Implications of the COVID-19 Lockdown on Dengue Transmission in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Temporal Analysis of Malaysia Dengue Incidences during Lockdown
2.3. Mosquito Occurrences
3. Results
3.1. Temporal Analysis of Dengue Incidences during Lockdown
3.2. Mosquito Occurrences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 24 June 2020).
- Hii, Y.L.; Zaki, R.A.; Aghamohammadi, N.; Rocklöv, J. Research on Climate and Dengue in Malaysia: A Systematic Review. Curr. Environ. Health Rep. 2016, 3, 81–90. [Google Scholar] [CrossRef]
- Sulaiman, S.; Pawanchee, Z.A.; Jeffery, J.; Ghauth, I.; Busparani, V. Studies on the distribution and abundance of Aedes aegypti (L.) and Aedes albopictus (Skuse) (Diptera: Culicidae)in an endemic area of dengue/dengue hemorrhagic fever in Kuala Lumpur. Mosq. Borne Dis. Bull. 1991, 8, 35–39. [Google Scholar]
- Sault, A. Why Lockdowns Can Halt the Spread of COVID-19. World Economic Forum 2020 March 21. Available online: https://www.weforum.org/agenda/2020/03/why-lockdowns-work-epidemics-coronavirus-covid19/ (accessed on 10 May 2020).
- World Health Organization (WHO). Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. Available online: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 22 April 2020).
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008, 5. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Lezama, J.A.; Martínez-Vega, R.A.; Kuri-Morales, P.A.; Ramos-Castañeda, J.; Adams, B. Day-to-Day Population Movement and the Management of Dengue Epidemics. Bull. Math. Biol. 2016, 78, 2011–2033. [Google Scholar] [CrossRef]
- Stoddard, S.T.; Forshey, B.M.; Morrison, A.C.; Paz-Soldan, V.A.; Vazquez-Prokopec, G.M.; Astete, H.; Reiner, R.C.; Vilcarromero, S.; Elder, J.P.; Halsey, E.S.; et al. House-to-house human movement drives dengue virus transmission. Proc. Natl. Acad. Sci. USA 2013, 110, 994–999. [Google Scholar] [CrossRef]
- Hector, C. Operating Businesses during MCO Must Be Classified as Offence. Available online: https://www.malaysiakini.com/letters/519902 (accessed on 3 May 2020).
- Zhang, X.; Liu, Y.; Yang, M.; Zhang, T.; Young, A.A.; Li, X. Comparative study of four-time series methods in forecasting typhoid fever incidence in China. PLoS ONE 2013, 8, e63116. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Ren, M.; Xie, S.; Wang, P. Predicting Seasonal Influenza Based on SARIMA Model, in Mainland China from 2005 to 2018. Int. J. Environ. Res. Public Health 2019, 16, 4760. [Google Scholar] [CrossRef]
- Reiner, R.C., Jr.; Stoddard, S.T.; Scott, T.W. Socially structured human movement shapes dengue transmission despite the diffusive effect of mosquito dispersal. Epidemics 2014, 6, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.G. Urbanization Increases Aedes albopictus Larval Habitats and Accelerates Mosquito Development and Survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef] [PubMed]
- Rozilawati, H.; Zairi, J.; Adanan, C.R. Seasonal abundance of Aedes albopictus in selected urban and suburban areas in Penang, Malaysia. Trop. Biomed. 2007, 24, 83–94. [Google Scholar]
- De Moura Rodrigues, M.; Marques, G.R.A.M.; Serpa, L.L.N.; de Brito Arduino, M.; Voltolini, J.C.; Barbosa, G.L.; Andrade, V.R.; de Lima, V.L.C. Density of Aedes aegypti and Aedes albopictus and its association with number of residents and meteorological variables in the home environment of dengue endemic area, São Paulo, Brazil. Parasit. Vectors 2015, 8, 115. [Google Scholar] [CrossRef]
- Martin, E.; Medeiros, M.C.; Carbajal, E.; Valdez, E.; Juarez, J.G.; Garcia-Luna, S.; Salazar, A.; Qualls, W.A.; Hinojosa, S.; Borucki, M.K.; et al. Surveillance of Aedes aegypti indoors and outdoors using Autocidal Gravid Ovitraps in South Texas during local transmission of Zika virus, 2016 to 2018. Acta Trop. 2019, 192, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Dieng, H.; Saifur, R.G.; Hassan, A.A.; Salmah, M.C.; Boots, M.; Satho, T.; Jaal, Z.; AbuBakar, S. Indoor-Breeding of Aedes albopictus in Northern Peninsular Malaysia and Its Potential Epidemiological Implications. PLoS ONE 2010, 5, e11790. [Google Scholar] [CrossRef] [PubMed]
- Malaysia Reports 130,000 Dengue Cases in 2019, Highest Since 2015. Available online: https://codeblue.galencentre.org/2020/01/03/malaysia-reports-130000-dengue-cases-in-2019-highest-since-2015/ (accessed on 3 May 2020).
- iDengue. Available online: http://idengue.arsm.gov.my/ (accessed on 22 June 2020).
- Martinez, E.Z.; Silva, E.A.S.D.; Fabbro, A.L.D. A SARIMA forecasting model to predict the number of cases of dengue in Campinas, State of São Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Prime Minister’s Office of Malaysia. Coronavirus Disease 2019 (Covid-19). Available online: https://www.pmo.gov.my/special-contents/2019-novel-coronavirus-2019-ncov/ (accessed on 22 April 2020).
- Only Eight Dengue Hotspots in Penang. Available online: https://www.thestar.com.my/news/community/2005/10/27/only-eight-dengue-hotspots-in-penang (accessed on 2 April 2020).
- MCO: Travel Limited to 10 km from Home. Available online: https://www.malaysiakini.com/news/518198 (accessed on 30 April 2020).
- Service, M.W. A critical review of procedures for sampling populations of adult mosquitos. Bull. Entomol. Res. 1977, 67, 343–382. [Google Scholar] [CrossRef]
- Scott, T.W.; Morrison, A.C. Vector Dynamics and Transmission of Dengue Virus: Implications for Dengue Surveillance and Prevention Strategies Vector Dynamics and Dengue Prevention. Curr. Top. Micrbiol. Immunol. 2010, 338, 115–128. [Google Scholar]
- Myer, M.H.; Fizer, C.M.; Mcpherson, K.R.; Neale, A.C.; Pilant, A.N.; Rodriguez, A.; Whung, P.Y.; Johnston, J.M. Mapping Aedes aegypti (Diptera: Culicidae) and Aedes albopictus Vector Mosquito Distribution in Brownsville, TX. J. Med. Entomol. 2020, 57, 231–240. [Google Scholar] [CrossRef]
- Trash in Penang Drops by Almost 20%. Available online: https://www.thestar.com.my/news/nation/2020/03/26/trash-in-penang-drops-by-almost-20 (accessed on 30 April 2020).
- Sim, B.L.H.; Chidambaram, S.K.; Wong, X.C.; Pathmanathan, M.D.; Peariasamy, K.M.; Hor, C.P.; Chua, H.J.; Goh, P.P. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: A nationwide observational study. Lancet Ref. Health West Pacif. 2020, 4, 100055. [Google Scholar] [CrossRef]
- Olive, M.M.; Baldet, T.; Devillers, J.; Fite, J.; Paty, M.C.; Paupy, C.; Quénel, P.; Quillery, E.; Raude, J.; Stahl, J.P.; et al. The COVID-19 pandemic should not jeopardize dengue control. PLoS Negl. Trop. Dis. 2020, 14, e0008716. [Google Scholar] [CrossRef]
- Smith, N. Worst Dengue Outbreak for Seven Years in Singapore Linked to Coronavirus Lockdown. Available online: https://www.telegraph.co.uk/global-health/science-and-disease/worst-dengue-outbreak-seven-years-singapore-linked-coronavirus/ (accessed on 30 April 2020).
- Jindal, A.; Rao, S. Lockdowns to Contain COVID-19 Increase Risk and Severity of Mosquito-Borne Disease Outbreaks. medRxiv 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.04.11.20061143v1.full.pdf+html (accessed on 2 June 2020). [CrossRef]
- Harrington, L.C.; Scott, T.W.; Lerdthusnee, K.; Coleman, R.C.; Costero, A.; Clark, G.G.; Jones, J.J.; Kitthawee, S.; Kittayapong, P.; Sithiprasasna, R.; et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 2005, 72, 209–220. [Google Scholar] [CrossRef]
- Nacher, M.; Douine, M.; Gaillet, M.; Flamand, C.; Rousset, D.; Rousseau, C.; Mahdaoui, C.; Carroll, S.; Valdes, A.; Passard, N.; et al. Simultaneous dengue and COVID-19 epidemics: Difficult days ahead? PLoS Negl. Trop. Dis. 2020, 14, e0008426. [Google Scholar] [CrossRef]
- Rahim, J.; Ahmad, A.H.; Maimusa, A.H.; Irfan, S. Updated abundance and distribution of Aedes albopictus (Skuse) (Diptera: Culicidae) in Penang Island, Malaysia. Trop. Biomed. 2018, 35, 308–320. [Google Scholar]
- Reegan, A.D.; Gandhi, M.R.; Asharaja, A.C.; Devi, C.; Shanthakumar, S.P. COVID-19 lockdown: Impact assessment on Aedes larval indices, breeding habitats, effects on vector control programme and prevention of dengue outbreaks. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Dengue Increase Likely during Rainy Season: WHO Warns. Available online: https://www.who.int/westernpacific/news/detail/11-06-2019-dengue-increase-likely-during-rainy-season-who-warns (accessed on 30 April 2020).
- Scott, T.W.; Morrison, A.C. Aedes aegypti density and the risk of dengue-virus transmission. In Ecological Aspects for Application of Genetically Modified Mosquitoes; Wageningen UR Frontis Series; Springer: Amsterdam, The Netherlands; Volume 2, ISBN 978-1-4020-1585-4.
- Nur Aida, H.; Abu Hassan, A.; Nurita, A.T.; Che Salmah, M.R.; Norasmah, B. Population analysis of Aedes albopictus (Skuse) (Diptera:Culicidae) under uncontrolled laboratory conditions. Trop. Biomed. 2008, 25, 117–125. [Google Scholar] [PubMed]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Dengue Control—The Mosquito. Available online: https://www.who.int/denguecontrol/mosquito/en/ (accessed on 3 June 2020).
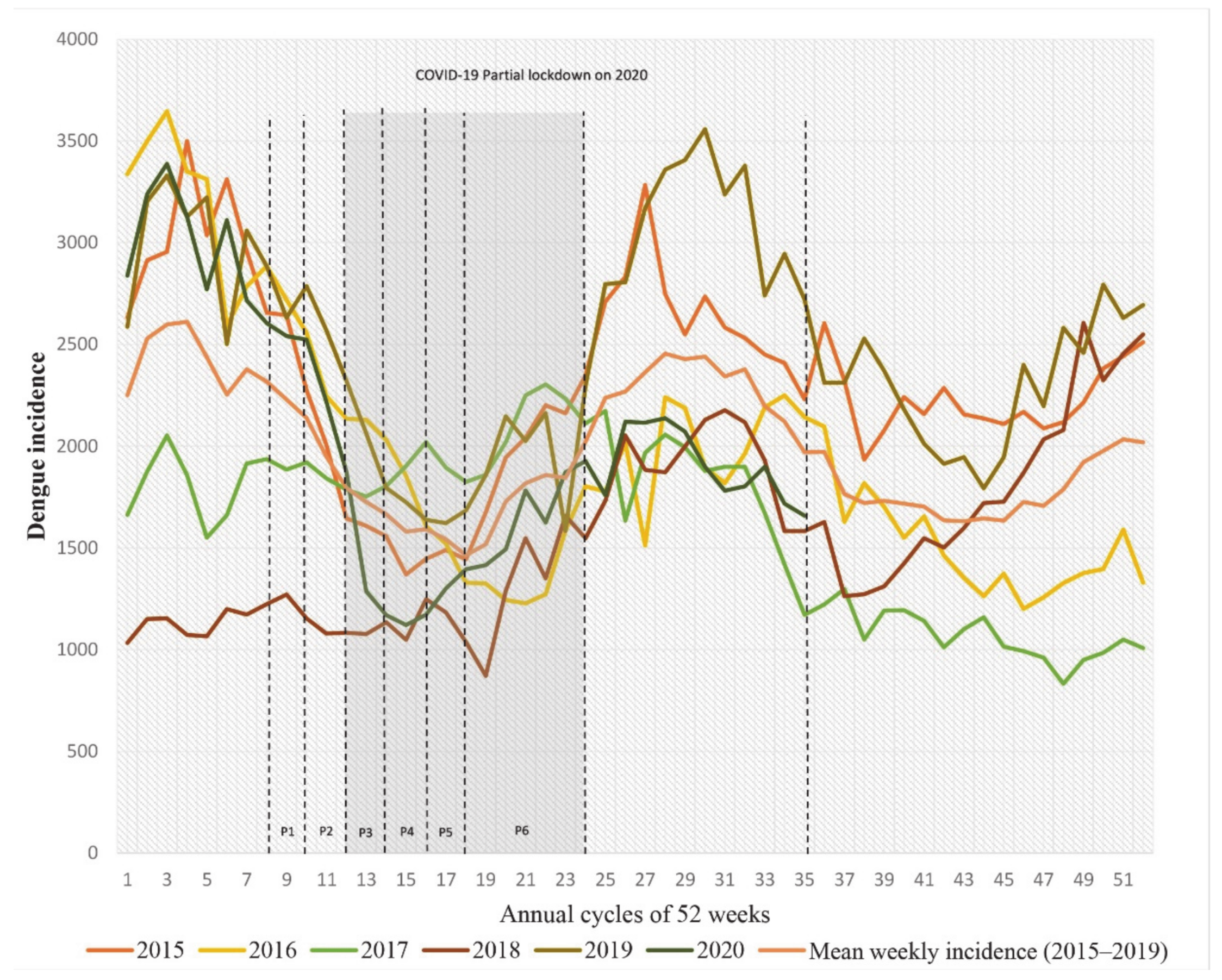
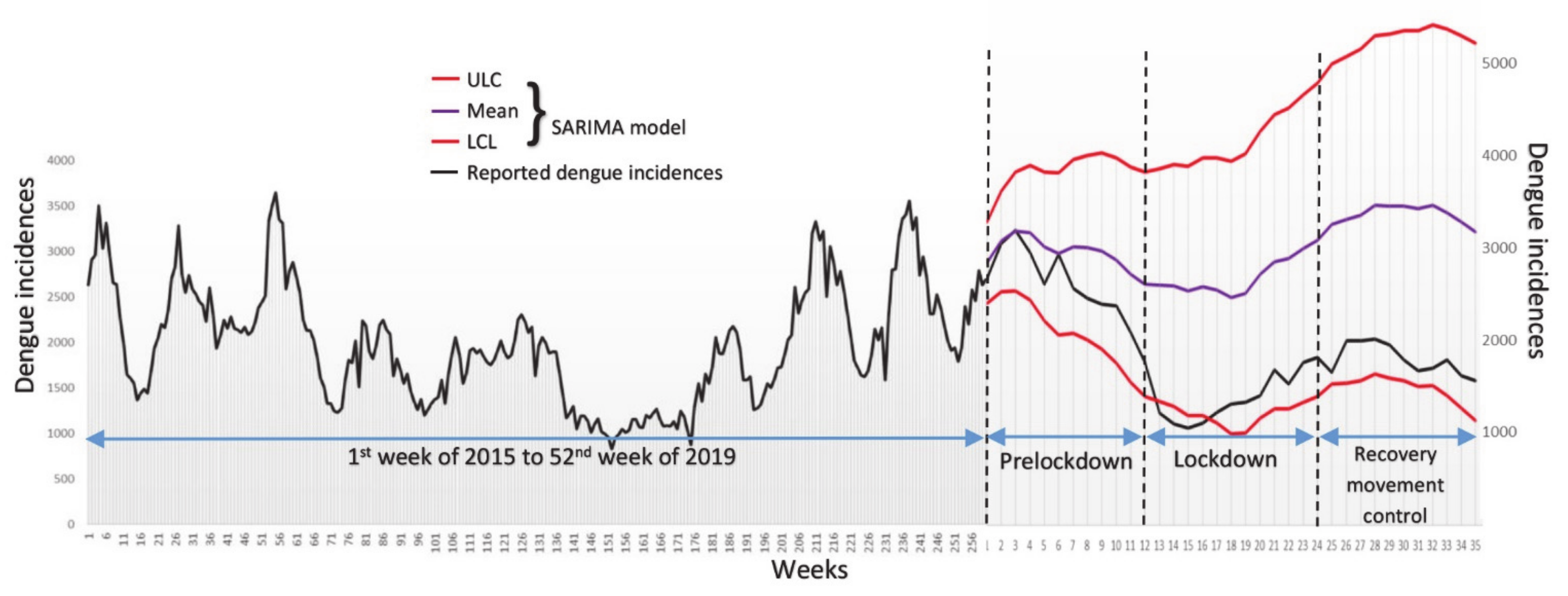
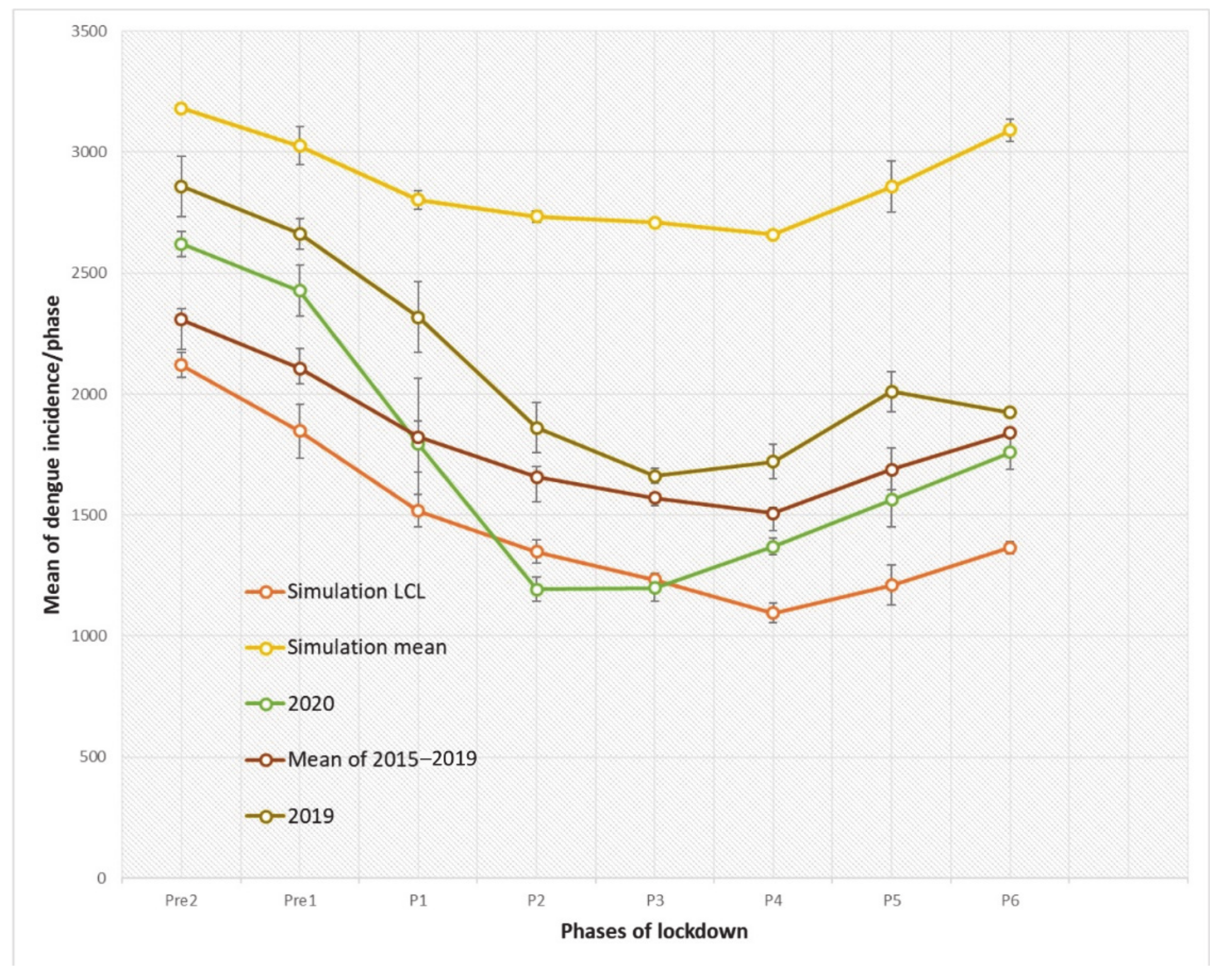
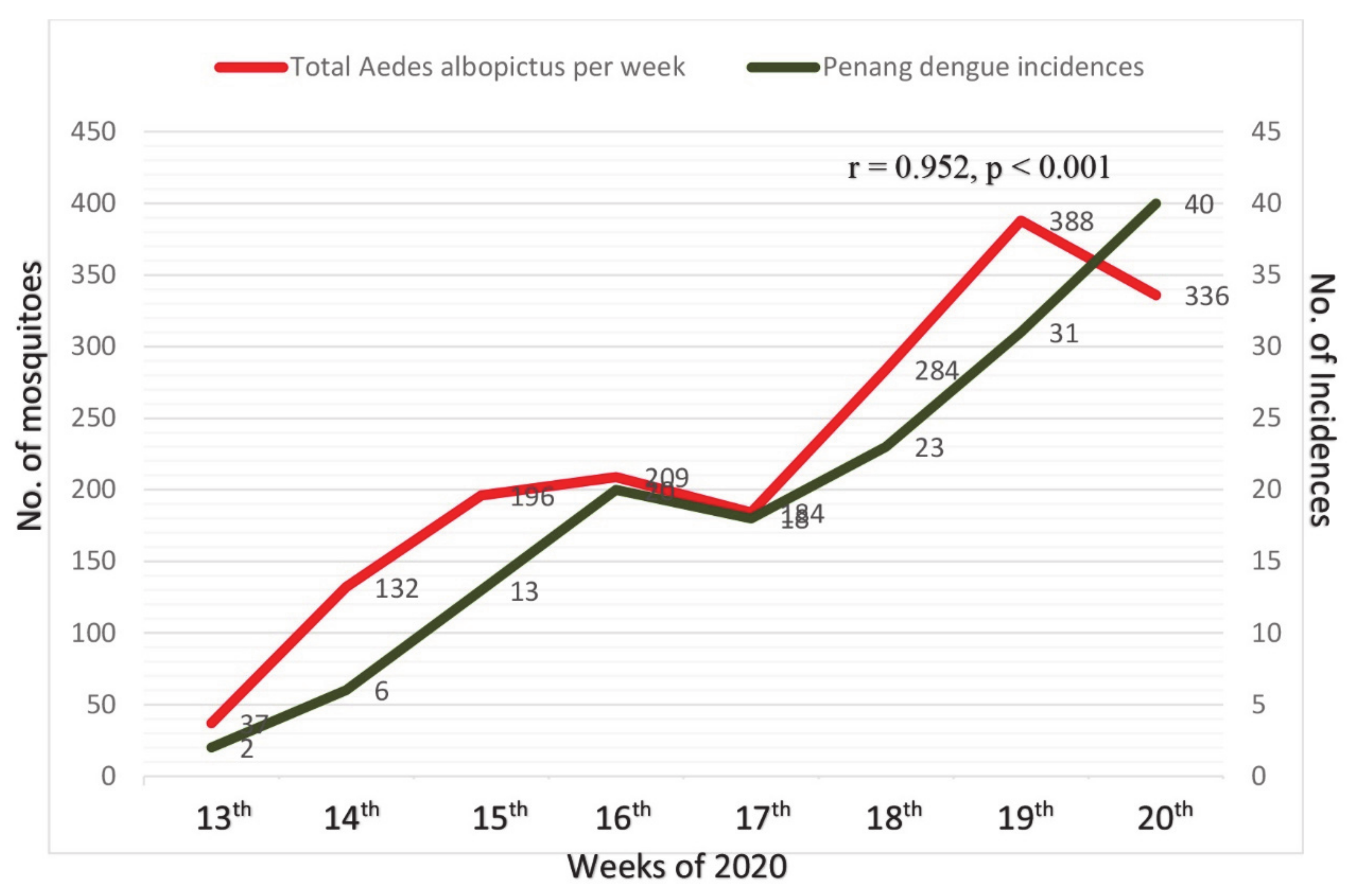
| Stage | Terms | Start and End Date (Year 2020) | Corresponding Weeks |
|---|---|---|---|
| Pre-2 | - | 19 February to 3 March | 8th to 9th |
| Pre-1 | - | 4 March to 17 March | 10th to 11th |
| P1 * | MCO | 18 March to 31 March | 12th to 13th |
| P2 | MCO | 1 April to 14 April | 14th to 15th |
| P3 | MCO | 15 April to 28 April | 16th to 17th |
| P4 | MCO | 29 April to 12 May | 18th to 19th |
| P5 | CMCO | 13 May to 9 June | 20th to 23rd |
| P6 | RMCO1 | 10 June to 31 August | 24th to 35th |
| P7 | RMCO2 | 1 September to 31 December | 36th to 52nd |
| SARIMA Model | Ljung-Box Q Test | ||||||
|---|---|---|---|---|---|---|---|
| Statistics (z) | DF | p-Value | NBIC | RMSE | MAPE | R2 | |
| (0,1,0) (0,1,0) | 47.293 | 18 | 1.92 × 10−4 | 11.615 | 324.382 | 13.107 | 0.743 |
| (0,1,0) (1,1,0) | 31.481 | 17 | 0.017 | 11.432 | 292.239 | 11.734 | 0.792 |
| (0,1,0) (1,1,1) | 32.411 | 16 | 0.009 | 11.387 | 282.053 | 11.311 | 0.808 |
| (1,1,0) (0,1,0) | 20.851 | 17 | 0.223 | 11.526 | 306.225 | 12.865 | 0.772 |
| (1,1,0) (1,1,0) | 21.299 | 16 | 0.167 | 11.396 | 283.304 | 11.771 | 0.806 |
| (1,1,0) (1,1,1) | 19.782 | 15 | 0.180 | 11.343 | 272.410 | 11.344 | 0.821 |
| (1,1,1) (0,1,0) | 20.640 | 16 | 0.193 | 11.554 | 306.533 | 12.844 | 0.773 |
| (1,1,1) (1,1,0) | 21.149 | 15 | 0.132 | 11.427 | 284.056 | 11.766 | 0.806 |
| (1,1,1) (1,1,1) | 19.746 | 14 | 0.138 | 11.374 | 273.117 | 11.333 | 0.821 |
| Slope * | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pre2 | Pre1 | P1 | P2 | P3 | P4 | P5 | P6 | |
| SARIMA model | −25.60 | −134.62 | −62.11 | −33.75 | 4.96 | −18.36 | 182.13 | 74.75 |
| Year 2020 | −88 | −161 | −466 | −83 | 90 | 57 | 183.5 | 44.5 |
| Mean of 2015–2019 | −74.1 | −140.6 | −111.2 | −73.2 | −19 | −11.9 | 149.5 | 13.6 |
| Year 2019 | −214 | −31 | −253 | −169 | −51.5 | 118 | 83.5 | −221 |
| Phases * | Aedes aegypti | Aedes albopictus |
|---|---|---|
| P3 | 4 | 6 |
| P4 | 3 | 5 |
| P5 | 7 | 11 |
| P6 | 5 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.-Q.; Ahmad, H.; Mohd Ngesom, A.M. Implications of the COVID-19 Lockdown on Dengue Transmission in Malaysia. Infect. Dis. Rep. 2021, 13, 148-160. https://doi.org/10.3390/idr13010016
Ong S-Q, Ahmad H, Mohd Ngesom AM. Implications of the COVID-19 Lockdown on Dengue Transmission in Malaysia. Infectious Disease Reports. 2021; 13(1):148-160. https://doi.org/10.3390/idr13010016
Chicago/Turabian StyleOng, Song-Quan, Hamdan Ahmad, and Ahmad Mohiddin Mohd Ngesom. 2021. "Implications of the COVID-19 Lockdown on Dengue Transmission in Malaysia" Infectious Disease Reports 13, no. 1: 148-160. https://doi.org/10.3390/idr13010016
APA StyleOng, S.-Q., Ahmad, H., & Mohd Ngesom, A. M. (2021). Implications of the COVID-19 Lockdown on Dengue Transmission in Malaysia. Infectious Disease Reports, 13(1), 148-160. https://doi.org/10.3390/idr13010016






