8) | 25 (12) | 17 (20) | 7 (19) | 74 (8) | |
| other Pseudomonas sp. | 9 (3) | 9 (1) | ||||
| Burkholderia cepacia | 38 (14) | 1 (0.5) | 18 (22) | 1 (3) | 58 (6) | |
| Stenotrophomonas maltophilia | 6 (7) | 6 (0.6) | ||||
| Acinetobacter baumannii | 11 (5) | 16 (19) | 2 (5) | 29 (3) | ||
| Acinetobacter sp. | 17 (6) | 17 (2) | ||||
| Other non-fermenter | 25 (8) | 11 (4) | 3 (4) | 39 (4) | ||
| Staphylococcus aureus | 19 (7) | 81 (38) | 4 (5) | 9 (24) | 113 (12) | |
| Coagulase-negative Staphylococci | 7(2) | 23 (8) | 17 (8) | 4 (11) | 51 (5) | |
| Streptococcus pyogenes | 1 (3) | 1 (0.1) | ||||
| Streptococcus sp. | 2 (1) | 4 (1) | 4 (2) | 10 (1) | ||
| Enterococcus sp. | 40 (13) | 4 (1) | 2 (1) | 46 (5) | ||
| Candida sp. | 36 (11) | 53 (19) | 2 (1) | 1 (1) | 1 (3) | 93 (10) |
| Cryptococcus neoformans | 1 (0.4) | 1 (0.1) | ||||
| Yeast/mold | 1 (3) | 1 (0.1) | ||||
| total | 315 (100) | 281 (100) | 216 (100) | 83 (100) | 37 (100) | 932 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, T.; Aung, M.S.; San, N.; Aung, M.M.Z.; Mon, W.L.Y.; Thazin, T.E.; Kobayashi, N. Bacterial Species and Antimicrobial Resistance of Clinical Isolates from Pediatric Patients in Yangon, Myanmar, 2020. Infect. Dis. Rep. 2022, 14, 26-32. https://doi.org/10.3390/idr14010004
San T, Aung MS, San N, Aung MMZ, Mon WLY, Thazin TE, Kobayashi N. Bacterial Species and Antimicrobial Resistance of Clinical Isolates from Pediatric Patients in Yangon, Myanmar, 2020. Infectious Disease Reports. 2022; 14(1):26-32. https://doi.org/10.3390/idr14010004
Chicago/Turabian StyleSan, Thida, Meiji Soe Aung, Nilar San, Myat Myint Zu Aung, Win Lei Yi Mon, Thin Ei Thazin, and Nobumichi Kobayashi. 2022. "Bacterial Species and Antimicrobial Resistance of Clinical Isolates from Pediatric Patients in Yangon, Myanmar, 2020" Infectious Disease Reports 14, no. 1: 26-32. https://doi.org/10.3390/idr14010004
APA StyleSan, T., Aung, M. S., San, N., Aung, M. M. Z., Mon, W. L. Y., Thazin, T. E., & Kobayashi, N. (2022). Bacterial Species and Antimicrobial Resistance of Clinical Isolates from Pediatric Patients in Yangon, Myanmar, 2020. Infectious Disease Reports, 14(1), 26-32. https://doi.org/10.3390/idr14010004






