COVID-19 mRNA Vaccination Mimicking Heart Attack in a Healthy 56-Year-Old Physician
Abstract
:1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slaoui, M.; Hepburn, M. Developing Safe and Effective Covid Vaccines—Operation Warp Speed’s Strategy and Approach. N. Engl. J. Med. 2020, 383, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration (FDA). Emergency use authorization for Moderna COVID-19. Vaccine 2020. Available online: https://www.fda.gov/media/153912/download (accessed on 16 December 2021).
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethynol glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Settin. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Shiravi, A.A.; Ardekani, A.; Sheikhbahaei, E.; Heshmat-Ghahdarijani, K. Cardiovascular Complications of SARS-CoV-2 Vaccines: An Overview. Cardiol. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, M.; Ahmad, H.; Mohamed, M.; Saidahmed, O.; Elhassan, A.E. From Muscles to Wires: Report of Two Cases and Literature Review on COVID-19 Vaccination and Cardiac Conduction Disturbance. Cureus 2021, 13, e18805. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, G.; Mehta, J.L.; De Gregorio, C.; Butera, G.; Neroni, P.; Fanos, V.; Bassareo, P.P. COVID 19 Vaccine for Adolescents. Concern about Myocarditis and Pericarditis. Pediatr. Rep. 2021, 13, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine after Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 977. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Chaker, A.M.; Cuevas, M. Allergische Reaktionen auf COVID-19-Impfungen—Was HNO-Ärzte wissen sollten. Laryngorhinootologie 2021, 100, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Centres for Diseases Control and Prevention (CDC). Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 7 March 2021).
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar]
- Kadali, R.A.; Janagama, R.; Peruru, S.; Malayala, S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stahel, V.P. Review the safety of Covid-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 20. [Google Scholar] [CrossRef] [PubMed]
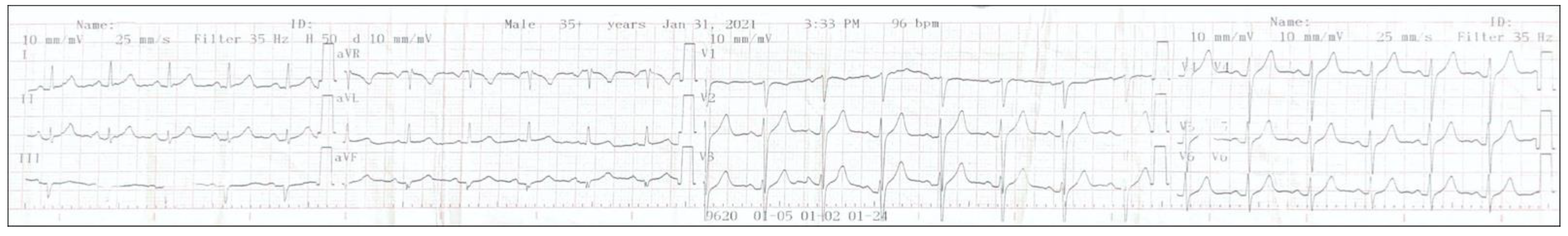
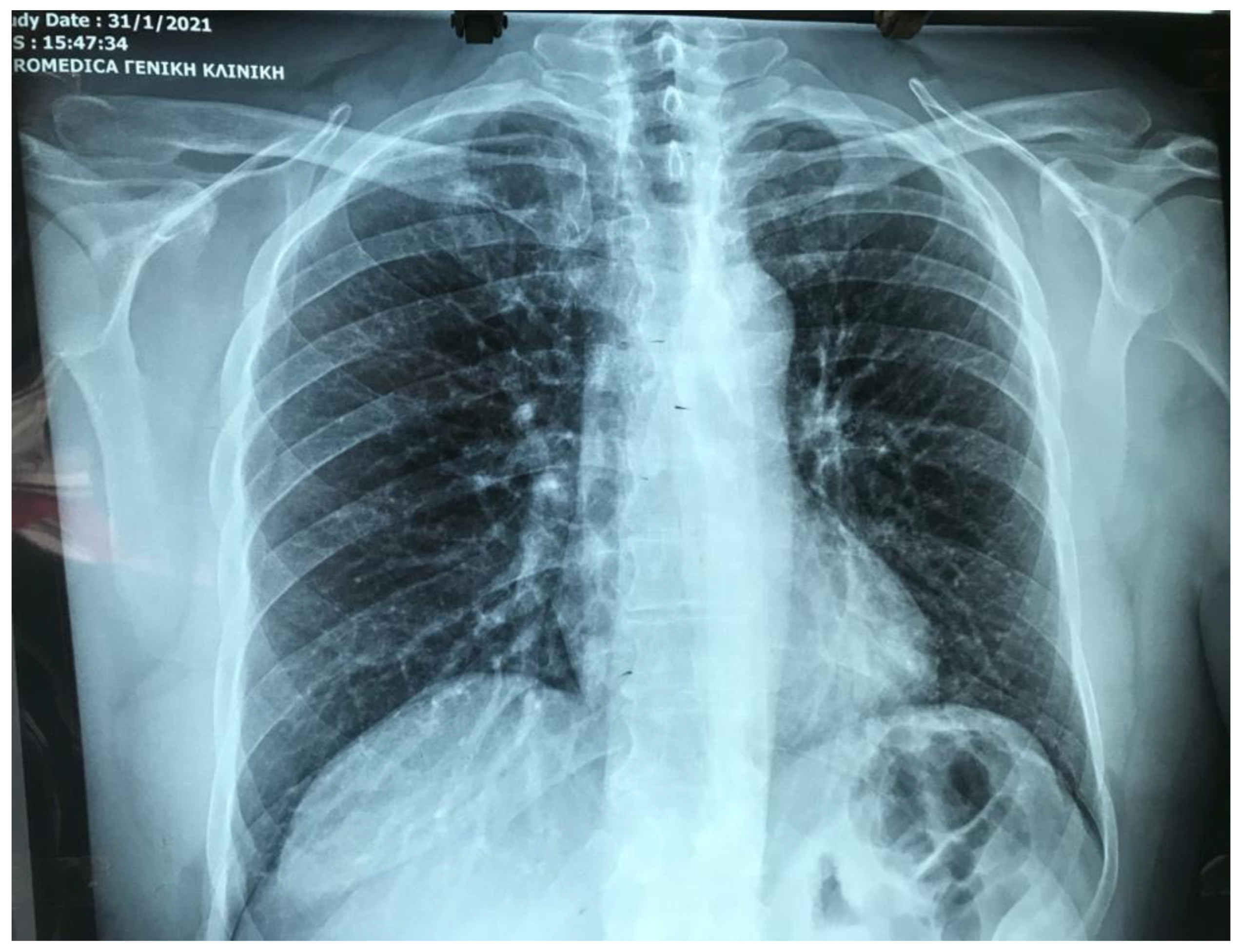
| Test | Result | Normal Levels |
|---|---|---|
| Troponine (pg/mL) | 2,1 | <34,2 |
| CK MB (U/L) | 16 | <25 |
| WBC (/μL) | 10,260 (PMN: 77,3%) | 4000–10,000 |
| Triglycerides (mg/dL) | 63 | <150 |
| CRP (mg/dL) | 0,91 | <0,3 |
| LDL (mg/dL) | 118 | <115 |
| HDL (mg/dL) | 69 | 32–68 |
| Urea (mg/dL) | 29 | 10–50 |
| Creatinine (mg/dL) | 1,02 | 0,70–1,30 |
| Glucose (mg/dL) | 110 | 70–110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xinias, I.; Mavroudi, A.; Theodorou, G.-T.; Roilidis, I. COVID-19 mRNA Vaccination Mimicking Heart Attack in a Healthy 56-Year-Old Physician. Infect. Dis. Rep. 2022, 14, 93-97. https://doi.org/10.3390/idr14010011
Xinias I, Mavroudi A, Theodorou G-T, Roilidis I. COVID-19 mRNA Vaccination Mimicking Heart Attack in a Healthy 56-Year-Old Physician. Infectious Disease Reports. 2022; 14(1):93-97. https://doi.org/10.3390/idr14010011
Chicago/Turabian StyleXinias, Ioannis, Antigoni Mavroudi, Georgios-Theofilos Theodorou, and Ioannis Roilidis. 2022. "COVID-19 mRNA Vaccination Mimicking Heart Attack in a Healthy 56-Year-Old Physician" Infectious Disease Reports 14, no. 1: 93-97. https://doi.org/10.3390/idr14010011
APA StyleXinias, I., Mavroudi, A., Theodorou, G.-T., & Roilidis, I. (2022). COVID-19 mRNA Vaccination Mimicking Heart Attack in a Healthy 56-Year-Old Physician. Infectious Disease Reports, 14(1), 93-97. https://doi.org/10.3390/idr14010011






