Clinical and Endoscopic Features of Upper Gastrointestinal Bleeding Associated with Helicobacter pylori Infection: A Retrospective Cohort Study in the Colombian Caribbean (2021–2023)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Study Outcomes
2.3. Definitions of Variables
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. General Characteristics
3.2. UGIB and H. pylori Infection
3.3. Comorbidities, Pathological Findings, and Complications
3.4. Characteristics According to Admission Diagnosis
3.5. Forrest Classification
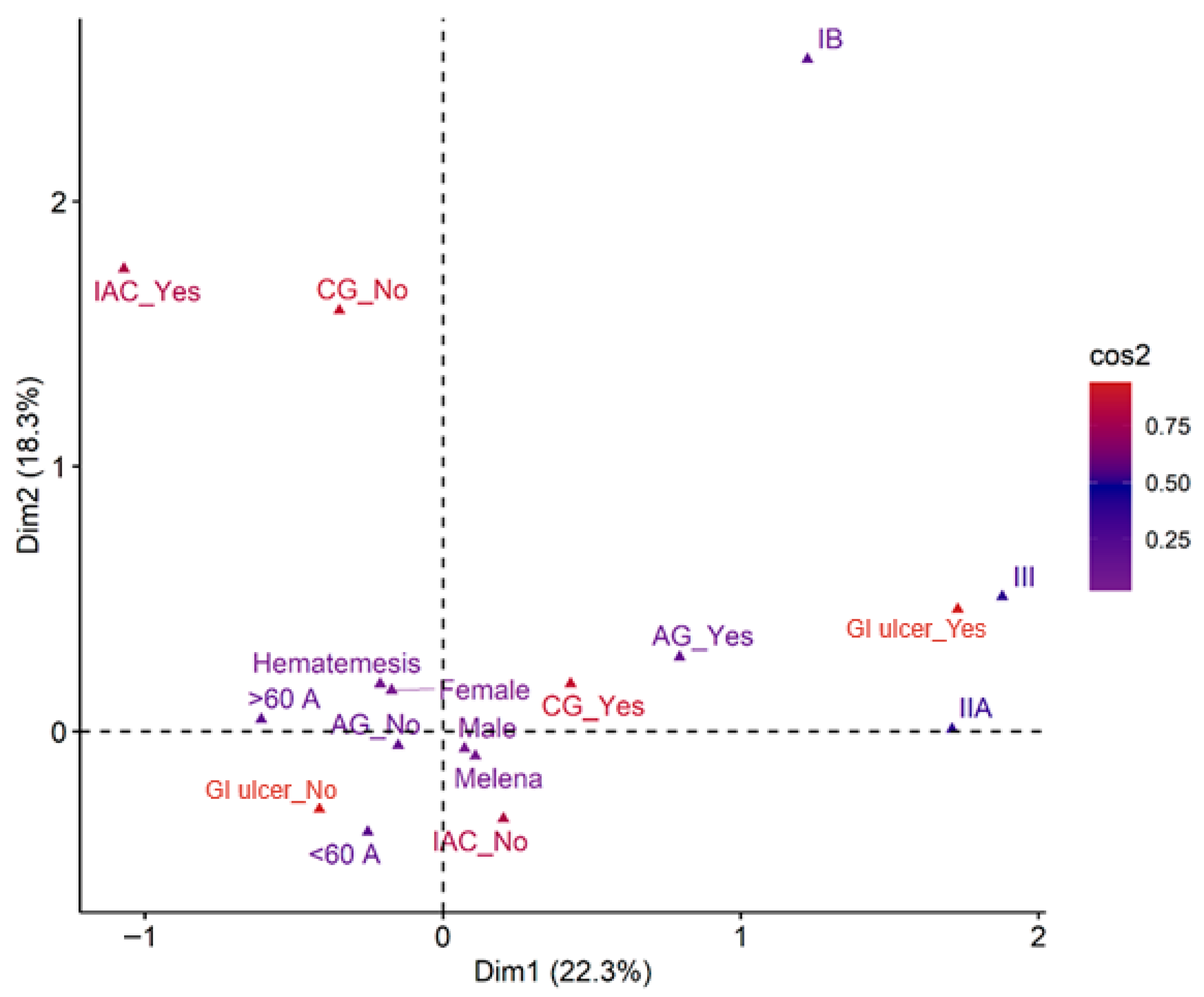
3.6. Relationship Between Clinical and Pathological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saydam, Ş.S.; Molnar, M.; Vora, P. The Global Epidemiology of Upper and Lower Gastrointestinal Bleeding in General Population: A Systematic Review. World J. Gastrointest. Surg. 2023, 15, 723–739. [Google Scholar] [CrossRef]
- Laine, L.; Barkun, A.N.; Saltzman, J.R.; Martel, M.; Leontiadis, G.I. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am. J. Gastroenterol. 2021, 116, 899–917. [Google Scholar] [CrossRef]
- Oakland, K. Changing Epidemiology and Etiology of Upper and Lower Gastrointestinal Bleeding. Best Pract. Res. Clin. Gastroenterol. 2019, 42–43, 101610. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-Y.; Xu, J.-L.; Sun, S.-P.; Wang, K.-J.; Lv, B. Helicobacter Pylori Eradication: Exploring Its Impacts on the Gastric Mucosa. World J. Gastroenterol. 2021, 27, 5152–5170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Guo, C.-G.; Cheung, K.S.; Leung, W.K. Long-Term Risk of Upper Gastrointestinal Bleeding after Helicobacter Pylori Eradication: A Population-Based Cohort Study. Aliment. Pharmacol. Ther. 2021, 54, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Curado, M.P.; de Oliveira, M.M.; de Araújo Fagundes, M. Prevalence of Helicobacter Pylori Infection in Latin America and the Caribbean Populations: A Systematic Review and Meta-Analysis. Cancer Epidemiol. 2019, 60, 141–148. [Google Scholar] [CrossRef]
- Yen, H.-H.; Wu, P.-Y.; Wu, T.-L.; Huang, S.-P.; Chen, Y.-Y.; Chen, M.-F.; Lin, W.-C.; Tsai, C.-L.; Lin, K.-P. Forrest Classification for Bleeding Peptic Ulcer: A New Look at the Old Endoscopic Classification. Diagnostics 2022, 12, 1066. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Stanley, A.J.; Morris, A.J.; Camus, M.; Lau, J.; Lanas, A.; Laursen, S.B.; Radaelli, F.; Papanikolaou, I.S.; Cúrdia Gonçalves, T.; et al. Endoscopic Diagnosis and Management of Nonvariceal Upper Gastrointestinal Hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 300–332. [Google Scholar] [CrossRef]
- Salazar Giraldo, B.E.; Gómez Villegas, S.I.; Vélez Gómez, D.E.; Ramírez Lopera, V.; Pérez Cala, T.L.; Martínez, A. Frequency of Helicobacter Pylori Infection in Patients Requiring GI Endoscopy in Seven Units in Three Antioquia Subregions. Rev. Colomb. Gastroenterol. 2023, 38, 290–303. [Google Scholar] [CrossRef]
- Porras, C.; Nodora, J.; Sexton, R.; Ferreccio, C.; Jimenez, S.; Dominguez, R.L.; Cook, P.; Anderson, G.; Morgan, D.R.; Baker, L.H.; et al. Epidemiology of Helicobacter Pylori Infection in Six Latin American Countries (SWOG Trial S0701). Cancer Causes Control 2013, 24, 209–215. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Gastric Cancer. Prevention in Latin America and the Caribbean. In Population-Based Helicobacter pylori Screen-and-Treat Strategies for Gastric Cancer Prevention: Guidance on Implementation; Park, J.Y., Ed.; International Agency for Research on Cancer: Lyon, France, 2025. [Google Scholar]
- Popa, D.G.; Obleagă, C.V.; Socea, B.; Serban, D.; Ciurea, M.E.; Diaconescu, M.; Vîlcea, I.D.; Meșină, C.; Mirea, C.; Florescu, D.N.; et al. Role of Helicobacter Pylori in the Triggering and Evolution of Hemorrhagic Gastro-Duodenal Lesions. Exp. Ther. Med. 2021, 22, 1147. [Google Scholar] [CrossRef]
- Torres Jiménez, F.; Torres Bayona, C. Molecular Pathophysiology in Infection by Helicobacter Pylori. Salud Uninorte 2021, 32, 500–512. [Google Scholar] [CrossRef]
- Sepulveda Copete, M.; Maldonado Gutiérrez, C.; Bravo Ocaña, J.C.; Satizabal, N.; Gempeler Rojas, A.; Castro Llanos, A.M.; Escobar Stein, J.; Herrera Mayor, J.; Rosso Suárez, F.; Rojas Rojas, N.E.; et al. Prevalencia de Helicobacter Pylori En Pacientes Llevados a Endoscopia de Vías Digestivas Altas En Un Hospital de Referencia En Cali, Colombia, En 2020. Rev. Colomb. Gastroenterol. 2022, 37, 355–361. [Google Scholar] [CrossRef]
- Tsang, S.H.; Avilés-Santa, M.L.; Abnet, C.C.; Brito, M.O.; Daviglus, M.L.; Wassertheil-Smoller, S.; Castañeda, S.F.; Minnerath, S.; Talavera, G.A.; Graubard, B.I.; et al. Seroprevalence and Determinants of Helicobacter Pylori Infection in the Hispanic Community Health Study/Study of Latinos. Clin. Gastroenterol. Hepatol. 2022, 20, e438–e451. [Google Scholar] [CrossRef] [PubMed]
- Strate, L.L.; Singh, P.; Boylan, M.R.; Piawah, S.; Cao, Y.; Chan, A.T. A Prospective Study of Alcohol Consumption and Smoking and the Risk of Major Gastrointestinal Bleeding in Men. PLoS ONE 2016, 11, e0165278. [Google Scholar] [CrossRef]
- Siebenhüner, K.; Blaser, J.; Nowak, A.; Cheetham, M.; Mueller, B.U.; Battegay, E.; Beeler, P.E. Comorbidities Associated with Worse Outcomes Among Inpatients Admitted for Acute Gastrointestinal Bleeding. Dig. Dis. Sci. 2022, 67, 3938–3947. [Google Scholar] [CrossRef]
- Zuluaga Arbelaez, N.; Sierra-Vargas, E.C.; Guevara-Casallas, L.G.; Pérez-Viana, S. Estrategias Terapéuticas Para Helicobacter Pylori En Colombia. CES Med. 2021, 35, 244–256. [Google Scholar] [CrossRef]
- Virdis, A.; Dell’Agnello, U.; Taddei, S. Impact of Inflammation on Vascular Disease in Hypertension. Maturitas 2014, 78, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M.; Vanhoutte, P.M. Endothelial Dysfunction: A Multifaceted Disorder (The Wiggers Award Lecture). Am. J. Physiol. Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef] [PubMed]
- Lanas, Á.; Carrera-Lasfuentes, P.; Arguedas, Y.; García, S.; Bujanda, L.; Calvet, X.; Ponce, J.; Perez-Aísa, Á.; Castro, M.; Muñoz, M.; et al. Risk of Upper and Lower Gastrointestinal Bleeding in Patients Taking Nonsteroidal Anti-Inflammatory Drugs, Antiplatelet Agents, or Anticoagulants. Clin. Gastroenterol. Hepatol. 2015, 13, 906–912.e2. [Google Scholar] [CrossRef]
- Hao, W.; Liu, A.; Zhu, H.; Yu, X.; Chen, G.; Xu, J. Risk Factors and Management of Gastrointestinal Bleeding in Patients with or without Antiplatelet and Anticoagulation Therapy: A Multicenter Real-World Prospective Study. BMC Gastroenterol. 2024, 24, 155. [Google Scholar] [CrossRef]
- García Rodríguez, L.A.; Lin, K.J.; Hernández-Díaz, S.; Johansson, S. Risk of Upper Gastrointestinal Bleeding with Low-Dose Acetylsalicylic Acid Alone and in Combination with Clopidogrel and Other Medications. Circulation 2011, 123, 1108–1115. [Google Scholar] [CrossRef]
- Suissa, S.; Bourgault, C.; Barkun, A.; Sheehy, O.; Ernst, P. Antihypertensive Drugs and the Risk of Gastrointestinal Bleeding. Am. J. Med. 1998, 105, 230–235. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Li, C.; Zhou, Y.-M.; Zeng, L.; Li, Y.-Y.; Huang, S.-L.; Zhu, C.-Y.; Wang, Y.; Wang, S.-N.; Chen, X.-D. Histopathological Features of Helicobacter Pylori Infection in Gastric Mucosa. J. Inflamm. Res. 2022, 15, 6231–6243. [Google Scholar] [CrossRef] [PubMed]
- Corral, J.E.; Mera, R.; Dye, C.W.; Morgan, D.R. Helicobacter Pylori Recurrence after Eradication in Latin America: Implications for Gastric Cancer Prevention. World J. Gastrointest. Oncol. 2017, 9, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Teutsch, B.; Rancz, A.; Tari, E.; Márta, K.; Veres, D.S.; Hosszúfalusi, N.; Mihály, E.; Hegyi, P.; Erőss, B. One in Four Patients with Gastrointestinal Bleeding Develops Shock or Hemodynamic Instability: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2023, 29, 4466–4480. [Google Scholar] [CrossRef] [PubMed]
| Parameter | n = 3291 |
|---|---|
| Age | 60 (18, 98) |
| Sex | |
| Female | 143 (43%) |
| Male | 186 (57%) |
| Hospital Service | |
| Outpatient | 12 (3.6%) |
| Hospitalization | 288 (88%) |
| UCI | 8 (2.4%) |
| Emergency | 21 (6.4%) |
| Admission Diagnosis | |
| Melena | 157 (48%) |
| Hematemesis | 148 (45%) |
| GIB (unspecified) | 24 (7.3%) |
| Length of Stay (Days) | 6 (0, 280) |
| H. pylori Positive | 44 (13%) |
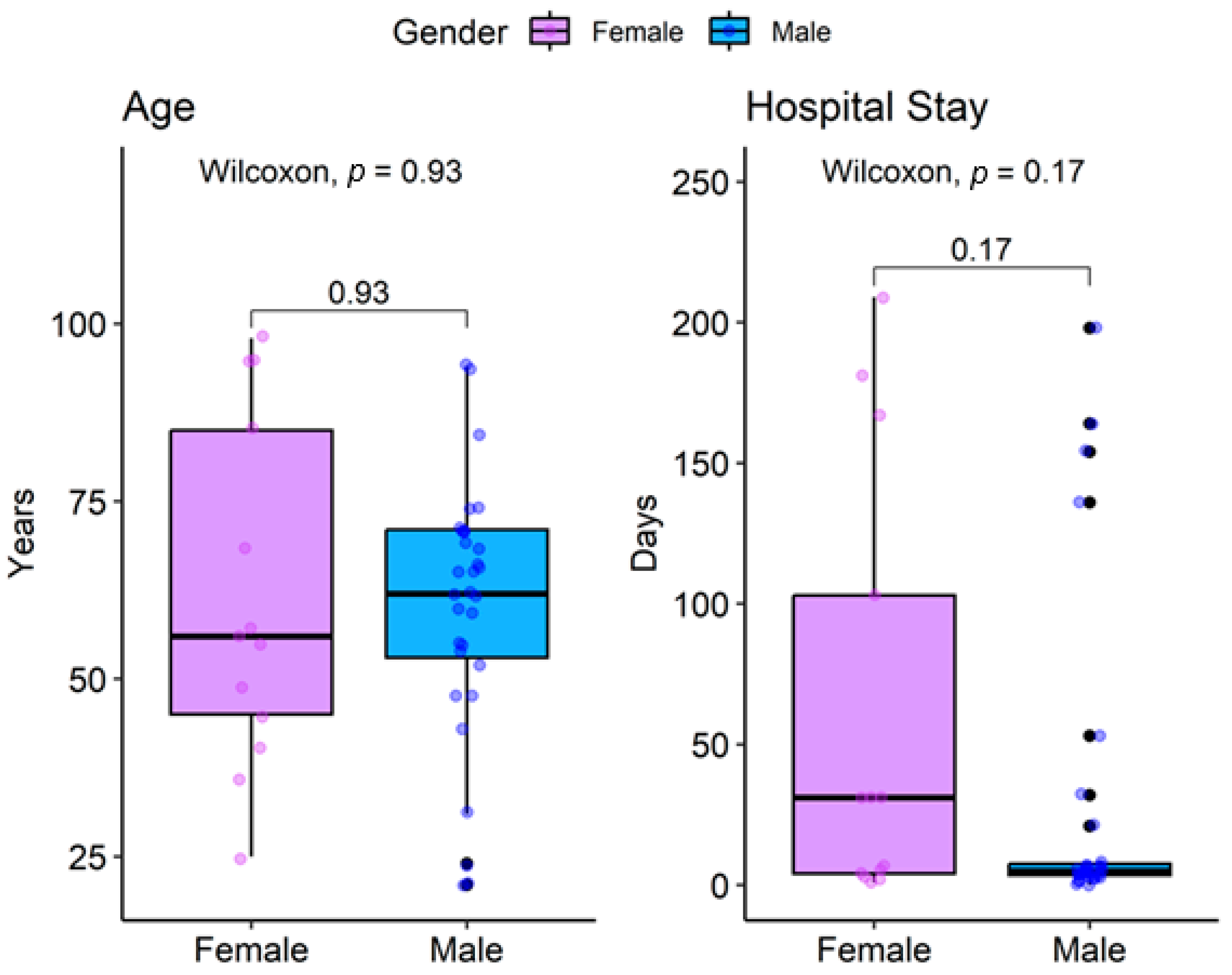
| Parameter | Overall n= 441 1 | Female n = 131 1 | Male n = 311 1 | p-Value |
|---|---|---|---|---|
| Age | 62 (49, 71) | 56 (45, 85) | 62 (53, 71) | >0.9 2 |
| Admission Diagnosis | 0.3 3 | |||
| Hematemesis | 15 (34%) | 6 (46%) | 9 (29%) | |
| Melena | 29 (66%) | 7 (54%) | 22 (71%) | |
| Length of Stay (days) | 6 (4, 31) | 31 (4, 103) | 5 (4, 8) | 0.2 2 |
| Outcome | >0.9 3 | |||
| Deceased | 1 (2.3%) | 0 (0%) | 1 (3.2%) | |
| Survived | 43 (98%) | 13 (100%) | 30 (97%) |
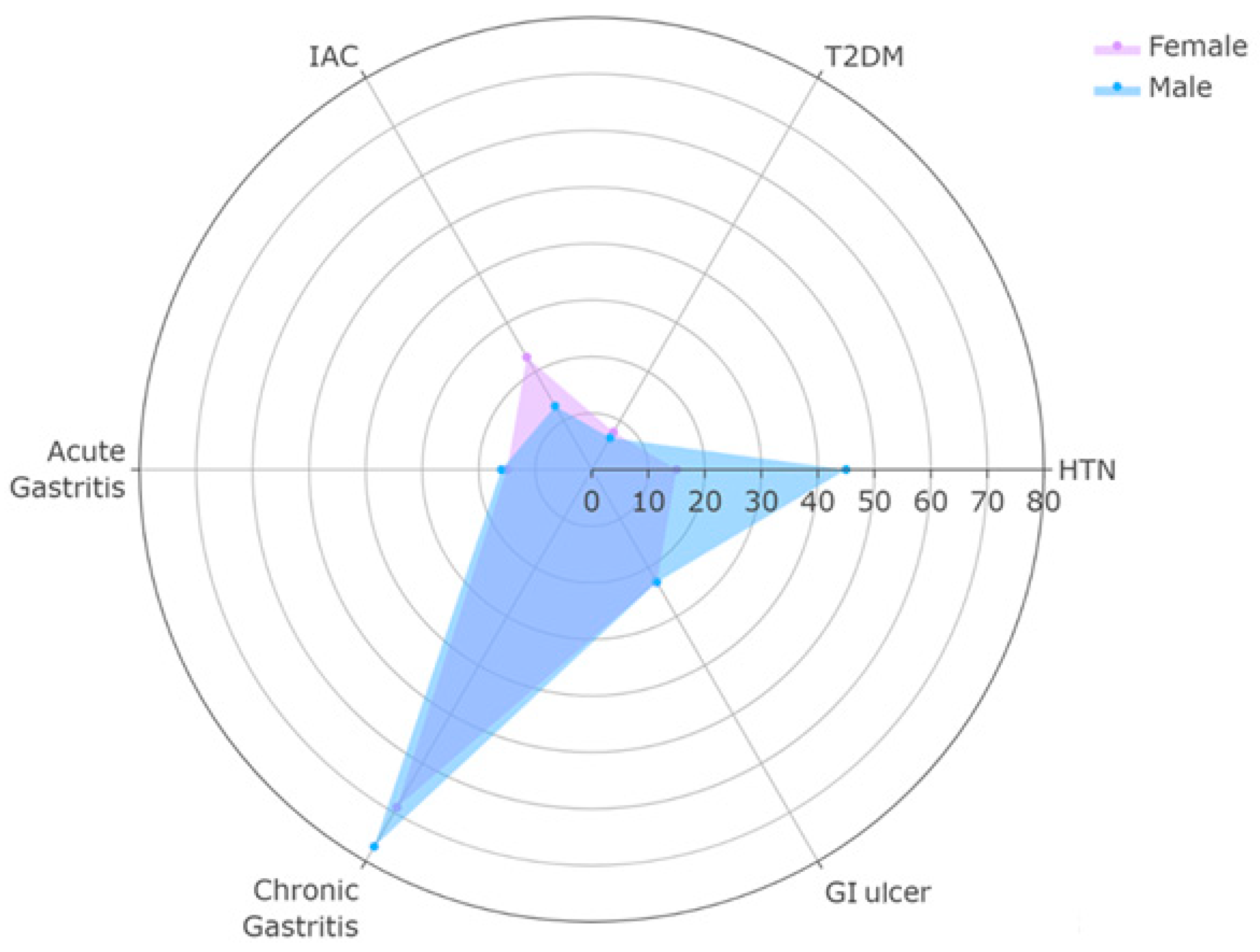
| Characteristics | Overall n = 44 1 | Female n = 13 1 | Male n = 31 1 | p-Value |
|---|---|---|---|---|
| Comorbidities | ||||
| HTN | 16 (36%) | 2 (15%) | 14 (45%) | 0.04 2 |
| DM | 3 (6.8%) | 1 (7.7%) | 2 (6.5%) | >0.9 2 |
| Pathological Findings | ||||
| Intestinal Adenocarcinoma (ACI) | 7 (16%) | 3 (23%) | 4 (13%) | 0.4 2 |
| Acute Gastritis | 7 (16%) | 2 (15%) | 5 (16%) | >0.9 2 |
| Congestive Gastritis | 5 (11%) | 1 (7.7%) | 4 (13%) | >0.9 2 |
| Chronic Gastritis | 33 (75%) | 9 (69%) | 24 (77%) | 0.7 2 |
| Follicular Gastritis | 8 (18%) | 3 (23%) | 5 (16%) | 0.7 2 |
| Gastroduodenal Ulcer | 10 (23%) | 3 (23%) | 7 (23%) | >0.9 2 |
| Complications | ||||
| Pneumonia | 5 (11%) | 2 (15%) | 3 (9.7%) | 0.6 2 |
| AKI | 2 (4.5%) | 0 (0%) | 2 (6.5%) | >0.9 2 |
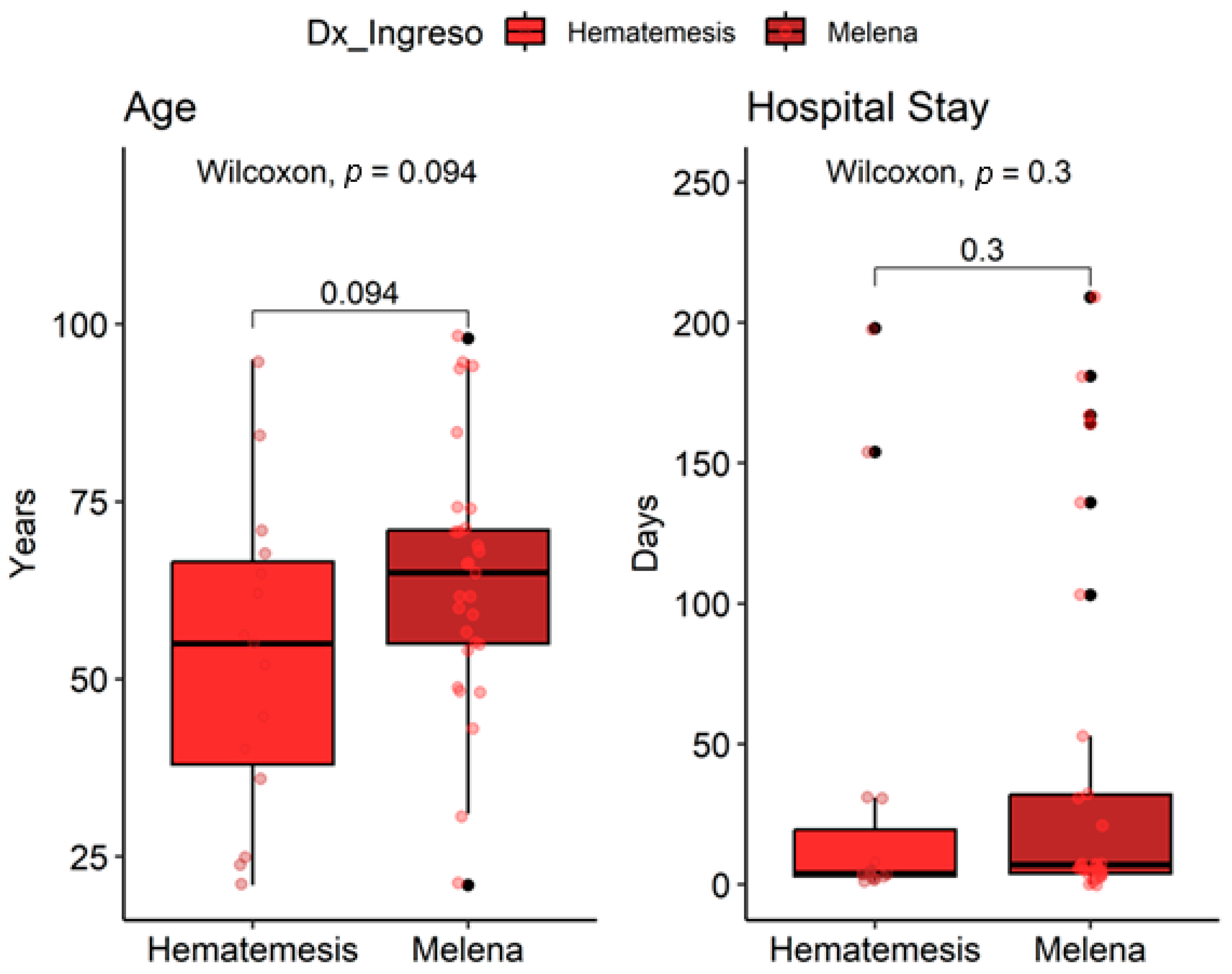
| Characteristics | Hematemesis n = 15 1 | Melena n = 29 1 | p-Value |
|---|---|---|---|
| Age | 55 (38, 67) | 65 (55, 71) | 0.094 2 |
| Sex | 0.3 3 | ||
| Female | 6 (40%) | 7 (24%) | |
| Male | 9 (60%) | 22 (76%) | |
| Length of stay (days) | 4 (3, 20) | 7 (4, 32) | 0.3 2 |
| Service | 0.8 3 | ||
| Emergency | 1 (6.7%) | 2 (6.9%) | |
| Hospitalization | 14 (93%) | 25 (86%) | |
| ICU | 0 (0%) | 2 (6.9%) | |
| Hypertension (HTA) | 3 (20%) | 13 (45%) | 0.10 4 |
| Intestinal Adenocarcinoma (ACI) | 2 (13%) | 5 (17%) | >0.9 3 |
| Acute gastritis | 2 (13%) | 5 (17%) | >0.9 3 |
| Chronic gastritis | 10 (67%) | 23 (79%) | 0.5 3 |
| Gastroduodenal ulcer | 3 (20%) | 7 (24%) |
| Forrest | Overall n = 441 | Female n = 131 | Male n = 311 | p-Value |
|---|---|---|---|---|
| IB | 1 (10%) | 0 (0%) | 1 (14%) | >0.92 |
| IIA | 5 (50%) | 2 (67%) | 3 (43%) | |
| III | 4 (40%) | 1 (33%) | 3 (43%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzón-Guerron, L.; Jiménez-Lacouture, C.; Cadena Bonfanti, A.; Dominguez Vargas, A.; González-Torres, H.J. Clinical and Endoscopic Features of Upper Gastrointestinal Bleeding Associated with Helicobacter pylori Infection: A Retrospective Cohort Study in the Colombian Caribbean (2021–2023). Gastroenterol. Insights 2025, 16, 48. https://doi.org/10.3390/gastroent16040048
Garzón-Guerron L, Jiménez-Lacouture C, Cadena Bonfanti A, Dominguez Vargas A, González-Torres HJ. Clinical and Endoscopic Features of Upper Gastrointestinal Bleeding Associated with Helicobacter pylori Infection: A Retrospective Cohort Study in the Colombian Caribbean (2021–2023). Gastroenterology Insights. 2025; 16(4):48. https://doi.org/10.3390/gastroent16040048
Chicago/Turabian StyleGarzón-Guerron, Lizeth, Carlos Jiménez-Lacouture, Andrés Cadena Bonfanti, Alex Dominguez Vargas, and Henry J. González-Torres. 2025. "Clinical and Endoscopic Features of Upper Gastrointestinal Bleeding Associated with Helicobacter pylori Infection: A Retrospective Cohort Study in the Colombian Caribbean (2021–2023)" Gastroenterology Insights 16, no. 4: 48. https://doi.org/10.3390/gastroent16040048
APA StyleGarzón-Guerron, L., Jiménez-Lacouture, C., Cadena Bonfanti, A., Dominguez Vargas, A., & González-Torres, H. J. (2025). Clinical and Endoscopic Features of Upper Gastrointestinal Bleeding Associated with Helicobacter pylori Infection: A Retrospective Cohort Study in the Colombian Caribbean (2021–2023). Gastroenterology Insights, 16(4), 48. https://doi.org/10.3390/gastroent16040048






