1. Introduction
Liver cirrhosis (LC) is a chronic disease that occurs as a result of chronic inflammation, causing healthy parenchyma to be replaced by fibrosis, ultimately leading to impaired liver function and the development of portal hypertension [
1,
2].
Alterations in blood biometrics are common findings in patients with LC. It has been reported that up to approximately 75% of such patients suffer from anemia, with multiple causal factors [
3]; most frequently, acute or chronic gastrointestinal bleeding can lead to consequent iron deficiency [
4]. In general, hematological disorders (anemia, leukopenia, and thrombocytopenia) are multifactorial; splanchnic sequestration, bone marrow suppression, and/or alterations in hematopoietic stimulating factors may be present or coexist [
5,
6,
7].
Dyspoiesis and peripheral cytopenia are common in cirrhosis. Dyspoiesis is a defect in the differentiation and maturation of cells in the bone marrow. In their study, Varadarajan A. et al. reported a 13.5% prevalence of dyspoiesis in LC patients. The authors also observed a greater effect on red blood cells (dyserythropoiesis), followed by megakaryocytes (dysmegakaryopoiesis) [
8]. Peripheral cytopenia is a common finding in LC and is defined as a peripheral white blood cell (WBC) count < 4.0 × 10
9/L, red blood cell (RBC) count < 3.5 × 10
12/L, or platelet (PLT) count < 100 × 10
9/L [
9]. Anemia and leukopenia are common biochemical findings in patients with LC, with multiple etiologies hypothesized, including gastrointestinal bleeding, folate deficiency, and hypersplenism, to name a few [
10]. Patients with LC and portal hypertension develop secondary hypersplenism, which leads to greater splenic retention of leukocytes, erythrocytes, and platelets, thus facilitating the capture, phagocytosis, or destruction of blood cells by phagocytes, resulting in peripheral cytopenia [
11]. The development of thrombocytopenia may also be influenced in LC by the impairment of thrombopoietin (TPO), which is produced almost exclusively by hepatocytes [
12]. The presence of cirrhosis decreases the number of functional liver cells that secrete TPO, causing an imbalance between TPO production and destruction; in the absence of TPO, immature megakaryocytes are destroyed [
12,
13].
The World Health Organization (WHO) defines anemia as a condition in which the number of red blood cells is insufficient to transport the oxygen required by cells for normal functioning [
14]. The WHO defines anemia as a hemoglobin level < 12 g/dL in women and <13 g/dL in men, serum ferritin levels < 15–100 ng/mL (depending on the presence of inflammation), and transferrin saturation (TSAT) < 16–20%, factors considered indicative of iron deficiency (ID) [
4].
Ohki et al. contend that, in patients with LC, the condition has no direct effect on the proliferation of CFU-E (erythroid colony-forming units) and BFU-E (erythroid burst-forming units); however, the condition does impact erythroid differentiation. Similarly, in another study, in vitro suppression of CFU-GMC (colony-forming units of granulocytes and macrophages) colony formation from healthy bone marrow was observed, and the degree of suppression correlated with the severity of granulocytopenia [
15].
Siddique A. et al. found in their study that, in one-third of patients with non-alcoholic fatty liver disease (NAFLD), the prevalence of iron deficiency (ID) (TSAT < 20%) stood at approximately 34%; the risk factors for the presence of ID were noted as female gender, obesity, increased body mass index (BMI), non-White race, and increased levels of IL-6 and IL-1ß. Hepcidin levels were also found to be significantly lower in patients with ID [
16]. Rivera-Álvarez M. et al. examined 123 patients with NAFLD, with their results showing that 20 patients (16%) suffered from thrombocytopenia and only 9 patients (7%) suffered from neutropenia (<5 × 10
9/L granulocytes); splenomegaly was not identified in any of these patients, and statistical significance was observed between the presence of thrombocytopenia and leukopenia/granulocytopenia. One possible explanation is the fact that fat deposition in hepatocytes causes damage, which is associated with a decrease in TPO production [
17].
Qamar A.A. et al. evaluated 213 patients with compensated LC without esophageal varices (EV) with a portal venous pressure gradient (PVPG) of at least 6 mmHg. Follow-up was carried out until the patients developed varices or variceal bleeding or until the follow-up period was completed. The most common type of cytopenia found was thrombocytopenia (59%), followed by anemia (35%) and leukopenia (21%). The presence of thrombocytopenia at the start of the study was associated with an increased likelihood of death or liver transplantation; patients with leukopenia and thrombocytopenia at the start of the study were more likely to experience clinical decompensation and had a higher HVPG than those in the group with thrombocytopenia alone [
18].
Similarly to other cytopenias, multiple factors have been considered to contribute to the development of leukopenia in patients with LC, which is characterized by a reduction in white blood cell count [
19]. One of these factors is explained by a decrease in WBC production, which may be due to bone marrow suppression, ineffective granulopoiesis, or genetic abnormalities. It has also been suggested that immunological conditions or the use of certain drugs (which prevent granulopoiesis) may have an influence, particularly affecting neutrophil production. Lastly, the condition may be associated with the sequestration and destruction of circulating WBCs [
2,
20]. The development of neutropenia is concerning due to the risk of these patients acquiring bacterial infections, thereby increasing mortality [
21].
Patients with LC present high morbidity and mortality rates, and cytopenias can influence the prognosis of these patients, either in the medium and/or long term. However, to date, researchers have yet to determine whether any difference exists between compensated and decompensated function and whether this factor influences the presence of different cytopenias. To address this gap, we chose to conduct this study with the aim of determining the hematological alterations present in patients with compensated vs. decompensated cirrhosis.
2. Materials and Methods
This prospective, observational study was conducted at a tertiary care hospital. The protocol was submitted to the hospital’s Ethics and Research Committee and approved under file number R-2024-3601-138, approval date: 12 June 2024. The diagnosis of liver cirrhosis was established using clinical and biochemical criteria, abdominal ultrasound, and endoscopy. The inclusion criteria were patients aged > 18 years with an established diagnosis of compensated and decompensated liver cirrhosis of any etiology, followed up in the Gastroenterology Department of the CMN SXXI Hospital de Especialidades, between June 2024 and May 2025, who agreed to sign an informed consent form and consented to serum sampling. Demographic data, comorbidities, functional class at the time of evaluation (Child–Pugh A, B, and C), history of jaundice, gastrointestinal bleeding, encephalopathy, spontaneous bacterial peritonitis, or any other infection were collected and biochemical data (complete blood count, liver function tests, blood chemistry, coagulation times, alpha-fetoprotein, erythrocyte sedimentation rate, and C-reactive protein) obtained from a venous serum sample via antecubital fossa puncture. The blood sample obtained from patients in a decompensated state was taken upon admission to the hospital. Exclusion criteria were as follows: patients who did not agree to participate or sign the informed consent form, or who did not agree to have a serum sample taken, in addition to patients with any type of neoplasm. A total of 22 patients were ultimately included.
Statistical Analysis
Descriptive statistics were performed. Categorical variables were reported as proportions, and for quantitative variables, the Shapiro–Wilk normality test was performed; the mean, median, standard deviation, or 25th–75th percentile were determined for discrete or continuous quantitative variables; and parametric and nonparametric statistics were used according to their distribution and variance. The X2 test was performed for the comparative analysis between categorical variables (gender and compensated or decompensated cirrhosis), and Student’s t-test or Mann–Whitney U test was used, as appropriate, for the analysis of quantitative variables. SPSS Statistics version 21 was used, and a statistical significance of p < 0.05 was considered.
3. Results
A total of 22 patients were included, of whom 8 suffered from compensated LC (CLC) and 14 suffered from decompensated LC (DLC). Based on the Child–Pugh score at the time of evaluation, 10 patients were categorized as Child–Pugh class A, 2 patients were categorized as class B, and 10 patients were categorized as Child–Pugh class C. Of the patients with DLC, four suffered from spontaneous bacterial peritonitis (SBP), two suffered from SBP and acute kidney injury (AKI), four suffered from hepatic encephalopathy (HE), three suffered from hemorrhagic portal hypertension (HPH), and one suffered from non-variceal upper gastrointestinal bleeding and AKI. The average age among the patients was 53 ± 12.20, with a predominance of females (77%). The main etiology was metabolic dysfunction-associated liver disease (MASLD) (27%), and 73% suffered from other comorbidities, mainly type 2 diabetes mellitus. The median body mass index (BMI) was 23.6. Lastly, the average hemoglobin level was 10.5 g/dL, the average platelet count was 105,000 × 10
3/μL, and the median WBC count was 4.82 × 10
3/μL (
Table 1 and
Table 2).
Subsequently, an analysis was performed by group for compensated cirrhosis (
Table 3) and decompensated cirrhosis (
Table 4).
A comparison was made between both groups with regard to blood biometry figures, erythrocyte sedimentation rate (ESR), C-reactive protein, and BMI, with the findings revealing that hemoglobin, hematocrit, lymphocyte, C-reactive protein, and ESR values were statistically significant between both groups.
When performing Student’s
t-test, a statistically significant difference was observed in hemoglobin, hematocrit, and ESR levels; a statistical difference was observed using the Mann–Whitney U test in lymphocyte and C-reactive protein levels between compensated and decompensated patients (
Table 5).
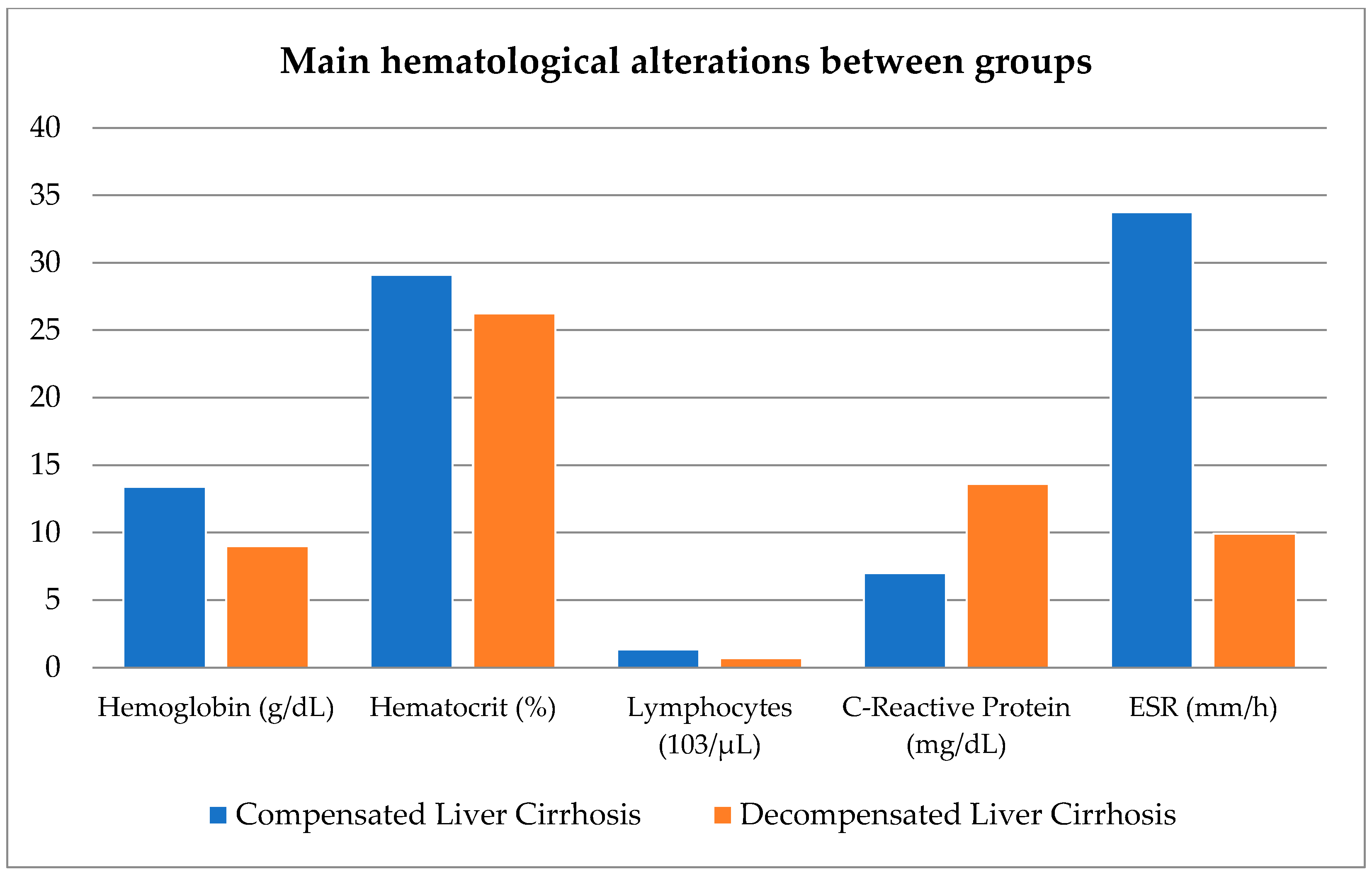
Figure 1 shows the main hematological alterations between the compensated vs. decompensated groups.
Based on the results presented in
Table 6, patients were included in the decompensated group because they were hospitalized for hemorrhagic portal hypertension, which was confirmed via endoscopy. The results presented in
Table 6 demonstrate that cytopenia is more common in patients with decompensated disease than in those with compensated disease.
The results presented in
Table 7 show the combination of different cytopenias with respect to Child–Pugh score; for the compensated group, we found that 50% of these patients exhibited alterations in two cell lines; in the 14 patients in the decompensated group, 50% were found to have involvement of all three cell lines, followed by 35.7% with bicytopenia.
4. Discussion
In our study, females predominated, with the most common etiology in the study population being metabolic dysfunction-associated liver disease (MASLD) and one of the most common comorbidities in these patients being type 2 diabetes mellitus.
Hematological complications in LC are due to multiple factors that predispose to the development of anemia (without obvious evidence of bleeding), leukopenia, and thrombocytopenia [
2]; the relevance of these cytopenias is due to the fact that they increase morbidity and mortality. In the article by Tantia et al. 2024, the authors refer to the presence of anemia in both compensated and decompensated patients, reporting that anemia is present in 66–75% of patients, with a higher risk of development in cirrhosis with a higher Child–Pugh score, and it was found that the predominant type of anemia is normocytic normochromic anemia [
2].
Similarly to the results of previous studies, the main cytopenias observed in our patients were thrombocytopenia (dysmegalocaryopoiesis) and anemia (dyserythropoiesis), noted in 73% of patients [
8,
18]. The values that showed a statistically significant difference between the two groups (compensated and decompensated LC) were hemoglobin, hematocrit, lymphocytes, C-reactive protein, and ESR. The statistically significant difference observed between the compensated and decompensated groups is likely explained by the low- and high-grade inflammatory status of patients with compensated vs. decompensated LC, respectively [
22]. In the article by Qamar et al., 2009, only patients with compensated cirrhosis were included, totaling 213 patients, 84% with thrombocytopenia, 42% with leukopenia, 37% with anemia, and 6.6% with pancytopenia. None of these patients showed evidence of esophagogastric varices. HVPG was performed, with results > 10 mmHg recorded in 89% of patients with thrombocytopenia plus leukopenia and 61% with thrombocytopenia alone, and 32% of these patients with this gradient did not present any hematological alteration or cytopenia [
18]. In our study, the group of patients with CLC were only categorized into thrombocytopenia vs. decompensated groups, which showed involvement in all three hematological cell lines; however, our findings differ in that these patients did not suffer from neutropenia but rather lymphopenia, and with regard to acute-phase reactants (C-reactive protein and ESR), the compensated group showed a slight increase in C-reactive protein levels and a significant increase in erythrocyte sedimentation rate. As expected, the decompensated group showed a greater increase in C-reactive protein levels, but not in ESR levels, with the average even falling within the normal range.
In another study including 242 patients with compensated cirrhosis (n = 53 (21.9%)) and decompensated cirrhosis (n = 189 (78.1%)), 128 (52.9%) of the patients suffered anemia. Based on the Child–Pugh score, a higher prevalence was determined in those with Child–Pugh class C (CPS; A: 26.5%, B: 59.2%, C: 69%;
p < 0.001) and when comparing compensated vs. decompensated patients, it was more prevalent in the latter group (62.4% vs. 18.8%,
p < 0.001). Anemia was associated with a significant risk of hepatic decompensation and/or long-term mortality [
23].
Both ESR and C-reactive protein are acute-phase reactants, generated in the liver in response to inflammation. It is important to note that there are multiple factors that can influence elevated ESR levels, such as the quality and quantity of red blood cells [
24], being obese or overweight, age, and gender, all of which could explain why this determination is elevated in the population studied. ESR and C-reactive protein are marked by low sensitivity and specificity; therefore, their measurements and clinical significance will depend on the patient’s condition [
24,
25].
The persistent elevation of C-reactive protein is explained by its ability to recognize damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) [
26,
27,
28]; it is important to note that patients with LC, regardless of its etiology, exhibit increased intestinal permeability that predisposes them to the presence of PAMPs and DAMPs secondary to hepatocyte damage. The latter factor causes a non-infectious or sterile inflammatory state, which explains why patients with no evidence of infection exhibit elevated levels of this acute-phase reactant. In their study, Pieri et al. reported a significant increase in C-reactive protein levels in patients with cirrhosis associated with chronic liver inflammation, and contend that persistently elevated levels are useful for identifying patients at higher risk of short-term mortality [
29]. In our study, patients with CLC may have experienced a slight increase in CRP associated with low inflammation, as none of them presented with bleeding or infection.
Differing from the results of previous studies, in which the authors note that patients with LC present leukopenia/granulocytopenia [
17,
21], in our study, we mainly observed leukopenia/lymphopenia, but only in patients with DLC, not in those with CLC. This finding could be explained by greater histological damage and secondary hypersplenism, since the development of leukopenia has been primarily described in more advanced stages of liver disease. It has been observed that patients with decompensated cirrhosis and acute-on-chronic liver failure (ACLF) present lymphopenia. Contrasting with previous findings, in our study, we found that patients with DLC present reduced leukocyte and lymphocyte counts with increased C-reactive protein levels. This finding could be explained by the fact that they suffer from a higher degree of inflammation and damage to hepatocytes, which predisposes these patients to the development of ACLF with the presence of lymphopenia and severe systemic inflammation [
30].
In Ximenes’ retrospective study, which included 149 patients with LC induced by excessive alcohol consumption, 47% of whom were categorized as Child–Pugh class C, bacterial infections were reported in 48% (72/149), and the variables associated with the risk of developing such infections were lymphopenia and C-reactive protein > 59 mg/L [
31]. One aspect observed in our study was the fact that the presence of lymphopenia and higher C-reactive protein levels were seen in patients with DLC who were also categorized as Child–Pugh class C; the percentage of patients with DLC who presented with an infectious process was 27% (6/22 patients), their average lymphocyte level was 0.74 10
3/μL, and their C-reactive protein level was 102.8 mg/dL. The average C-reactive protein level in patients with functional class decompensation was 56 mg/dL. Of note, sixty-six percent of patients (4/6) suffering from infection (SPB) died.
The relationship between CRP levels and liver function severity has been evaluated in two studies using the Child–Pugh scale. A significant difference between the three Child–Pugh groups was found in neither study [
32], nor was it determined whether they had suffered from an infection [
33]. Janum S.H. et al. concluded that mortality was associated with the degree of liver dysfunction or a higher score on the Child–Pugh scale.
As observed in our study, three patients in the decompensated group were classified as Child–Pugh class A. In light of these results, it is important to note that the Child–Pugh score can change over time; a patient can progress from Child–Pugh class A to B or C during acute decompensation and potentially return to a better class if the triggering factor is successfully treated and the acute damage is reversible. Child–Pugh A classification indicates chronic stability; however, the onset of a triggering factor can lead to acute decompensation. Although it is used to determine compensated versus decompensated status, this scale was developed for prognostic purposes.
Hematological alterations in liver cirrhosis have multiple etiologies and can be observed from the compensated phase of the disease; however, in the advanced stages of the disease, they will be more pronounced or the patient will suffer from more severe cytopenia. Acute-phase reactants are only markers of inflammation, whether acute or chronic; however, they do not play a prognostic role in these patients. It is important to identify these cytopenias in a timely manner due to the risk of developing infections; however, it is also necessary to bear in mind that these cytopenias are associated with increased morbidity and mortality, without neglecting the fact that the Child–Pugh score continues to offer good prognostic value in these patients.
One limitation of this study is the sample size. In addition, we present preliminary results from one population; thus, validation in a larger cohort is required.