Effects of Nutrients and Alcoholic Beverages on Gastrointestinal Tract Morphology
Abstract
1. Introduction
- 1.
- Describe the normal human GIT morphology.
- 2.
- Describe the main characteristics of SCs, DF, proteins, fats, and alcoholic beverages.
- 3.
- Describe the effects of consumption of SCs, DF, proteins, fats, and alcoholic beverages on the morphology of the human GIT.
- 4.
- Describe the associations of the previously mentioned substances with the development of pathologies of the GIT.
- 1.
- Published in peer-reviewed scientific journals.
- 2.
- Employed observational study designs (cohort, case–control, or cross-sectional), randomised controlled trials, or reviews.
- 3.
- Published in the English language.
- 4.
- Full-text articles available for review.
- Studies were excluded if they did not fulfil any of the inclusion criteria.
2. Literature Review
2.1. Normal Morphology of the Human Gastrointestinal Tract
2.2. Description of Nutrients and Beverages
2.2.1. Simple Carbohydrates
2.2.2. Fibre
2.2.3. Proteins
2.2.4. Fats
2.2.5. Alcoholic Beverages
2.3. Effects of Nutrients and Beverages on Gastrointestinal Morphology
2.3.1. Simple Carbohydrates
2.3.2. Fibre
2.3.3. Proteins
2.3.4. Fats
2.3.5. Alcoholic Beverages
2.4. Nutrients and Beverages in Relation to Gastrointestinal Pathologies
2.4.1. Simple Carbohydrates
2.4.2. Fibre
2.4.3. Proteins
2.4.4. Fats
2.4.5. Alcoholic Beverages
3. Discussion
4. Conclusions
5. Recommendations
- (1)
- A high SC consumption, especially fructose and glucose, should be perceived with caution, as it may be a factor contributing to intestinal wall permeability. Practising a low-FODMAP diet is one of the recommendations to help relieve the symptoms for patients suffering from IBD. A high-sugar diet can contribute to development of tooth caries; their substitution with caloric sweeteners should be performed carefully, as it can lead to an osmotic diarrhoea.
- (2)
- WHO guidelines suggest consuming > 20 g per day of non-starch polysaccharides and >25 g per day of total DF. Wholegrain cereals, fruits, and vegetables are highlighted as the preferred sources of the nutrient. For IBS patients, insoluble and fermentable DFs should be reduced or consumed with caution as they can cause bloating, distention, flatulence, and cramping. Resistant starches and soluble DFs, such as psyllium, are some DFs that may be beneficial or well-tolerated. Due to the common clinical overlap of IBS and IBD, IBS diet guidelines have proved to be beneficial for IBD patients too and are advisable for implementation. In any case, DFs should be gradually integrated and consumed in moderation, adjusting the intake to the body’s tolerance.
- (3)
- For healthy individuals, firstly, protein intake should be accompanied by sufficient fermentable carbohydrates (DFs), which promote beneficial bacterial metabolism and reduce harmful protein fermentation products such as ammonia and amines. Secondly, both animal and plant proteins can be included, but a higher proportion of plant-derived proteins may better support microbial diversity and lower inflammatory potential.
- (4)
- For individuals suffering from IBD or colitis, moderately increased protein intake could protect intestinal mucosa, while overconsumption of proteins could exacerbate inflammation. Emphasis should be put on fish and legumes, while avoiding processed or red meat. Chronic kidney disease patients also should be mindful about consuming proteins because protein intake above 0.6–0.8 g/kg/day can damage intestinal mucosa and increase permeability. Proteins with complete essential amino acid profiles, such as egg, dairy, soy, and lean meats, should be prioritised to support mucosal recovery. To prevent villous atrophy and restore intestinal integrity, patients with coeliac disease need to avoid gluten and substitute gluten-containing grains with alternative sources (legumes, quinoa, amaranth, buckwheat).
- (5)
- Daily fat intake should not surpass 30% of the total daily energy consumption, but intake of saturated fats should not exceed 10%. As for trans-fats, they should not be more than 1% of the total energy caloric intake for adults. Unsaturated fats, for example, in fish, nuts, avocados, sunflower, canola, soybean, and olive oil, are preferable and have more positive effects than saturated fats in lard, fatty meat, palm oil, coconut oil, butter, cheese, and trans-fats sourced, for instance, from baked, fried, and pre-packaged foods, as well as in the meat and dairy of ruminant species, including cows, sheep, and goats. The long-term effects of an HFD are increased intestinal permeability and dysbiosis, leading to chronic, low-grade inflammation. These changes are responsible for many metabolic disorders, including type 2 diabetes, obesity, cardiovascular diseases, as well as IBD. Furthermore, an HFD alters bile acid metabolism, elevating the risk for colorectal cancer.
- (6)
- There is no amount of alcohol consumption that is considered completely safe and that does not have adverse effects on health. The risks increase with both the amount and duration of alcohol consumption. Long-term gastrointestinal effects of alcohol consumption include alcoholic liver disease, Barrett’s oesophagus, oesophageal adenocarcinoma, gastric mucosal atrophy and ulceration, intestinal dysbiosis and malabsorption, salivary gland impairment, and oral mucosal dysplasia with cellular damage, among other alcohol-related pathologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIDS | Acquired immune deficiency syndrome |
| AA | Amino acid |
| ALD | Alcohol-associated liver disease |
| BCFA | Branched-chain fatty acid |
| CMC | Carboxymethylcellulose |
| DF | Dietary fibre |
| DNA | Deoxyribonucleic acid |
| FA | Fatty acid |
| FITC-dextran | Fluorescein isothiocyanate dextran |
| FODMAP | Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols |
| GERD | Gastroesophageal reflux disease |
| GIT | Gastrointestinal tract |
| HFD | High-fat diet |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| LFD | Low-FODMAP diet |
| MEOS | Microsomal ethanol oxidation system |
| NAD | Nicotinamide adenine dinucleotide |
| PI-IBS | Post-infectious irritable bowel syndrome |
| ROS | Reactive oxygen species |
| SC | Simple carbohydrate |
| SCFA | Short-chain fatty acid |
| TDA | Traditional dietary advice diet |
| TNF-α | Tumour necrosis factor alpha |
References
- Bertin, L.; Zanconato, M.; Crepaldi, M.; Marasco, G.; Cremon, C.; Barbara, G.; Barberio, B.; Zingone, F.; Savarino, E.V. The Role of the FODMAP Diet in IBS. Nutrients 2024, 16, 370. [Google Scholar] [CrossRef]
- Fajstova, A.; Galanova, N.; Coufal, S.; Malkova, J.; Kostovcik, M.; Cermakova, M.; Pelantova, H.; Kuzma, M.; Sediva, B.; Hudcovic, T.; et al. Diet Rich in Simple Sugars Promotes Pro-Inflammatory Response via Gut Microbiota Alteration and TLR4 Signaling. Cells 2020, 9, 2701. [Google Scholar] [CrossRef]
- Merino, B.; Fernández-Díaz, C.M.; Cózar-Castellano, I.; Perdomo, G. Intestinal Fructose and Glucose Metabolism in Health and Disease. Nutrients 2019, 12, 94. [Google Scholar] [CrossRef]
- Melchior, C.; Douard, V.; Coëffier, M.; Gourcerol, G. Fructose and irritable bowel syndrome. Nutr. Res. Rev. 2020, 33, 235–243. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme; Report of the 30th Session of the Codex Committee on Nutrition and Foods for Special Dietary Uses; Food and Agriculture Organization of the United Nations (FAO): Cape Town, South Africa, 2008; p. 5. [Google Scholar]
- Hedemann, M.S.; Eskildsen, M.; Lærke, H.N.; Pedersen, C.; Lindberg, J.E.; Laurinen, P.; Knudsen, K.E.B. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J. Anim. Sci. 2006, 84, 1375–1386. [Google Scholar] [CrossRef]
- Wang, H.; Gong, J.; Wang, W.; Long, Y.; Fu, X.; Fu, Y.; Qian, W.; Hou, X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE 2014, 9, e90153. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Lim, M.T.; Pan, B.J.; Toh, D.W.K.; Sutanto, C.N.; Kim, J.E. Animal Protein versus Plant Protein in Supporting Lean Mass and Muscle Strength: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.T.; Morya, S.; Kasankala, L.M. Emerging trends in bioavailability and pharma-nutraceutical potential of whey bioactives. J. Nutr. Metab. 2024, 2024, 1–14. [Google Scholar] [CrossRef]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv. Nutr. 2019, 11, 77–91. [Google Scholar] [CrossRef]
- Ocvirk, S.; O’Keefe, S.J. Dietary fat, bile acid metabolism and colorectal cancer. Semin. Cancer Biol. 2021, 73, 347–355. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Li, Y. Is alcohol consumption associated with gastroesophageal reflux disease? J. Zhejiang Univ. Sci. B 2010, 11, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Ito, H.; Matsui, H.; Hyodo, I. Acetaldehyde is an oxidative stressor for gastric epithelial cells. J. Clin. Biochem. Nutr. 2014, 55, 26–31. [Google Scholar] [CrossRef]
- Paulsen, D.F. Chapter 15: Digestive Tract. In Histology and Cell Biology: Examination & Board Review, 6th ed; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Zhang, L.; Shao, H.; Alkan, S. (Eds.) Diagnostic Pathology of Hematopoietic Disorders of Spleen and Liver, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Seeberger, P.H. Monosaccharide Diversity. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; NCBI Bookshelf; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579981/ (accessed on 4 November 2024).
- Qi, X.; Tester, R.F. Fructose, galactose and glucose—In health and disease. Clin. Nutr. ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Lanaspa, M.A.; Sanchez-Lozada, L.G.; Tolan, D.; Nakagawa, T.; Ishimoto, T.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Stenvinkel, P. The fructose survival hypothesis for obesity. Philos. Trans. R Soc. Lond. B Biol. Sci. 2023, 378, 1885. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.-J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of Joint WHO/FAO Expert Consultation; WHO Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2002; 58p. [Google Scholar]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets: A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- LaPelusa, A.; Kaushik, R. Physiology, Proteins; National Library of Medicine; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555990/ (accessed on 24 November 2024).
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Sevela, S.; Meisnerova, E.; Vecka, M.; Vavrova, L.; Rychlikova, J.; Lenicek, M.; Vitek, L.; Novakova, O.; Novak, F. High dose fish oil added to various lipid emulsions normalizes superoxide dismutase 1 activity in home parenteral nutrition patients. Nutrients 2024, 16, 485. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 19 October 2025).
- Ye, Z.; Li, R.; Cao, C.; Xu, Y.-J.; Cao, P.; Li, Q.; Liu, Y. Fatty acid profiles of typical dietary lipids after gastrointestinal digestion and absorption: A combination study between in-vitro and in-vivo. Food Chem. 2019, 280, 34–44. [Google Scholar] [CrossRef]
- Le Daré, B.; Lagente, V.; Gicquel, T. Ethanol and its metabolites: Update on toxicity, benefits, and focus on immunomodulatory effects. Drug Metab. Rev. 2019, 51, 545–561. [Google Scholar] [CrossRef]
- Lu, Y.; George, J. Interaction between fatty acid oxidation and ethanol metabolism in liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G483–G494. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health and Policy 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- McCallum, N.; Najlah, M. The anticancer activity of Monosaccharides: Perspectives and outlooks. Cancers 2024, 16, 2775. [Google Scholar] [CrossRef]
- Lai, K.K.; Horvath, B.; Xie, H.; Wu, X.; Lewis, B.L.; Pai, R.K.; Plesec, T.; Patil, D.T.; Gordon, I.O.; Wang, Y.; et al. Risk for Colorectal Neoplasia in Patients with Inflammatory Bowel Disease and Mucosa Indefinite for Dysplasia. Inflamm. Bowel Dis. 2015, 21, 378–384. [Google Scholar] [CrossRef]
- Mackie, A.; Rigby, N.; Harvey, P.; Bajka, B. Increasing dietary oat fibre decreases the permeability of intestinal mucus. J. Func. Foods 2016, 26, 418–427. [Google Scholar] [CrossRef]
- Steinberg, A.P.; Wang, Z.-G.; Ismagilov, R.F. Food Polyelectrolytes Compress the Colonic Mucus Hydrogel by a Donnan Mechanism. Biomacromolecules 2019, 20, 2675–2683. [Google Scholar] [CrossRef]
- Ma, S.; Yeom, J.; Lim, Y.H. Specific activation of hypoxia-inducible factor-2α by propionate metabolism via a β-oxidation-like pathway stimulates MUC2 production in intestinal goblet cells. Biomed. Pharmacother. 2022, 155, 113672. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; O, E.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim. Feed Sci. Technol. 2020, 260, 114335. [Google Scholar] [CrossRef]
- Fouhse, J.M.; Gänzle, M.G.; Beattie, A.D.; Vasanthan, T.; Zijlstra, R.T. Whole-Grain Starch and Fiber Composition Modifies Ileal Flow of Nutrients and Nutrient Availability in the Hindgut, Shifting Fecal Microbial Profiles in Pigs. J. Nutr. 2017, 11, 2031–2040. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.E.; Pethick, D.W.; Mullan, B.P.; Hampson, D.J. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth and stimulates proliferation of enterotoxigenic Escherichia coli in newly weaned pigs. Br. J. Nutr. 2001, 86, 487–498. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Protein and amino acid requirements in human nutrition: Report of a joint FAO/WHO/UNU expert consultation. WHO Tech. Rep. Ser. 2007, 935, 242–243. [Google Scholar]
- Witard, O.C.; Jackman, S.R.; Kies, A.K.; Jeukendrup, A.E.; Tipton, K.D. Effect of increased dietary protein on tolerance to intensified training. Med. Sci. Sports Exerc. 2011, 43, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Peacock, C.A.; Ellerbroek, A.; Fromhoff, B.; Silver, T. The effects of consuming a high protein diet (4.4 g/kg/d) on body composition in resistance-trained individuals. J. Int. Soc. Sports Nutr. 2014, 11, 19. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kawano, S.; Tsuji, S.; Fusamoto, H.; Kamada, T.; Sato, N. Mechanism of gastric mucosal damage induced by ammonia. Gastroenterology 1992, 102, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.; Protein-Energy Undernutrition (PEU). MSD Manual Professional Edition. Available online: https://www.msdmanuals.com/professional/nutritional-disorders/undernutrition/protein-energy-undernutrition-peu (accessed on 27 February 2025).
- Yu, D.; Zhu, W.; Hang, S. Effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs. Animals 2019, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, P.; Fan, P.; He, T.; Jacobs, D.; Levesque, C.L.; Johnston, L.J.; Ji, L.; Ma, N.; Chen, Y.; et al. Moderate Dietary Protein Restriction Optimized Gut Microbiota and Mucosal Barrier in Growing Pig Model. Front. Cell. Infect. Microbiol. 2018, 8, 246. [Google Scholar] [CrossRef]
- Kangwan, N.; Pratchayasakul, W.; Kongkaew, A.; Pintha, K.; Chattipakorn, N.; Chattipakorn, S.C. Perilla Seed Oil Alleviates Gut Dysbiosis, Intestinal Inflammation and Metabolic Disturbance in Obese-Insulin-Resistant Rats. Nutrients 2021, 13, 3141. [Google Scholar] [CrossRef]
- Stenman, L.K. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J. Gastroenterol. 2012, 18, 923. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.Y.; Storlien, L.H.; Hoffmann, J.M.A.; Oscarsson, J.; Dinudom, A.; Mather, T.J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity 2015, 23, 1429–1439. [Google Scholar] [CrossRef]
- Mogilevski, T.; Rosella, S.; Nguyen, A.; Fitzpatrick, J.; Parker, F.; Halmos, E.P.; Gibson, P.R. Characterisation of biomarkers of intestinal barrier function in response to a high fat/high carbohydrate meal and corticotropin releasing hormone. PLoS ONE 2024, 19, e0294918. [Google Scholar] [CrossRef]
- Silveira, B.K.S.; Rocha, D.M.U.P.; Martino, H.S.D.; Grancieri, M.; Gomes, M.J.C.; Mantovani, H.C.; Bressan, J.; Hermsdorff, H.H.M. Daily cashew and Brazil nut consumption modifies intestinal health in overweight women on energy-restricted intervention: A randomized controlled trial (Brazilian Nuts Study). J. Nutr. 2024, 154, 962–977. [Google Scholar] [CrossRef]
- Brizuela, M.; Winters, R. Histology, Oral Mucosa; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572115/ (accessed on 24 November 2024).
- Goldstein, B.Y.; Chang, S.C.; Hashibe, M.; La Vecchia, C.; Zhang, Z.F. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: An update. Eur. J. Cancer Prev. 2010, 19, 431–465. [Google Scholar] [CrossRef]
- Inenaga, K.; Ono, K.; Hitomi, S.; Kuroki, A.; Ujihara, I. Thirst sensation and oral dryness following alcohol intake. Jpn. Dent. Sci. Rev. 2017, 53, 78–85. [Google Scholar] [CrossRef]
- Li, G.; Zhu, L.; Cao, Z.; Wang, J.; Zhou, F.; Wang, X.; Li, X.; Nie, G. A new participant in the pathogenesis of alcoholic gastritis: Pyroptosis. Cell. Physiol. Biochem. 2018, 49, 406–418. [Google Scholar] [CrossRef]
- Ohashi, K.; Pimienta, M.; Seki, E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018, 2, 161–172. [Google Scholar] [CrossRef]
- Kosnicki, K.L.; Penprase, J.C.; Cintora, P.; Torres, P.J.; Harris, G.L.; Brasser, S.M.; Kelley, S.T. Effects of moderate, voluntary ethanol consumption on the rat and human gut microbiome. Addict. Biol. 2018, 24, 617–630. [Google Scholar] [CrossRef]
- Benahmed, A.G.; Gasmi, A.; Dadar, M.; Arshad, M.; Bjørklund, G. The role of sugar-rich diet and salivary proteins in dental plaque formation and oral health. J. Oral Biosci. 2021, 63, 134–141. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, N.; Pawar, A.P.; Birajdar, S.S.; Natt, A.S.; Singh, H.P. Role of Sugar and Sugar Substitutes in Dental Caries: A Review. ISRN Dent. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- IBS and the Low FODMAP Diet. Gloucestershire Hospitals NHS Foundation Trust. Available online: https://www.gloshospitals.nhs.uk/our-services/services-we-offer/nutrition-dietetics/irritable-bowel-syndrome-ibs/ibs-and-low-fodmap-diet/ (accessed on 18 October 2025).
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut microbiota in patients with irritable bowel syndrome—A systematic review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Goyal, O.; Batta, S.; Nohria, S.; Kishore, H.; Goyal, P.; Sehgal, R.; Sood, A. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea-predominant irritable bowel syndrome: A prospective, randomized trial. J. Gastroenterol. Hepatol. 2021, 36, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Nguyen, L.H.; Song, M.; Jovani, M.; Liu, P.H.; Cao, Y.; Tam, I.; Wu, K.; Giovannucci, E.L.; Strate, L.L.; et al. Intake of Dietary Fiber, Fruits, and Vegetables and Risk of Diverticulitis. Am. J. Gastroenterol. 2019, 114, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Boughton, R.S.; Brophy, C.; Corbett, G.; Murphy, S.; Clifford, J.; Hanly, A.; Fitzpatrick, M.; O’Brien, L. Haemorrhoids and Anal Fissures in Pregnancy: Predictive Factors and Effective Treatments. Cureus 2024, 16, e53773. [Google Scholar] [CrossRef]
- Yu, W.; Su, X.; Chen, W.; Tian, X.; Zhang, K.; Guo, G.; Zhou, L.; Zeng, T.; Han, B. Three types of gut bacteria collaborating to improve Kui Jie’an enema treat DSS-induced colitis in mice. Biomed. Pharmacother. 2019, 113, 108751. [Google Scholar] [CrossRef]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary factors modulating colorectal carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef]
- So, D.; Yao, C.K.; Gibson, P.R.; Muir, J.G. Evaluating tolerability of resistant starch 2, alone and in combination with minimally fermented fibre for patients with irritable bowel syndrome: A pilot randomised controlled cross-over trial. J. Nutr. Sci. 2022, 11, e15. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Yao, C.K.; Ardalan, Z.S.; Thwaites, P.A.; Kalantar-Zadeh, K.; Gibson, P.R.; Muir, J.G. Supplementing Dietary Fibers with a Low FODMAP Diet in Irritable Bowel Syndrome: A Randomized Controlled Crossover Trial. Clin. Gastr. Hep. 2022, 20, 2112–2120.e7. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.; Fried, M.; Gwee, K.A.; Khalif, I.; Hungin, A.P.S.; Lindberg, G.; Abbas, Z.; Fernandez, L.B.; Bhatia, S.J.; Schmulson, M.; et al. World Gastroenterology Organisation global guidelines: Irritable bowel syndrome. J. Clin. Gastroenterol. 2016, 50, 704–713. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Gordon, M.; Sinopoulou, V.; Radford, S.J.; Darie, A.M.; Vuyyuru, S.K.; Alrubaiy, L.; Arebi, N.; Blackwell, J.; Butler, T.D.; et al. British Society of Gastroenterology guidelines on inflammatory bowel disease in adults: 2025. Gut 2025, 74, s1–s101. [Google Scholar] [CrossRef]
- Vidal-Lletjós, S.; Andriamihaja, M.; Blais, A.; Grauso, M.; Lepage, P.; Davila, A.-M.; Viel, R.; Gaudichon, C.; Leclerc, M.; Blachier, F.; et al. Dietary protein intake level modulates mucosal healing and mucosa-adherent microbiota in mouse model of colitis. Nutrients 2019, 11, 514. [Google Scholar] [CrossRef]
- Kostovcikova, K.; Coufal, S.; Galanova, N.; Fajstova, A.; Hudcovic, T.; Kostovcik, M.; Prochazkova, P.; Jiraskova Zakostelska, Z.; Cermakova, M.; Sediva, B.; et al. Diet Rich in Animal Protein Promotes Pro-inflammatory Macrophage Response and Exacerbates Colitis in Mice. Front. Immunol. 2019, 10, 919. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Xu, K.; Han, H.; Liu, Z.; Wang, C.; Li, T.; Yin, Y. Protein Level and Infantile Diarrhea in a Post Weaning Piglet Model. Mediat. Inflamm. 2020, 2020, 1937387. [Google Scholar] [CrossRef]
- Dieterich, W.; Neurath, M.F.; Zopf, Y. Intestinal ex vivo organoid culture reveals altered programmed crypt stem cells in patients with celiac disease. Sci. Rep. 2020, 10, 3535. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, Z.K.; Huang, Z.B.; Chen, X.L.; Liu, F.B. Dietary and lifestyle factors related to gastroesophageal reflux disease: A systematic review. Ther. Clin. Risk Manag. 2021, 17, 305–323. [Google Scholar] [CrossRef]
- Paik, J.; Fierce, Y.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a−/− male mice. J. Nutr. 2013, 143, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chinnathambi, S.; Kumar, M.; Pandian, G.N. Food intake and colorectal cancer. Nutr. Cancer 2023, 75, 1710–1742. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, D.; Wang, L.; Zhang, H.; Jiang, F.; Zhang, R.; Xu, L.; Yang, N.; Dai, S.; Xu, X.; et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients 2023, 15, 730. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, M.; Dong, W.; Deng, B.; Wang, S.; Zhang, Y.; Wang, S.; Luo, S.; Wang, W.; Qi, Y.; et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 2017, 140, 2545–2556. [Google Scholar] [CrossRef]
- Dermadi, D.; Valo, S.; Ollila, S.; Soliymani, R.; Sipari, N.; Pussila, M.; Sarantaus, L.; Linden, J.; Baumann, M.; Nyström, M. Western diet deregulates bile acid homeostasis, cell proliferation, and tumorigenesis in colon. Cancer Res. 2017, 77, 3352–3363. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z. Unraveling the causal link: Fatty acids and inflammatory bowel disease. Front. Immunol. 2024, 15, 1405790. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2013, 27, 277–284. [Google Scholar]
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 2017, 17, 594–604. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Chakravarthi, S.; Jegasothy, R.; Seng, W.Y.; Fuloria, N.K.; Fuloria, S.; Hazarika, I.; Das, A. Alcohol-associated liver disease: A review on its pathophysiology, diagnosis and drug therapy. Toxicol. Rep. 2021, 8, 376–385. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2020, 73, 4–13. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2016, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Jördens, M.S.; Luedde, M.; Modest, D.P.; Labuhn, S.; Luedde, T.; Kostev, K.; Roderburg, C. Incidence of cancer in patients with irritable bowel syndrome. J. Clin. Med. 2021, 10, 5911. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Farkas, D.K.; Pedersen, L.; Erichsen, R.; de la Cour, Z.D.; Gregersen, H.; Sørensen, H.T. Irritable bowel syndrome and risk of colorectal cancer: A Danish nationwide cohort study. Br. J. Cancer 2011, 104, 1202–1206. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Porcine models of digestive disease: The future of large animal translational research. Transl. Res. 2015, 166, 12–27. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Elamin, E.; Jonkers, D.; Juuti-Uusitalo, K.; van Ijzendoorn, S.; Troost, F.; Duimel, H.; Broers, J.; Verheyen, F.; Dekker, J.; Masclee, A. Effects of ethanol and acetaldehyde on tight junction integrity: In vitro study in a three-dimensional intestinal epithelial cell culture model. PLoS ONE 2012, 7, e35008. [Google Scholar] [CrossRef] [PubMed]
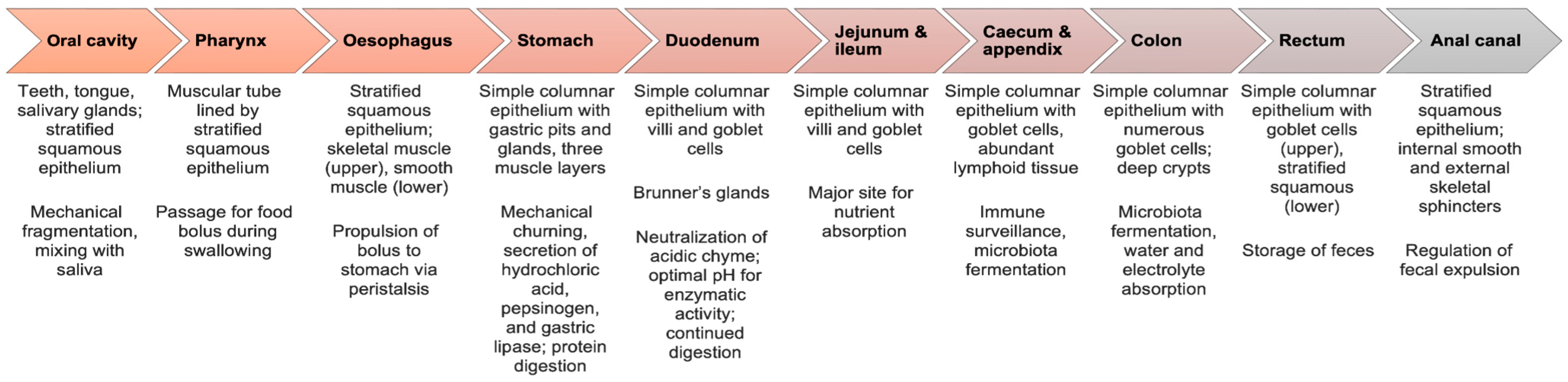
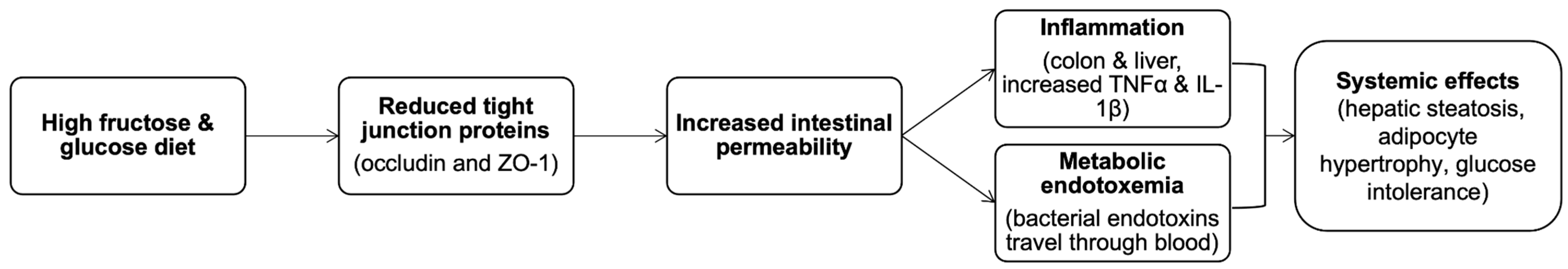
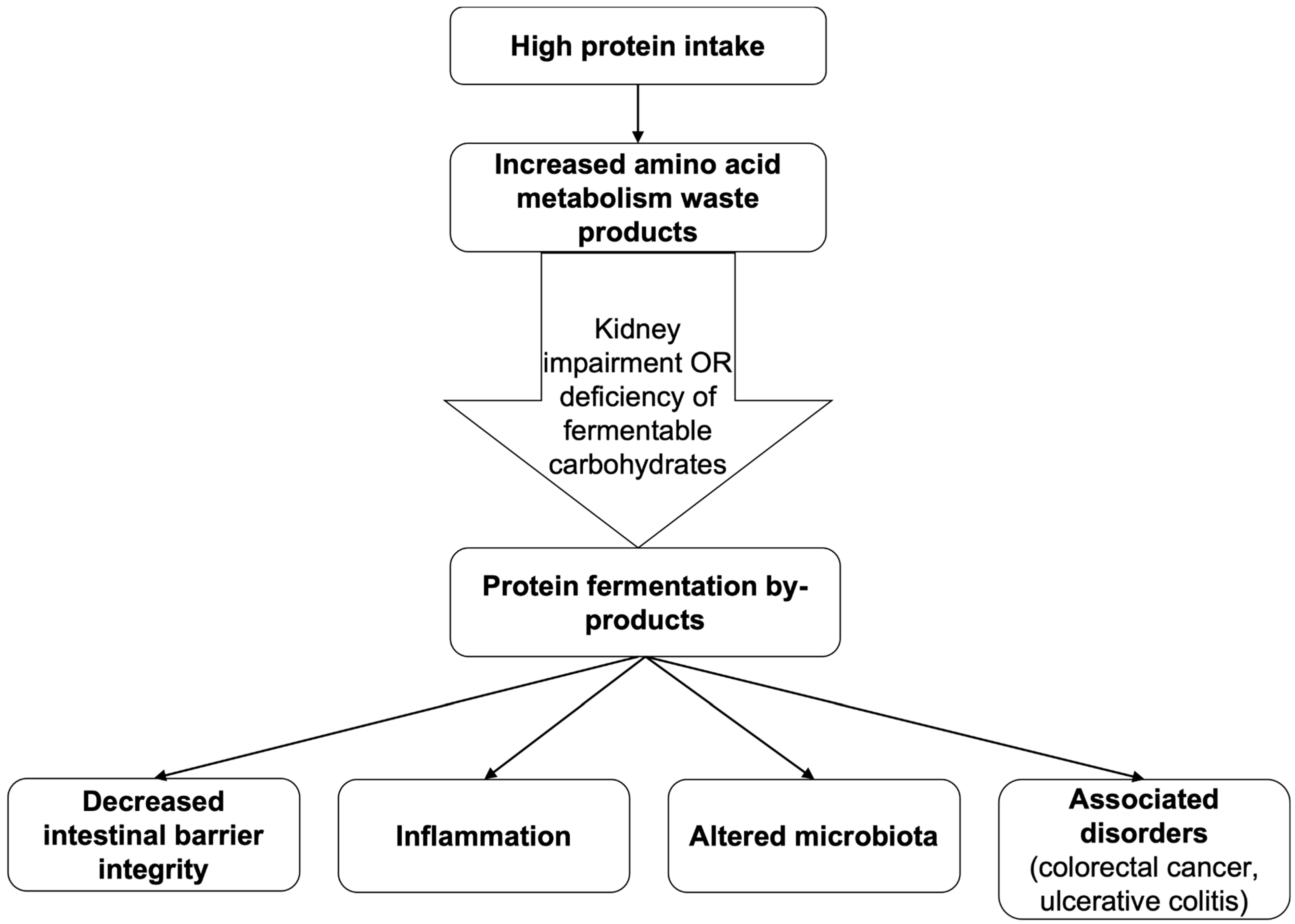
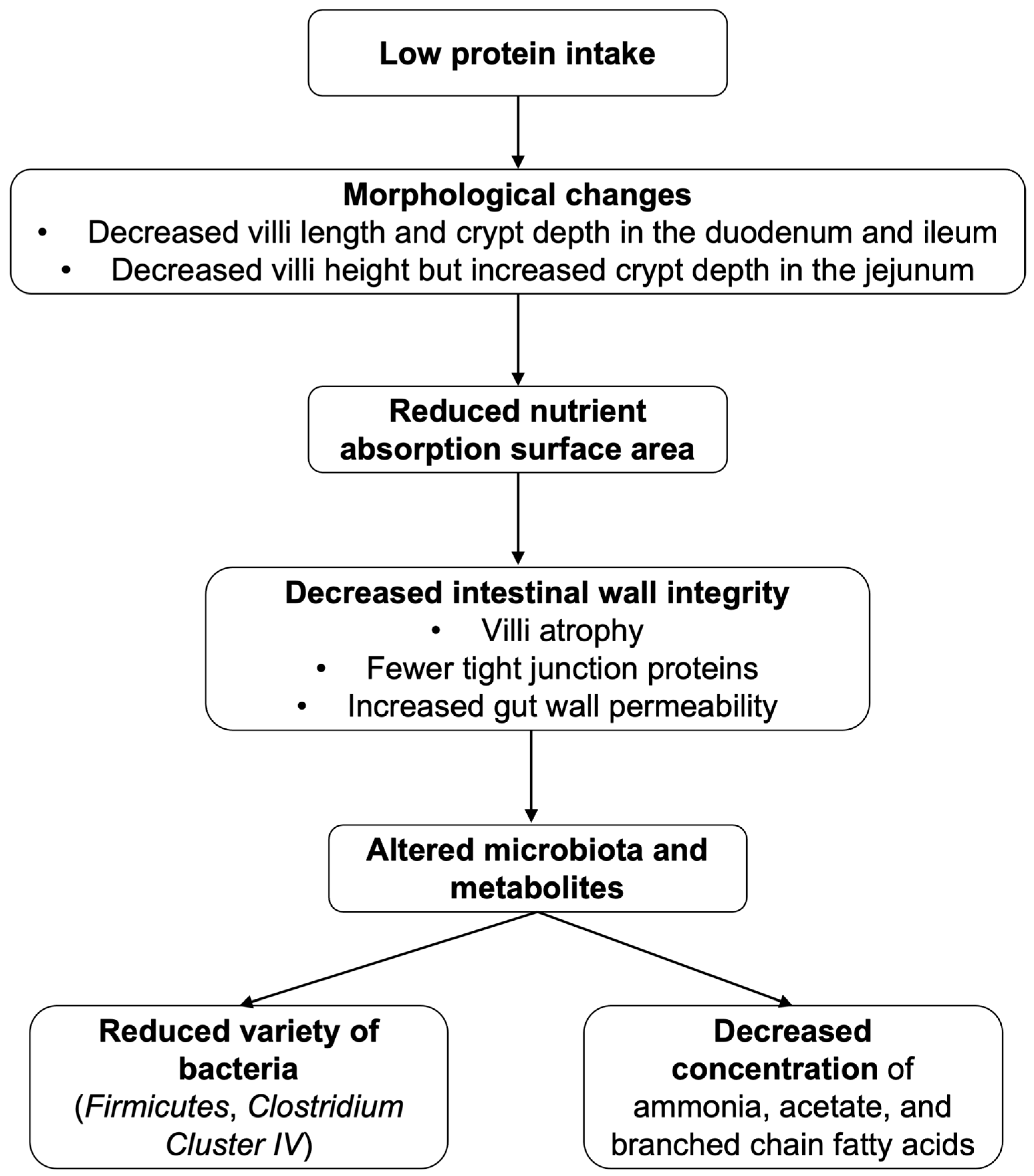

| Nutrients/Beverages | Structure | Digestion/Absorption | Sources |
|---|---|---|---|
| Simple carbohydrates | Monosaccharides (e.g., glucose and fructose). Single molecule with formula Cx(H2O)n. | Absorbed in the small intestine via SGLT1, GLUT5, and GLUT2 transporters. | Fruit, vegetables, honey, dairy products |
| Fibre | Carbohydrate polymers with at least 10 monomers. | Not hydrolysed by human enzymes. Fermented by bacteria in the large intestine. | Plant-based foods |
| Proteins | High-molecular-weight compounds of amino acids linked by peptide bonds. | Denatured in the stomach by hydrochloric acid. Pepsin and pancreatic enzymes break them into amino acids, dipeptides, and tripeptides in the duodenum. | Animal products (meat, eggs, dairy, seafood, fish) and plant-derived products (legumes, nuts, seeds, cereals, soy). |
| Fats | Triacyl glycerides are composed of glycerol and three fatty acid chains. | Digested in the duodenum with bile and lipases. Form micelles, which are absorbed into enterocytes. | Animal-derived products, red meat, butter, dairy, fish, vegetable oils, nuts |
| Alcoholic beverages | Contain ethanol (C2H5OH) | Absorbed in the stomach and small intestine by passive diffusion. | Obtained from fermentation processes involving yeast or bacteria |
| Effects On | Soluble Dietary Fibre | Insoluble Dietary Fibre |
|---|---|---|
| Intestinal content | Makes more viscous | Increases peristalsis |
| Transit time | Prolongs | Shortens |
| Microbiota | Supports the multiplication of some bacteria; reduces E. coli colonisation | Reduces the proliferation of pathogenic microorganisms |
| Fatty Acids | Microbiota | Inflammation | Permeability |
|---|---|---|---|
| Saturated | ↑ 1 hydrogen sulphide-producing bacteria (Desulfovibrio) ↓ 2 butyrate-producing bacteria (Faecalibacterium) | ↑ | Impairs butyrate oxidation, decreases intestinal barrier resistance. |
| Omega-3 | ↑ beneficial bacteria (Bifidobacteria, Ruminococcus) ↓ harmful bacteria (Enterobacteriaceae) reverses HFD-induced dysbiosis | ↓ | Increases expression of tight junction proteins, protects goblet cells Restores gut permeability |
| Gastrointestinal Organ | Effects of Alcoholic Beverages |
|---|---|
| Oral cavity | Cell membrane disruption, inflammation, reduced saliva production, and partial ethanol absorption |
| Oesophagus | Tissue damage and increased risk of apoptosis or dysplasia |
| Stomach | Reduced mucus secretion and increased tissue vulnerability |
| Intestines | Inflammation, oxidative stress, weakened tight junctions, and gut dysbiosis |
| Liver | Metabolism into toxic acetaldehyde, hepatic steatosis, oxidative stress, and inflammation |
| Nutrients/Beverages | Epithelium Integrity | Inflammation | Permeability | Microbiota |
|---|---|---|---|---|
| Simple carbohydrates | ↓ 1 | ↑ | ↑ | Dysbiosis |
| Fibre | ↑ 2 | ↓ | ↓ | Beneficial effect |
| Saturated fats | ↓ | ↑ | ↑ | Dysbiosis |
| Alcoholic beverages | ↓ | ↑ | ↑ | Dysbiosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vītola, M.E.; Eisāne, R.A.; Iļičuka, S.; Kļaviņa, K.A.; Junga, A.; Pilmane, M. Effects of Nutrients and Alcoholic Beverages on Gastrointestinal Tract Morphology. Gastroenterol. Insights 2025, 16, 42. https://doi.org/10.3390/gastroent16040042
Vītola ME, Eisāne RA, Iļičuka S, Kļaviņa KA, Junga A, Pilmane M. Effects of Nutrients and Alcoholic Beverages on Gastrointestinal Tract Morphology. Gastroenterology Insights. 2025; 16(4):42. https://doi.org/10.3390/gastroent16040042
Chicago/Turabian StyleVītola, Marta Elizabete, Rūta Anna Eisāne, Sofija Iļičuka, Krista Anna Kļaviņa, Anna Junga, and Māra Pilmane. 2025. "Effects of Nutrients and Alcoholic Beverages on Gastrointestinal Tract Morphology" Gastroenterology Insights 16, no. 4: 42. https://doi.org/10.3390/gastroent16040042
APA StyleVītola, M. E., Eisāne, R. A., Iļičuka, S., Kļaviņa, K. A., Junga, A., & Pilmane, M. (2025). Effects of Nutrients and Alcoholic Beverages on Gastrointestinal Tract Morphology. Gastroenterology Insights, 16(4), 42. https://doi.org/10.3390/gastroent16040042









