Root Causes of Fungal Coinfections in COVID-19 Infected Patients
Abstract
1. Introduction
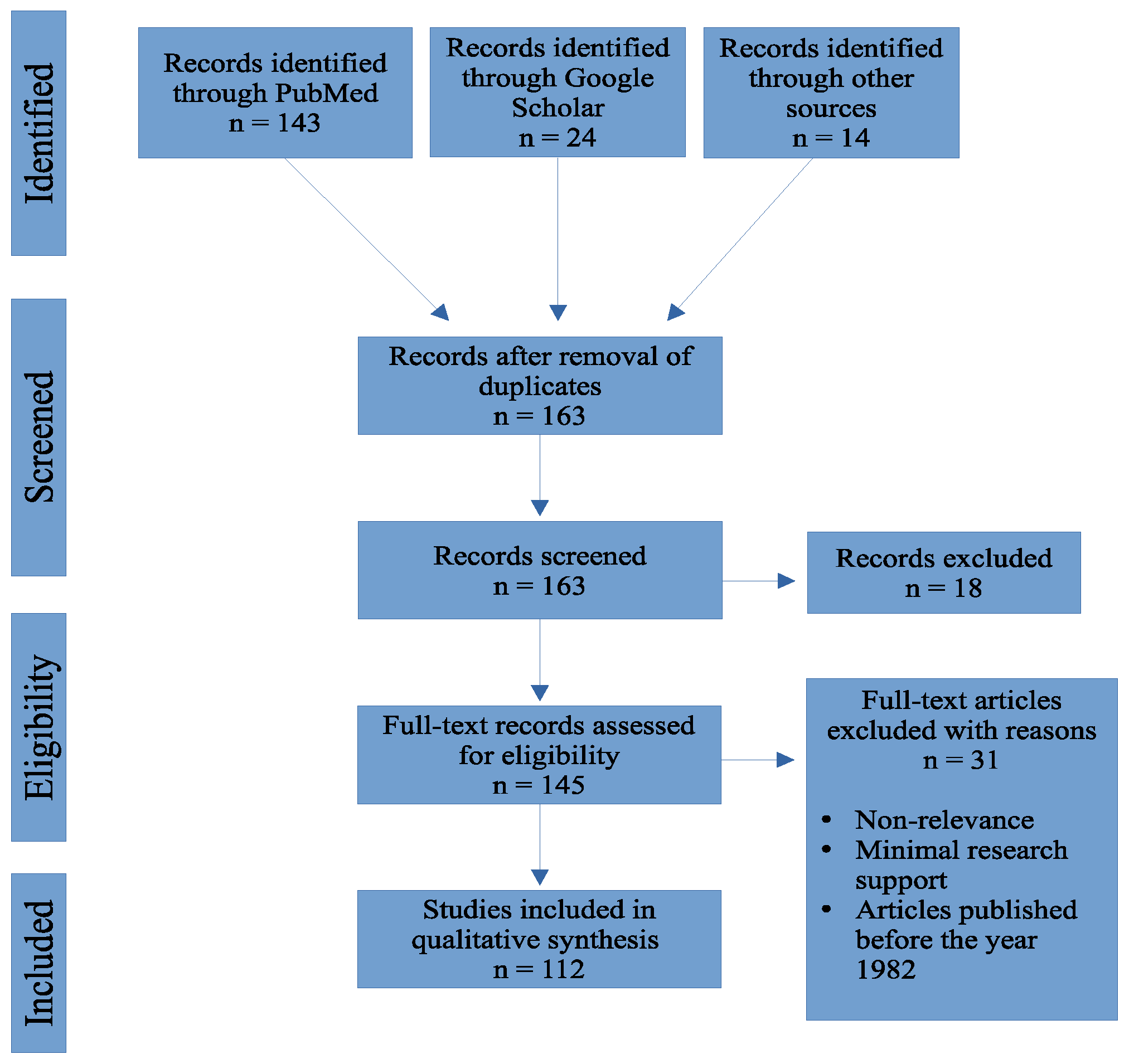
2. Methods
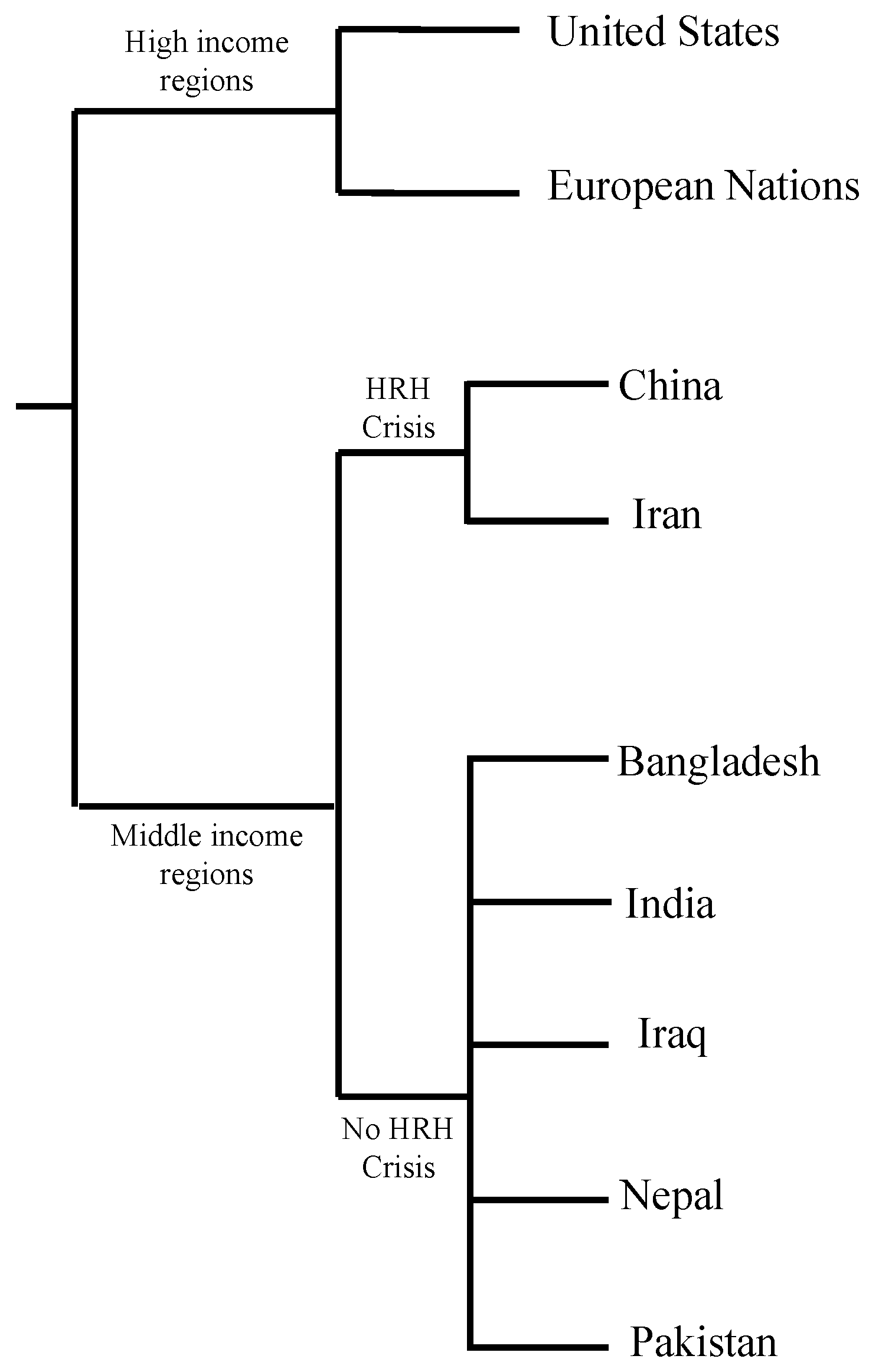
3. Countries with Cases of Fungal Infections
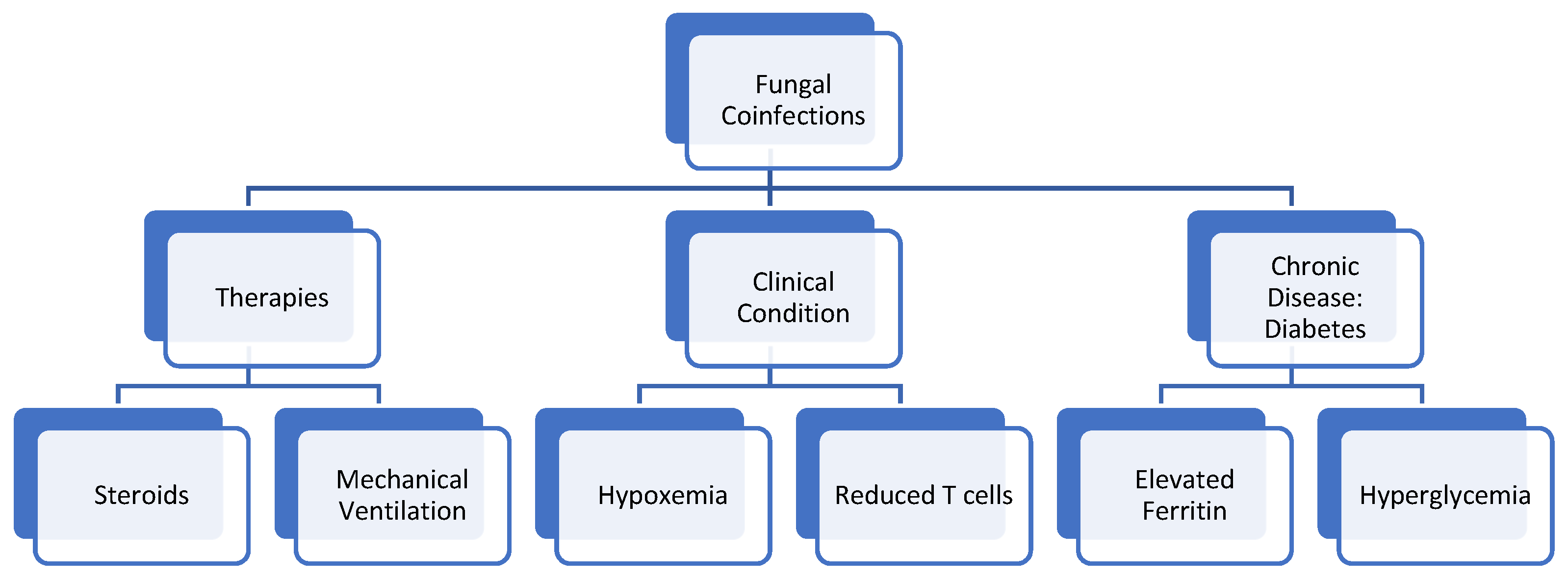
4. Root Cause of Coinfection
4.1. Oxygen/Hypoxia Induced
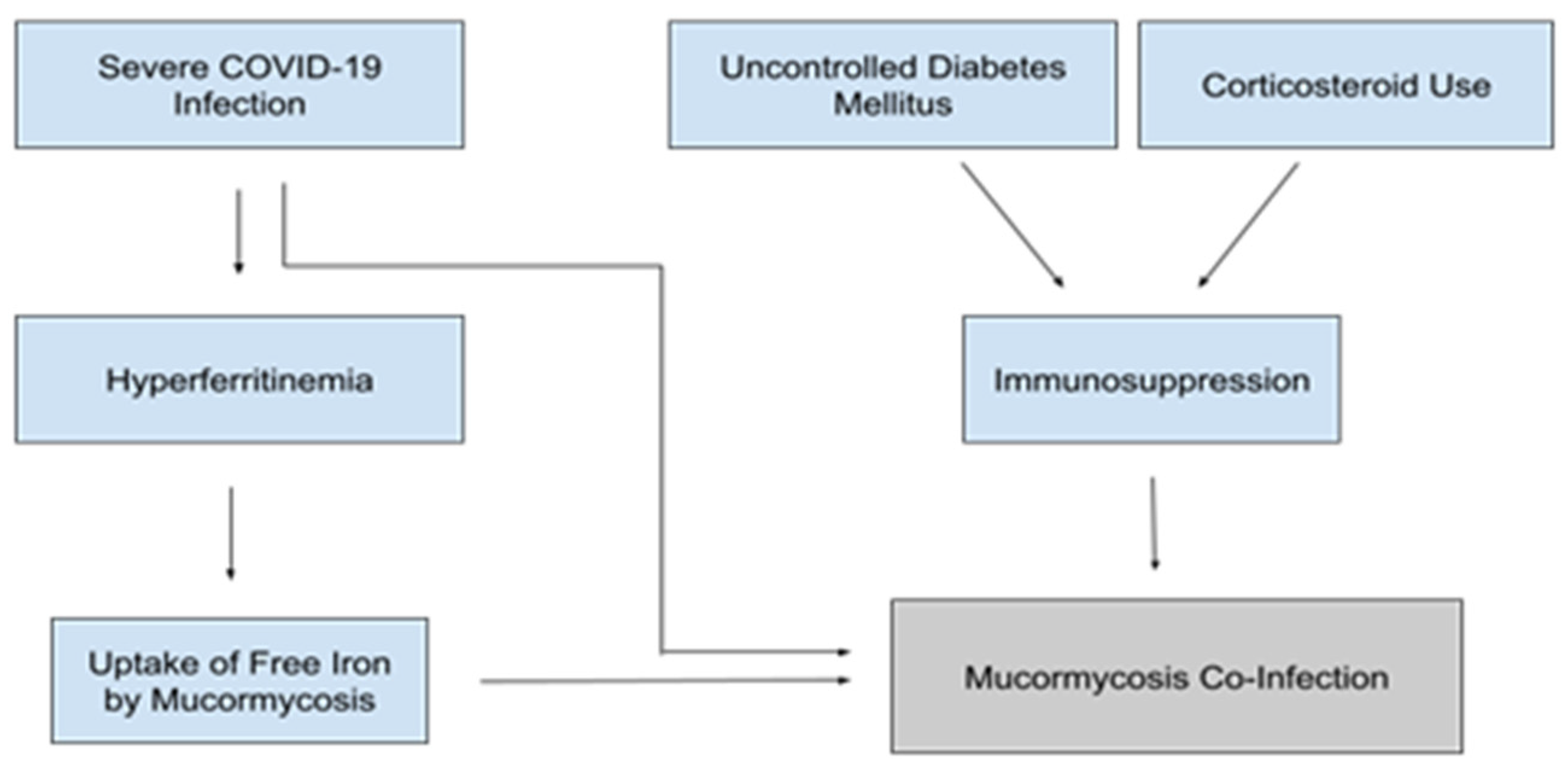
4.2. Diabetes
4.3. Steroids
4.4. Ferritin and Free Iron Levels
4.5. Mechanical Ventilation
4.6. T-Cell Lymphopenia
5. Fungal Coinfections
5.1. Aspergillosis
5.2. Candidiasis
5.3. Cryptococcosis
5.4. Mucormycosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Severe Acute Respiratory Syndrome Coronavirus 2 | (SARS-CoV-2) |
| Centers for Disease Control | (CDC) |
| United States | (US) |
| Interleukin-6 | (IL-6) |
| Nuclear Factor Kappa Beta | (NF-κB) |
| COVID-19 Associated Mucormycosis | (CAM) |
| Diabetic Ketoacidosis | (DKA) |
| Intensive Care Unit | (ICU) |
| Hypoxia inducible factor-1α | (HIF-1α) |
| Angiotensin-converting enzyme-2 | (ACE-2) |
| Diabetes Mellitus | (DM) |
| Tumor necrosis factor-alpha | (TNF-α) |
| Interleukin-10 | (IL-10) |
| Interferon gamma | (IFN-ℽ) |
| T helper 1 cell | (Th1) |
| Invasive Pulmonary Aspergillosis | (IPA) |
| COVID-19 Associated Pulmonary Aspergillosis | (CAPA) |
| COVID-19 Associated Candidiasis | (CAC) |
| Oropharyngeal Candidiasis | (OPC) |
| Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome | (HIV/AIDS) |
| Shortness of breath | (SOB) |
| Headache | (HA) |
| Gastrointestinal | (GI) |
| Thymosin alpha 1 | (Tα1) |
| All Trans Retinoic Acid | (ATRA) |
References
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A global challenge with old history, epidemiology and progress so far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis, and management of coronavirus disease 2019 (COVID-19). Postgrad Med. J. 2020, 96, 753–758. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. 2021. Available online: https://covid.cdc.gov/covid-data-tracker/#cases_totalcases (accessed on 3 August 2021).
- Fakhroo, A.D.; Al Thani, A.A.; Yassine, H.M. Markers associated with COVID-19 susceptibility, resistance, and severity. Viruses 2020, 13, 45. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). People with Certain Medical Conditions. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 18 August 2021).
- World Health Organization (WHO). Coronavirus Disease (COVID-19): How is it Transmitted? 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted (accessed on 18 August 2021).
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar] [CrossRef] [PubMed]
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Bougnoux, M.E.; Dannaoui, E.; Cornet, M.; Zahar, J.R. Invasive fungal diseases during COVID-19: We should be prepared. J. Mycol. Med. 2020, 30, 100971. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4⁺T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Arnold, F.W.; Fuqua, J.L. Viral respiratory infections: A cause of community-acquired pneumonia or a predisposing factor? Curr. Opin. Pulm. Med. 2020, 26, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Anderson, R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 2021, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Kapila, R.; Chmel, H.; Armstrong, D.A.; Louria, D.B. Superinfection: Another look. Am. J. Med. 1982, 73, 706–718. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Fungal Diseases and COVID-19. 2021. Available online: https://www.cdc.gov/fungal/covid-fungal.html (accessed on 18 August 2021).
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Iliev, I.D.; Hohl, T.M. Immunity against fungi. JCI Insight 2017, 2, e93156. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Guerra Del Castillo, R.; Sanchez-Gonzalez, M. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Aspergillosis. 2021. Available online: https://www.cdc.gov/fungal/diseases/aspergillosis/index.html (accessed on 10 October 2021).
- Centers for Disease Control and Prevention (CDC). Symptoms of COVID-19. 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 10 October 2021).
- Centers for Disease Control and Prevention (CDC). Candidiasis. 2021. Available online: https://www.cdc.gov/fungal/diseases/candidiasis/index.html (accessed on 10 October 2021).
- Centers for Disease Control and Prevention (CDC). Symptoms of C. neoformans Infection. 2021. Available online: https://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/symptoms.html (accessed on 10 October 2021).
- Centers for Disease Control and Prevention (CDC). Symptoms of Mucormycosis. 2021. Available online: https://www.cdc.gov/fungal/diseases/mucormycosis/symptoms.html (accessed on 10 October 2021).
- Devnath, P.; Dhama, K.; Tareq, A.M.; Emran, T.B. Mucormycosis coinfection in the context of global COVID-19 outbreak: A fatal addition to the pandemic spectrum. Int. J. Surg. 2021, 92, 106031. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and diagnosis of mucormycosis: An update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Dunachie, S.; Chamnan, P. The double burden of diabetes and global infection in low and middle-income countries. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 56–64. [Google Scholar] [CrossRef]
- The World Bank. High Income. 2019. Available online: https://data.worldbank.org/income-level/high-income (accessed on 10 October 2021).
- The World Bank. Middle Income. 2019. Available online: https://data.worldbank.org/income-level/middle-income (accessed on 10 October 2021).
- The World Bank. Low Income. 2019. Available online: https://data.worldbank.org/income-level/low-income (accessed on 10 October 2021).
- World Health Organization (WHO). List of 57 Countries Facing Human Resources for Health Crisis. 2006. Available online: https://www.who.int/workforcealliance/countries/57crisiscountries.pdf?ua=1 (accessed on 10 October 2021).
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. 2021, 15, 102146. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Wilkerson, R.G.; Adler, J.D.; Shah, N.G.; Brown, R. Silent hypoxia: A harbinger of clinical deterioration in patients with COVID-19. Am. J. Emerg. Med. 2020, 38, 2243.e5–2243.e6. [Google Scholar] [CrossRef]
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482316/ (accessed on 3 August 2021).
- Chung, H.; Lee, Y.H. Hypoxia: A double-edged sword during fungal pathogenesis? Front. Microbiol. 2020, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Kanbay, M.; Afsar, R.E. Hypoxia inducible factor-1 protects against COVID-19: A hypothesis. Med. Hypotheses 2020, 143, 109857. [Google Scholar] [CrossRef]
- Grahl, N.; Shepardson, K.M.; Chung, D.; Cramer, R.A. Hypoxia and fungal pathogenesis: To air or not to air? Eukaryot. Cell 2012, 11, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, Z.O.; Chong, E.Y.; Serebrovska, T.V.; Tumanovska, L.V.; Xi, L. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sinica 2020, 41, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, P.D. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J. Biol. Chem. 2020, 295, 18579–18588. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Gazzaz, Z.J. Diabetes and COVID-19. Open Life Sci. 2021, 16, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Al Hayek, A.A.; Robert, A.A.; Matar, A.B.; Algarni, A.; Alkubedan, H.; Alharbi, T.; Al Amro, A.; Alrashidi, S.A.; Al Dawish, M. Risk factors for hospital admission among COVID-19 patients with diabetes. A study from Saudi Arabia. Saudi Med. J. 2020, 41, 1090–1097. [Google Scholar] [CrossRef]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821, Erratum in 2020, 10, 1932296820932678. [Google Scholar] [CrossRef]
- Ilyas, R.; Wallis, R.; Soilleux, E.J.; Townsend, P.; Zehnder, D.; Tan, B.K.; Sim, R.B.; Lehnert, H.; Randeva, H.S.; Mitchell, D.A. High glucose disrupts oligosaccharide recognition function via competitive inhibition: A potential mechanism for immune dysregulation in diabetes mellitus. Immunobiology 2011, 216, 126–131. [Google Scholar] [CrossRef]
- Mazade, M.A.; Edwards, M.S. Impairment of type III group B Streptococcus-stimulated superoxide production and opsonophagocytosis by neutrophils in diabetes. Mol. Genet. Metab. 2001, 73, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Price, C.L.; Hassi, H.O.; English, N.R.; Blakemore, A.I.; Stagg, A.J.; Knight, S.C. Methylglyoxal modulates immune responses: Relevance to diabetes. J. Cell Mol. Med. 2010, 14, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef]
- Hostetter, M.K. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes 1990, 39, 271–275. [Google Scholar] [CrossRef]
- Mattos-Silva, P.; Felix, N.S.; Silva, P.L.; Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.; Cruz, F.F. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir. Physiol. Neurobiol. 2020, 280, 103492. [Google Scholar] [CrossRef]
- Matsuyama, S.; Kawase, M.; Nao, N.; Shirato, K.; Ujike, M.; Kamitani, W.; Shimojima, M.; Fukushi, S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J. Virol. 2020, 95, e01648-20. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.; et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Segrelles-Calvo, G.; de SAraújo, G.R.; Frases, S. Systemic mycoses: A potential alert for complications in COVID-19 patients. Future Microbiol. 2020, 15, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.; Dong, X.; Li, Z.; Xu, Q.; Feng, H.; Cai, J.; Huang, S.; Guo, J.; Zhang, L.; et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J. Clin. Investig. 2020, 130, 6417–6428. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Sosale, A.; Sosale, B.; Kesavadev, J.; Chawla, M.; Reddy, S.; Saboo, B.; Misra, A. Steroid use during COVID-19 infection and hyperglycemia—What a physician should know. Diabetes Metab. Syndr. 2021, 15, 102167. [Google Scholar] [CrossRef]
- Vargas-Vargas, M.; Cortés-Rojo, C. Ferritin levels and COVID-19. Rev. Panam. Salud Pública 2020, 44, e72. [Google Scholar] [CrossRef]
- Son, N.E. Influence of ferritin levels and inflammatory markers on HbA1c in the Type 2 diabetes mellitus patients. Pak. J. Med. Sci. 2019, 35, 1030–1035. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021, 136, 111228. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Limaye, A.P.; Ko, C.W.; Bronner, M.P.; Kowdley, K.V. Association of hepatic iron overload with invasive fungal infection in liver transplant recipients. Liver Transpl. 2006, 12, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Van Asbeck, B.S.; Marx, J.J.; Struyvenberg, A.; Verhoef, J. Functional defects in phagocytic cells from patients with iron overload. J. Infect. 1984, 8, 232–240. [Google Scholar] [CrossRef]
- Moorthy, A.; Gaikwad, R.; Krishna, S.; Hegde, R.; Tripathi, K.K.; Kale, P.G.; Rao, P.S.; Haldipur, D.; Bonanthaya, K. SARS-CoV-2, uncontrolled diabetes and corticosteroids—An unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J. Maxillofac. Oral Surg. 2021, 20, 418–425. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E., Jr.; Spellberg, B.J. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Investig. 2007, 117, 2649–2657. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Edwards, J.E., Jr.; Fu, Y.; Spellberg, B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J. Antimicrob. Chemother. 2006, 58, 1070–1073. [Google Scholar] [CrossRef]
- Boelaert, J.R.; de Locht, M.; Van Cutsem, J.; Kerrels, V.; Cantinieaux, B.; Verdonck, A.; Van Landuyt, H.W.; Schneider, Y.J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 1993, 91, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Nseir, S.; Zerimech, F.; Jaillette, E.; Artru, F.; Balduyck, M. Microaspiration in intubated critically ill patients: Diagnosis and prevention. Infect. Disord. Drug Targets 2011, 11, 413–423. [Google Scholar] [CrossRef]
- Meawed, T.; Ahmed, S.; Mowafy, S.; Samir, G.; Anis, R. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Fekkar, A.; Lampros, A.; Mayaux, J.; Poignon, C.; Demeret, S.; Constantin, J.M.; Marcelin, A.G.; Monsel, A.; Luyt, C.E.; Blaize, M. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021, 203, 307–317. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Netea, M.G. T-cell subsets and antifungal host defenses. Curr. Fungal Infect. Rep. 2010, 4, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Baddley, J.W. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011, 49, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Brüggemann, R.J.M.; Vos, S.; De Hertogh, G.; Wauters, J.; Reijers, M.H.E.; Netea, M.G.; Schouten, J.A.; Verweij, P.E. COVID-19-associated Aspergillus tracheobronchitis: The interplay between viral tropism, host defence, and fungal invasion. Lancet Respir. Med. 2021, 9, 795–802. [Google Scholar] [CrossRef]
- Khorramdelazad, H.; Kazemi, M.H.; Najafi, A.; Keykhaee, M.; Zolfaghari Emameh, R.; Falak, R. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection. Microb. Pathog. 2021, 152, 104554. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: A prospective study. Clin. Infect. Dis. 2020, 1065. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Koehler, P.; Cornely, O.A.; Böttiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef] [PubMed]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 73, e1634–e1644. [Google Scholar] [CrossRef] [PubMed]
- Dellière, S.; Dudoignon, E.; Fodil, S.; Voicu, S.; Collet, M.; Oillic, P.A.; Salmona, M.; Dépret, F.; Ghelfenstein-Ferreira, T.; Plaud, B.; et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: A French multicentric retrospective cohort. Clin. Microbiol. Infect. 2020, 27, 790.e1–790.e5. [Google Scholar] [CrossRef] [PubMed]
- Cenci, E.; Mencacci, A.; Casagrande, A.; Mosci, P.; Bistoni, F.; Romani, L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J. Infect. Dis. 2001, 184, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. Mian Yu Gan Ran Za Zhi 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Gaziano, R.; Pistoia, E.S.; Campione, E.; Fontana, C.; Marino, D.; Favaro, M.; Pica, F.; Di Francesco, P. Immunomodulatory agents as potential therapeutic or preventive strategies for Covid-19. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4174–4184. [Google Scholar] [CrossRef]
- Rolling, T.; Hohl, T.M.; Zhai, B. Minority report: The intestinal mycobiota in systemic infections. Curr. Opin. Microbiol. 2020, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-associated candidiasis (CAC): An underestimated complication in the absence of immunological predispositions. J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, A.S.; Durmaz, Ş.Ö. Fungal infections in COVID-19 intensive care patients. Pol. J. Microbiol. 2021, 70, 395–400. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020; WHO/2019-nCoV/clinical/2020.5; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 17 October 2021).
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020, 19, 102564. [Google Scholar] [CrossRef]
- Van Enckevort, F.H.J.; Netea, M.G.; Hermus, A.R.M.M.; Sweep, C.G.J.; Meis, J.F.G.M.; Van der Meer, J.W.M.; Kullberg, B.J. Increased susceptibility to systemic candidiasis in interleukin-6 deficient mice. Med Mycol. 1999, 37, 419–426. [Google Scholar] [CrossRef]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The landscape of candidemia during the COVID-19 pandemic. Clin. Infect. Dis. 2021, ciab562. [Google Scholar] [CrossRef]
- Ghannoum, M.; Roilides, E.; Katragkou, A.; Petraitis, V.; Walsh, T.J. The role of echinocandins in Candida biofilm–related vascular catheter infections: In vitro and in vivo model systems. Clin. Infect. Dis. 2015, 61, S618–S621. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Timsit, J.F.; Tafflet, M.; de Lassence, A.; Darmon, M.; Zahar, J.R.; Adrie, C.; Garrouste-Orgeas, M.; Cohen, Y.; Mourvillier, B.; et al. Outcomerea Study Group. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 2006, 129, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, A.; Jaiswal, A.; Verma, P.K.; Singhal, R. A prospective study of fungal colonization and invasive fungal disease in long-term mechanically ventilated patients in a respiratory intensive care unit. Indian J. Crit. Care Med. Peer Rev. Off. Publ. Indian Soc. Crit. Care Med. 2018, 22, 597–601. [Google Scholar] [CrossRef]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis. Clin. N. Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Jean, S.S.; Fang, C.T.; Shau, W.Y.; Chen, Y.C.; Chang, S.C.; Hsueh, P.R.; Hung, C.C.; Luh, K.T. Cryptococcaemia: Clinical features and prognostic factors. QJM Mon. J. Assoc. Physicians 2002, 95, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, V.C.; Perosa, A.H.; de Souza Luna, L.K.; Conte, D.D.; Nascimento, O.A.; Ota-Arakaki, J.; Bellei, N. Detected SARS-CoV-2 in ascitic fluid followed by cryptococcemia: A case report. SN Compr. Clin. Med. 2020, 2414–2418. [Google Scholar] [CrossRef]
- Khatib, M.Y.; Ahmed, A.A.; Shaat, S.B.; Mohamed, A.S.; Nashwan, A.J. Cryptococcemia in a patient with COVID-19: A case report. Clin. Case Rep. 2020, 9, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Shattock, R.J.; Harrison, T.S. (2006). Role of capsule and interleukin-6 in long-term immune control of Cryptococcus neoformans infection by specifically activated human peripheral blood mononuclear cells. Infect. Immun. 2006, 74, 5302–5310. [Google Scholar] [CrossRef]
- Ghanem, H.; Sivasubramanian, G. Cryptococcus neoformans meningoencephalitis in an immunocompetent patient after COVID-19 infection. Case Rep. Infect. Dis. 2021, 2021, 5597473. [Google Scholar] [CrossRef]
- Jeong, W.; Keighley, C.; Wolfe, R.; Lee, W.L.; Slavin, M.A.; Kong, D.C.; Chen, S.C.-A. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin. Microbiol. Infect. 2019, 25, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium; Mucormycosis ECMM MSG Global Guideline Writing Group. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Fenves, A.Z.; Coburn, J.W. Deferoxamine therapy and mucormycosis in dialysis patients: Report of an international registry. Am. J. Kidney Dis. 1991, 18, 660–667. [Google Scholar] [CrossRef]
- Chander, J.; Singla, N.; Kaur, M.; Punia, R.S.; Attri, A.; Alastruey-Izquierdo, A.; Stchigel, A.M.; Cano-Lira, J.F.; Guarro, J. Saksenaea erythrospora, an emerging mucoralean fungus causing severe necrotizing skin and soft tissue infections—A study from a tertiary care hospital in north India. Infect. Dis. 2017, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021, 64, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: Transferrin and iron availability. Diabetes 1982, 31, 1109–1114. [Google Scholar] [CrossRef]
- Gleissner, B.; Schilling, A.; Anagnostopolous, I.; Siehl, I.; Thiel, E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma 2004, 45, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
| Fungus | Infection | CDC-Main Fungal Symptoms Overlapping with COVID-19 | CDC-Main Fungal Symptoms Differing from COVID-19 |
|---|---|---|---|
| Aspergillus genera | Aspergillosis | Shortness of breath (SOB), cough, fever, fatigue, runny nose, headache (HA), chest pain, congestion, loss of smell | Wheezing, hemoptysis |
| Candida auris | Candidiasis | Fever, chills, loss of taste, sore throat | Odynophagia, oral thrush, vaginal candidiasis |
| Cryptococcus neoformans | Cryptococcosis | Cough, SOB, fever, HA, nausea, vomiting, confusion, chest pain | Light sensitivity |
| Mucorales order | Mucormycosis | HA, nasal congestion, fever, cough, chest pain, SOB, nausea, vomiting | Unilateral facial swelling, black lesions on nasal bridge or inside the mouth, gastrointestinal (GI) bleeding |
| Author | Country | Type of Fungal Infection | Severity (ICU, Floor, or Mixed) | Study Type | Total Patients (n) | Fungal Co-Infection (%) | Death (%) |
|---|---|---|---|---|---|---|---|
| Bartoletti et al. | Italy | Aspergillosis | ICU | Prospective | 108 | 27.7 | 44 |
| Koehler et al. | Germany | Aspergillosis | ICU | Retrospective | 19 | 26.3 | 60 |
| White et al. | United Kingdom | Aspergillosis | ICU | Prospective | 135 | 14.1 | 57.9 |
| Dellière et al. | France | Aspergillosis | ICU | Retrospective | 366 | 5.7 | 71.4 |
| Lai & Yu | Multiple
| Aspergillosis | Mixed | Review | Total: 34
| 100 | 64.7 |
| Musuuza et al. | Multiple | Candidiasis | Mixed | Systematic Review and Meta-analysis | N/A | 18.8 | N/A |
| Arastehfar et al. | Multiple
| Candidiasis | Mixed | Review |
|
|
|
| Villanueva-Loza no et al. | Mexico | Candidiasis | ICU | Retrospective | 12 | 50 | 83.3 |
| Coşkun et al. | Turkey | Candidiasis | ICU | Retrospective | 627 | 2.6 | 80 |
| Antinori et al. | Italy | Candidiasis | Mixed | Prospective | 43 | 6.9 | N/A |
| Seagle et al. | United States | Candidiasis | Mixed | Case-level analysis | 64 | 100 | 60 |
| Passarelli et al. | United States | Cryptococcosis | ICU | Case report | 1 | 100 | 100 |
| Khatib et al. | Qatar | Cryptococcosis | ICU | Case report | 1 | 100 | 100 |
| Ghanem & Sivasubramanian | United States | Cryptococcosis | Mixed | Case Report | 1 | 100 | 0 |
| Pal et al. | Multiple
| Mucormycosis | Mixed | Systematic review and meta-analysis | Total: 99
| 100 | 34 |
| Jeong et al. | Multiple
| Mucormycosis | Mixed | Systematic review and Meta-analysis |
| 14 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, A.; Vartanian, A.; Poladian, N.; Voloshko, A.; Yegiazaryan, A.; Al-Kassir, A.L.; Venketaraman, V. Root Causes of Fungal Coinfections in COVID-19 Infected Patients. Infect. Dis. Rep. 2021, 13, 1018-1035. https://doi.org/10.3390/idr13040093
Amin A, Vartanian A, Poladian N, Voloshko A, Yegiazaryan A, Al-Kassir AL, Venketaraman V. Root Causes of Fungal Coinfections in COVID-19 Infected Patients. Infectious Disease Reports. 2021; 13(4):1018-1035. https://doi.org/10.3390/idr13040093
Chicago/Turabian StyleAmin, Arman, Artin Vartanian, Nicole Poladian, Alexander Voloshko, Aram Yegiazaryan, Abdul Latif Al-Kassir, and Vishwanath Venketaraman. 2021. "Root Causes of Fungal Coinfections in COVID-19 Infected Patients" Infectious Disease Reports 13, no. 4: 1018-1035. https://doi.org/10.3390/idr13040093
APA StyleAmin, A., Vartanian, A., Poladian, N., Voloshko, A., Yegiazaryan, A., Al-Kassir, A. L., & Venketaraman, V. (2021). Root Causes of Fungal Coinfections in COVID-19 Infected Patients. Infectious Disease Reports, 13(4), 1018-1035. https://doi.org/10.3390/idr13040093







