Perspectives on Mail-Based Fecal Testing for Colorectal Cancer Screening in Bulgaria: A Survey of Gastroenterologists
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Clinical Experience and Screening Perspectives
3.3. Attitudes Toward Personal and Family Use of FOBT
4. Discussion
4.1. Use of FOBT in Clinical Practice
4.2. Colorectal Cancer Screening
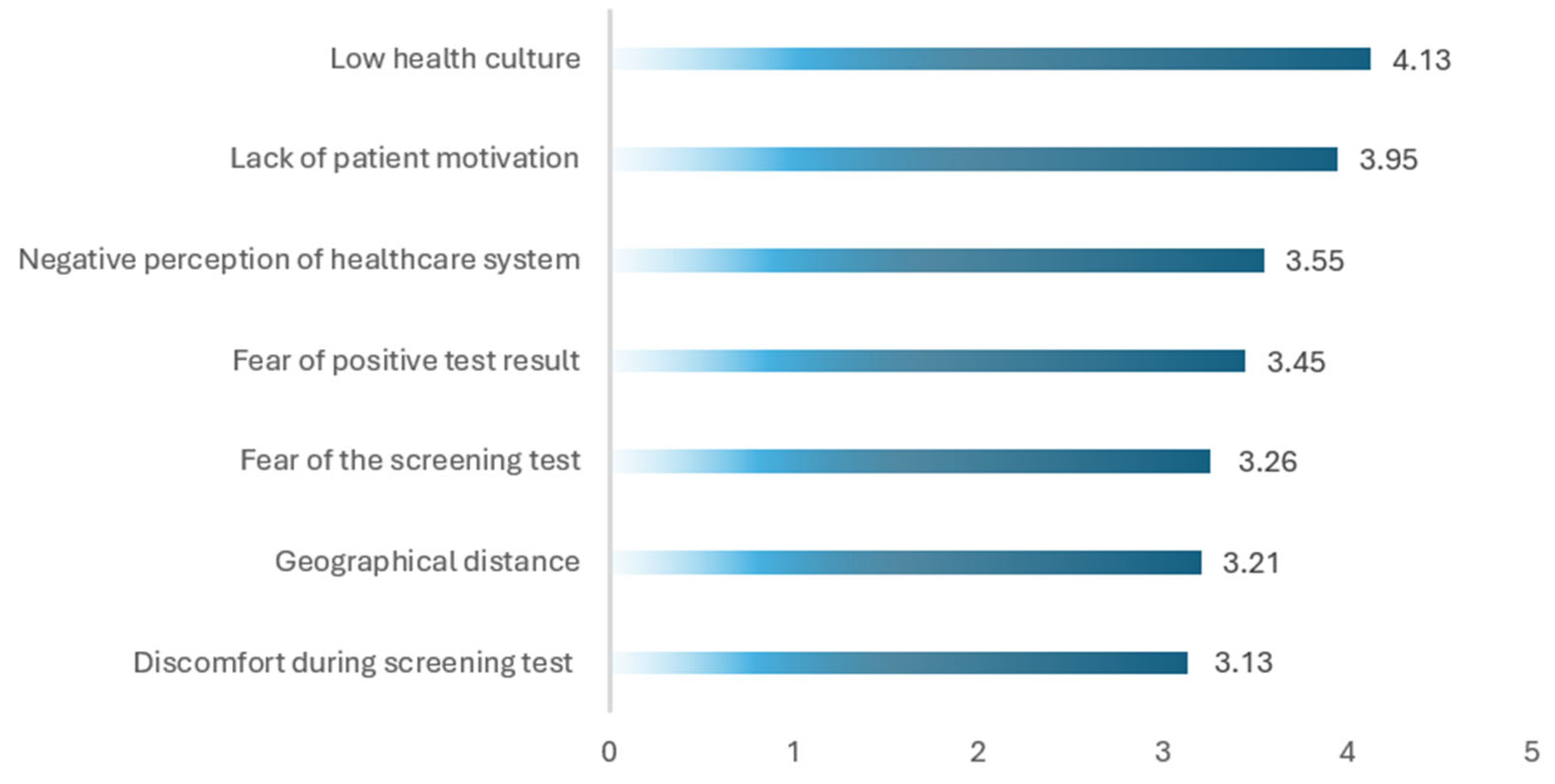
4.3. Barriers to CRC Screening in Bulgaria
4.4. Feasibility of Mail-Based FOBT Distribution
4.5. Lessons from Successful International Mail-Based FOBT Programs
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal Cancer |
| FOBT | Fecal Occult Blood Test |
| FIT | Fecal Immunochemical Test |
| gFOBT | Guaiac-based Fecal Occult Blood Test |
| iFOBT | Immunochemical Fecal Occult Blood Test (alternative term for FIT) |
| NHIF | National Health Insurance Fund |
| ASR | Age-Standardized Incidence Rate |
| ASMR | Age-Standardized Mortality Rate |
| GP | General Practitioner |
| RCT | Randomized Controlled Trial |
| QALY | Quality-Adjusted Life Year |
| ICER | Incremental Cost-Effectiveness Ratio |
| NBCSP | National Bowel Cancer Screening Program |
| NHS | National Health Service |
| IQR | Interquartile Range |
| SD | Standard Deviation |
| RR | Relative Risk |
| CI | Confidence Interval |
| MISCAN | Microsimulation Screening Analysis (Colon Model) |
| Hb | Hemoglobin |
| EU | European Union |
References
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef]
- Roshandel, G.; Ghasemi-Kebria, F.; Malekzadeh, R. Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers 2024, 16, 1530. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.who.int/today (accessed on 25 June 2025).
- Vekic, B.; Dragojevic-Simic, V.; Jakovljevic, M.; Kalezic, M.; Zagorac, Z.; Dragovic, S.; Zivic, R.; Pilipovic, F.; Simic, R.; Jovanovic, D.; et al. Correlation Study of the Colorectal Cancer Statistics and Economic Indicators in Selected Balkan Countries. Front. Public Health 2020, 8, 29. [Google Scholar] [CrossRef]
- Pinheiro, M.; Moreira, D.N.; Ghidini, M. Colon and rectal cancer: An emergent public health problem. World J. Gastroenterol. 2024, 30, 644–651. [Google Scholar] [CrossRef]
- Eng, C.; Jácome, A.A.; Agarwal, R.; Hayat, M.H.; Byndloss, M.X.; Holowatyj, A.N.; Bailey, C.; Lieu, C.H. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 2022, 23, e116–e128. [Google Scholar] [CrossRef]
- Altobelli, E.; Lattanzi, A.; Paduano, R.; Varassi, G.; di Orio, F. Colorectal cancer prevention in Europe: Burden of disease and status of screening programs. Prev. Med. 2014, 62, 132–141. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Gogenur, I.; Qvortrup, C. Colorectal cancer screening in Europe: What are the next steps? Lancet Oncol. 2021, 22, 898–899. [Google Scholar] [CrossRef]
- European Commission. Europe’s Beating Cancer Plan. In Communication From the Commission to the European Parliament and the Council. Available online: https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (accessed on 25 June 2025).
- Ministry of Health, Republic of Bulgaria. National Cancer Control Plan of the Republic of Bulgaria 2027. Adopted by Decision No. 3/04.01.2023. Published January 10, 2023. 2023. Available online: https://www.mh.government.bg/bg/strategii/nacionalni-planove (accessed on 26 June 2025).
- Lachezar Tsotsorkov Foundation. National Pilot Program: Colorectal Cancer Screening in Bulgaria (March–June 2024). Sofia: Tsotsorkov Foundation. 2025. Available online: https://tsotsorkovfoundation.bg/wp-content/uploads/2025/03/National-CRC-screening-campaign-Bulgaria-2024-report.pdf (accessed on 26 June 2025).
- Ministry of Health of Bulgaria. National Colorectal Cancer Screening Program. Sofia: Ministry of Health. 2024. Available online: https://www.mh.government.bg/bg/informaciya-za-grazhdani/skriningovi%20kampanii/main/skrining-colorectalen-rak (accessed on 26 June 2025).
- Emile, S.H.; Barsom, S.H.; Wexner, S.D. An updated review of the methods, guidelines of, and controversies on screening for colorectal cancer. Am. J. Surg. 2022, 224 Pt B, 339–347. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Robertson, D.J.; Senore, C.; Rex, D.K. Optimizing the Quality of Colorectal Cancer Screening Worldwide. Gastroenterology 2020, 158, 404–417. [Google Scholar] [CrossRef]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.; Young, G.P.; Kuipers, E.J. Colorectal cancer screening: A global overview of existing programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef]
- Marijnissen, F.E.; Rijnders, E.E.C.; Tielemans, M.M.; van Noord, D.; Wolters, L.M.M.; Jansen, J.M.; Schot, I.; Bekkering, F.C.; Reijm, A.N.; van Baalen, S.M.; et al. Reducing outpatient visits for FIT-positive participants of colorectal cancer screening programs with home-based digital counselling. NPJ Digit. Med. 2025, 8, 285. [Google Scholar] [CrossRef]
- Massat, N.J.; Dibden, A.; Parmar, D.; Cuzick, J.; Sasieni, P.D.; Duffy, S.W. Impact of Screening on Breast Cancer Mortality: The UK Program 20 Years On. Cancer Epidemiol. Biomark. Prev. 2016, 25, 455–462. [Google Scholar] [CrossRef]
- Ploukou, S.; Birtsou, C.; Gavana, M.; Tsakiridou, K.; Dandoulakis, M.; Symintiridou, D.; Moraiti, E.; Parisis, A.; Smyrnakis, E. General Practitioners’ attitudes and beliefs on barriers to colorectal cancer screening: A qualitative study. Popul. Med. 2023, 5, 7. [Google Scholar] [CrossRef]
- Honein-AbouHaidar, G.N.; Kastner, M.; Vuong, V.; Perrier, L.; Daly, C.; Rabeneck, L.; Straus, S.; Baxter, N.N. Systematic Review and Meta-study Synthesis of Qualitative Studies Evaluating Facilitators and Barriers to Participation in Colorectal Cancer Screening. Cancer Epidemiol. Biomark. Prev. 2016, 25, 907–917. [Google Scholar] [CrossRef]
- Gupta, S.; Coronado, G.D.; Argenbright, K.; Brenner, A.T.; Castañeda, S.F.; Dominitz, J.A.; Green, B.; Issaka, R.B.; Levin, T.R.; Reuland, D.S.; et al. Mailed fecal immunochemical test outreach for colorectal cancer screening: Summary of a Centers for Disease Control and Prevention-sponsored Summit. CA Cancer J. Clin. 2020, 70, 283–298. [Google Scholar] [CrossRef]
- Santare, D.; Kojalo, I.; Huttunen, T.; Rikacovs, S.; Rucevskis, P.; Boka, V.; Leja, M. Improving uptake of screening for colorectal cancer: A study on invitation strategies and different test kit use. Eur. J. Gastroenterol. Hepatol. 2015, 27, 536–543. [Google Scholar] [CrossRef]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 1978–1998. [Google Scholar] [CrossRef]
- Segnan, N.; Armaroli, P.; Bonelli, L.; Patnick, J.; Atkin, W.; Halloran, S.; Malila, N.; Minozzi, S.; Quirke, P.; Steele, R.J.; et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Endoscopy 2013, 45, 51–59. [Google Scholar] [CrossRef]
- Robertson, D.J.; Lee, J.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Lieberman, D.; Levin, T.R.; Rex, D.K. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1217–1237.e3. [Google Scholar] [CrossRef]
- Alzoubi, M.M.; Al-Ghabeesh, S.H. Knowledge, Attitude, Practice, and Perceived Barriers Regarding Colorectal Cancer Screening Practices Among Healthcare Practitioners: A Systematic Review. Cureus 2024, 16, e54381. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Klabunde, C.N.; Yuan, G.; McNeel, T.S.; Brown, M.L.; Casciotti, D.; Buckman, D.W.; Taplin, S. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J. Gen. Int. Med. 2011, 26, 177–184. [Google Scholar] [CrossRef]
- Kolligs, F.T. Diagnostics and Epidemiology of Colorectal Cancer. Visc. Med. 2016, 32, 158–164. [Google Scholar] [CrossRef]
- Franco, D.L.; Leighton, J.A.; Gurudu, S.R. Approach to Incomplete Colonoscopy: New Techniques and Technologies. Gastroenterol. Hepatol. 2017, 13, 476–483. [Google Scholar] [PubMed]
- Jain, S.; Maque, J.; Galoosian, A.; Osuna-Garcia, A.; May, F.P. Optimal Strategies for Colorectal Cancer Screening. Curr. Treat Options Oncol. 2022, 23, 474–493. [Google Scholar] [CrossRef]
- Latos, W.; Aebisher, D.; Latos, M.; Krupka-Olek, M.; Dynarowicz, K.; Chodurek, E.; Cieślar, G.; Kawczyk-Krupka, A. Colonoscopy: Preparation and Potential Complications. Diagnostics 2022, 12, 747. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Colonoscopic perforation: Incidence, risk factors, management and outcome. World J. Gastroenterol. 2010, 16, 425–430. [Google Scholar] [CrossRef]
- Wernli, K.J.; Brenner, A.T.; Rutter, C.M.; Inadomi, J.M. Risks Associated With Anesthesia Services During Colonoscopy. Gastroenterology 2016, 150, 888–894, quiz e18. [Google Scholar] [CrossRef]
- Waddingham, W.; Kamran, U.; Kumar, B.; Trudgill, N.J.; Tsiamoulos, Z.P.; Banks, M. Complications of colonoscopy: Common and rare—Recognition, assessment and management. BMJ Open Gastroenterol. 2023, 10, e001193. [Google Scholar] [CrossRef]
- Bailey, S.E.R.; Abel, G.A.; Atkins, A.; Byford, R.; Davies, S.-J.; Mays, J.; McDonald, T.J.; Miller, J.; Neck, C.; Renninson, J.; et al. Diagnostic performance of a faecal immunochemical test for patients with low-risk symptoms of colorectal cancer in primary care: An evaluation in the South West of England. Br. J. Cancer 2021, 124, 1231–1236. [Google Scholar] [CrossRef]
- OECD. EU Country Cancer Profile: Bulgaria 2023. In EU Country Cancer Profiles; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Landy, R.; Pesola, F.; Castañón, A.; Sasieni, P. Impact of cervical screening on cervical cancer mortality: Estimation using stage-specific results from a nested case-control study. Br. J. Cancer 2016, 115, 1140–1146. [Google Scholar] [CrossRef]
- Council Recommendation of 9 December 2022 on Strengthening Prevention Through Early Detection: Anew EU Approach on Cancer Screening Replacing Council Recommendation, 2003/878/EC. Official Journal of the European Union, C 473. 13 December 2022, pp. 1–9. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.C_.2022.473.01.0001.01.ENG (accessed on 1 July 2025).
- Jager, M.; Demb, J.; Asghar, A.; Selby, K.; Mello, E.M.; Heskett, K.M.; Lieberman, A.J.; Geng, Z.; Bharti, B.; Singh, S.; et al. Mailed Outreach Is Superior to Usual Care Alone for Colorectal Cancer Screening in the USA: A Systematic Review and Meta-analysis. Dig. Dis. Sci. 2019, 64, 2489–2496. [Google Scholar] [CrossRef]
- Goodwin, B.C.; Ireland, M.J.; March, S.; Myers, L.; Crawford-Williams, F.; Chambers, S.K.; Aitken, J.F.; Dunn, J. Strategies for increasing participation in mail-out colorectal cancer screening programs: A systematic review and meta-analysis. Syst. Rev. 2019, 8, 257. [Google Scholar] [CrossRef]
- Peterse, E.F.P.; Osoro, C.B.; Bardou, M.; Lansdorp-Vogelaar, I. Comparative benefit and cost-effectiveness of mailed-out faecal immunochemical tests vs collection at the general practitioner. Aliment. Pharmacol. Ther. 2021, 53, 1118–1125. [Google Scholar] [CrossRef]
- UK Health Security Agency. Bowel Cancer Screening Standards Data Report 2023 to 2024. London: Department of Health and Social Care. 25 Feb 2025. Available online: https://www.gov.uk/government/publications/bowel-cancer-screening-annual-report-2023-to-2024/bowel-cancer-screening-standards-data-report-2023-24 (accessed on 2 July 2025).
- Strachan, H.; Maclean, B. Wealth of Evidence Highlights the Benefits of Bowel Cancer Screening. UK National Screening Committee Blog. 11 April 2025. Available online: https://nationalscreening.blog.gov.uk/2025/04/11/wealth-of-evidence-highlights-the-benefits-of-bowel-cancer-screening/ (accessed on 2 July 2025).
- He, E.; Lew, J.B.; Egger, S.; Banks, E.; Ward, R.L.; Beral, V.; Canfell, K. Factors associated with participation in colorectal cancer screening in Australia: Results from the 45 and Up Study cohort. Prev. Med. 2018, 106, 185–193. [Google Scholar] [CrossRef]
- Hoffmeister, M. Interim evaluation of the colorectal cancer screening programme in the Netherlands. Lancet Gastroenterol. Hepatol. 2022, 7, 8–9. [Google Scholar] [CrossRef]
- Therkildsen, S.B.; Larsen, P.T.; Njor, S.H. Screening participants with inflammatory bowel disease or high colorectal cancer risk in Denmark: A cohort study. J. Public Health Policy 2024, 45, 727–739. [Google Scholar] [CrossRef]
- Danish Cancer Society. Colorectal Cancer Screening. Retrieved 2 June 2025. Available online: https://www.cancer.dk/forebyg-kraeft/screening/tarmkraeft/in-english/ (accessed on 3 July 2025).
- Olesen, T.B.; Jensen, H.; Møller, H.; Jensen, J.W.; Andersen, B.; Rasmussen, M. Nationwide participation in FIT-based colorectal cancer screening in Denmark during the COVID-19 pandemic: An observational study. Elife 2023, 12, e81808. [Google Scholar] [CrossRef]
- Carethers, J.M. Improving Noninvasive Colorectal Cancer Screening. N. Engl. J. Med. 2024, 390, 1045–1046. [Google Scholar] [CrossRef]
- NHS England. Bowel Cancer Screening. London: NHS. Available online: https://www.nhs.uk/conditions/bowel-cancer-screening/ (accessed on 4 July 2025).
- European Commission Initiative on Colorectal Cancer (ECICC). European Guidelines on Colorectal Cancer Screening and Diagnosis. Ispra: European Commission, Joint Research Centre. Available online: https://cancer-screening-and-care.jrc.ec.europa.eu/en/ecicc/european-colorectal-cancer-guidelines (accessed on 4 July 2025).
- Anderson, L.E.; Myers, L.; Collins, K.; Vicario, J.; Viljoen, B.; Ireland, M.J.; Goodwin, B.C. Co-designing planning interventions to facilitate participation in mail-out bowel cancer screening. BMC Public Health 2024, 24, 2418. [Google Scholar] [CrossRef]
- Robb, K.A.; Young, B.; Murphy, M.K.; Duklas, P.; McConnachie, A.; Hollands, G.J.; McCowan, C.; Macdonald, S.; O’Carroll, R.E.; O’Connor, R.C.; et al. Behavioural interventions to increase uptake of FIT colorectal screening in Scotland (TEMPO): A nationwide, eight-arm, factorial, randomised controlled trial. Lancet 2025, 405, 1081–1092. [Google Scholar] [CrossRef]

| Category | Variable | Value |
|---|---|---|
| Sex Distribution | Male | 21 (55.26%) |
| Female | 17 (44.74%) | |
| Age (years) | Mean (SD) | 36.45 (8.99) |
| Median | 34.50 | |
| Interquartile Range (IQR) | 30.75–40.25 | |
| Years of Professional Experience | Mean (SD) | 11.04 (9.10) |
| Median | 8.50 | |
| Interquartile Range (IQR) | 5.00–13.25 | |
| Practice Setting | Hospital-based practice | 38 (100.00%) |
| Concurrent outpatient practice | 15 (39.47%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, K.Y.; Velchev, V.; Danailova, N.; Staneva, E.; Koparanov, T.; Diankov, T.; Gencheva, T.; Valkov, B.; Hristova-Atanasova, E.; Iskrov, G.; et al. Perspectives on Mail-Based Fecal Testing for Colorectal Cancer Screening in Bulgaria: A Survey of Gastroenterologists. Gastroenterol. Insights 2025, 16, 25. https://doi.org/10.3390/gastroent16030025
Dimitrov KY, Velchev V, Danailova N, Staneva E, Koparanov T, Diankov T, Gencheva T, Valkov B, Hristova-Atanasova E, Iskrov G, et al. Perspectives on Mail-Based Fecal Testing for Colorectal Cancer Screening in Bulgaria: A Survey of Gastroenterologists. Gastroenterology Insights. 2025; 16(3):25. https://doi.org/10.3390/gastroent16030025
Chicago/Turabian StyleDimitrov, Kostadin Yordanov, Vladislav Velchev, Nely Danailova, Elena Staneva, Teodor Koparanov, Trifon Diankov, Teodora Gencheva, Bozhidar Valkov, Eleonora Hristova-Atanasova, Georgi Iskrov, and et al. 2025. "Perspectives on Mail-Based Fecal Testing for Colorectal Cancer Screening in Bulgaria: A Survey of Gastroenterologists" Gastroenterology Insights 16, no. 3: 25. https://doi.org/10.3390/gastroent16030025
APA StyleDimitrov, K. Y., Velchev, V., Danailova, N., Staneva, E., Koparanov, T., Diankov, T., Gencheva, T., Valkov, B., Hristova-Atanasova, E., Iskrov, G., & Stefanov, R. (2025). Perspectives on Mail-Based Fecal Testing for Colorectal Cancer Screening in Bulgaria: A Survey of Gastroenterologists. Gastroenterology Insights, 16(3), 25. https://doi.org/10.3390/gastroent16030025







