Abstract
Introduction: Autism spectrum disorder (ASD) comprises a group of developmental disorders characterized by deficits in social interaction and behavioral patterns. Children with ASD may face nutritional challenges, primarily due to their restrictive behaviors and frequent gastrointestinal issues. Objective: The objective of the present study was to assess nutritional status, dietary habits, and intestinal permeability in a sample of individuals with ASD. Methods: A cross-sectional study was conducted with 24 children and adolescents (both sexes), aged 4 to 18 years, living in two cities of Mexico (Aguascalientes and Querétaro). Weight and height were measured, and body mass index for age was calculated and compared using WHO Z-scores. Diet was assessed through three 24 h dietary recalls and a food frequency questionnaire. Intestinal permeability was evaluated using a lactulose/mannitol test by HPLC. Results: A high prevalence of malnutrition was observed; 12.5% of the participants were underweight, and 45.8% were overweight or obese. Children had a low intake of fiber, vitamin E, folate, potassium, zinc, and phosphorus and a high intake of sodium. On average, the intestinal permeability ratio was 0.09 ± 0.05, with 54.2% of the children exhibiting high intestinal permeability. Conclusions: It is advisable to develop food counseling strategies for children with ASD to prevent micronutrient deficiencies, promote healthy weight, and improve gastrointestinal integrity.
1. Introduction
Autism spectrum disorder (ASD) is a group of neurodevelopmental disabilities that affect communication and social interaction and are characterized by restrictive, repetitive, and stereotyped behavior patterns [1]. According to the Centers for Disease Control and Prevention (CDC), the prevalence of ASD in the United States is 1 in 36 children [2], while in Mexico, 1 case in 115 children has been reported [3]. México has similar prevalences to those reported globally, with an estimated rate of 1 in 100 children [4].
Many children with ASD face nutritional challenges, primarily because of restrictive behaviors applied to food, such as rejection or refusal to accept new foods [5,6]. Consequently, they have a lower intake of fruits and vegetables and an increased consumption of high-energy, dense foods, with deterioration in their nutritional status [7].
Additionally, children with ASD commonly experience gastrointestinal disorders (GDs) [8]. GDs can affect overall nutritional status and are associated with altered microbiota composition [9]. It has been suggested that GDs in children with ASD are associated with autism severity [10,11].
The interaction between the intestine and the brain has been called the “gut-brain axis” and can be modified by increased intestinal permeability, known as leaky gut [12]. Previous studies have reported that children with ASD have a higher prevalence of leaky gut than healthy children, allowing digested products to pass through [13,14,15]. The risk factors for intestinal permeability have been related to alterations in gut microbiota, food allergies, inflammatory bowel diseases, and infectious diarrhea [16,17,18,19].
As previously mentioned, ASD has been associated with dietary challenges and a higher prevalence of GDs, which can be linked to nutritional risks or vice versa. The objective of the present study was to assess the nutritional status, dietary habits, and intestinal permeability of Mexican children and adolescents with ASD. To our knowledge, this is the first trial to investigate the nutrition and gastrointestinal function of Mexican children with ASD.
2. Materials and Methods
2.1. Study Design and Sample
A cross-sectional study was carried out in 24 ASD children and adolescents, 21 boys (87.5%) and 3 girls (12.5%), aged 4 to 18 years (average, 11.13 ± 4.05 y). The mean age at which they were diagnosed was 4.40 ± 3.46 years.
The inclusion criteria for the sample were (1) a previous diagnosis of ASD issued by qualified personnel such as a pediatrician, psychologist, or psychiatrist, according to the criteria of the DSM-5 [1]; (2) an age range from 4 to 18 years; (3) no history of gastrointestinal diseases in the past 2 weeks or chronic gastrointestinal problems, such as inflammatory bowel disease or irritable bowel syndrome; (4) voluntary participation; and (5) signing the informed consent letter. The exclusion criteria were incomplete records or refusal of the child or family to continue in the study.
Subjects were recruited from two educational centers in different Mexican cities that provide special therapies for people with ASD or give support to their parents. One institution was the Autism Aguascalientes Association, a non-governmental organization for supporting ASD children and their families, located in the city of Aguascalientes (79.8%, n = 15). The other center was Ana Cristina Juárez Diez Marina Institution, a private school that provides attention to children and young people with ASD in the city of Querétaro (20.8%, n = 9). Table 1 shows the subjects’ characteristics in each center.

Table 1.
Psychopharmacotherapy in children and adolescents with ASD.
2.2. Medical History Assessment
The child’s medical history was gathered from the parents’ interview, including information about the family history of diseases and mental health, the child’s history of illnesses and allergies, the vaccination schedule, and any previous or current pharmacological treatments. Additionally, the parents were asked if the child had been sick in the last 2 weeks and about his or her treatment.
2.3. Anthropometric Measurement
Participants in the two educational centers were asked to attend early in the morning after an overnight fasting and wearing light clothes. Measurements of weight and height were performed two consecutive times by a trained nutritionist following procedures described previously [20]. Weight was measured on a bascule TANITA model UM-061, and height was measured with a Seca 213 model stadiometer. Z-scores were calculated for weight-for-age (W/A), height-for-age (H/A), and body mass index-for-age (BMI/A) using WHO AnthroPlus software version 1.0.4 and WHO child growth standards for children aged 0–5 years and WHO growth standard for children and adolescents aged 5–19 years [21]. Z-scores below −2 for W/A, H/A, and BMI/A indicated underweight, short height, and underweight, respectively. In children under 5 years of age, Z scores greater than +2 for W/A were defined as overweight, and Z scores greater than +3 were defined as obesity. At the same time, in children aged 5 to 18 years, Z scores above +1 for BMI/A indicated overweight, and Z scores greater than +2 indicated obesity. For H/A, Z scores greater than +2 indicated above-average height.
2.4. Diet Evaluation
To find habitual children’s and adolescents’ diets, the 24-h dietary recall (24hR) was applied by a trained nutritionist in face-to-face interviews with participants’ mothers, using home measures and the protocol of 3 days on non-consecutive days, two on weekdays, and one on a weekend day. The consumption of macro- and micronutrients was calculated using the USDA Food Composition Databases [22] and Mexican Food Equivalent System [23]. Diet evaluation results were compared with dietary reference intakes (DRIs) for the Mexican population [24] according to the age group (4 to 8 y, 9 to 13 y, and 14 to 18 y) to obtain the percentage of adequacy as follows:
Adequacy percentage = [(micronutrients consumption/DRIs) × 100].
Deficient consumption was considered if the adequacy percentage was lower than 90%; if the adequacy percentage was between 90 and 110%, then consumption was considered adequate; elevated consumption was considered when the adequacy rate was more than 110%.
A trained nutritionist evaluated the children’s habitual diet using a food frequency questionnaire (FFQ) that had been previously validated among parents of school-age Mexican children [25]. The questionnaire consisted of 150 items of local foods divided into nine categories: vegetables (24 items); fruits (23 items); legumes (5 items); dairy (27 items); animal source foods, including meat, poultry, and fish (15 items); cured meats (5 items); cereals (25 items); fats (7 items); and sugars and beverages with added sugars (19 items). The mothers reported the number of times per day/week/month/year that food was consumed by their children. Additionally, weighted food consumption was estimated using the average consumption reported in the 24hR, which was subsequently multiplied by the frequency of food consumption reported in the FFQ for the most consumed foods [26].
2.5. Intestinal Permeability Evaluation
Intestinal permeability was measured through a challenge test with lactulose/mannitol (L/M) ratio and subsequent urine collection for 5 h. After an overnight fast of at least 8 h, each participant was given mannitol and lactulose. Children under 12 years ingested 50 mL of a solution containing 2 g of mannitol and 5 g of lactulose solution dissolved in water. Those over 12 years consumed 100 mL of a solution with 5 g of mannitol and 10 g of lactulose in water. All subjects remained fasted for the next 2 h after taking the lactulose/mannitol solution. Urine was collected for 5 h after taking the solution. Urine volume was measured, and a 100 mL aliquot was added with 1 mL of chlorhexidine and stored at −20 °C until analysis.
The concentration of lactulose and mannitol in urine samples was measured by high-performance liquid chromatography (HPLC) according to the technique described by Miki et al. [27]. To determine if participants had abnormal intestinal permeability, subjects with a lactulose/mannitol ratio ≥ 0.03 were considered to have high intestinal permeability [14,27].
2.6. Statistical Analyses
All the analyses were conducted using SPSS software, version 26.0 for Windows. The quantitative variables are shown in frequencies, percentages, and mean ± standard deviation or confidence interval. For quantitative analyses, Shapiro–Wilk and Levene’s tests were used to evaluate the data’s normal distribution and homogeneity of variance, respectively. Descriptive analyses were performed to define participants’ characteristics by city, and comparisons between groups were made with the Student t-test or Mann–Whitney U-test.
Two quantitative analyses were performed for 24hR: (1) Comparisons of total energy intake, macronutrients, and fiber between age groups were made with ANOVA test. (2) The mean intake of fiber, vitamins, and minerals was compared with DRIs using a one-sample Student t-test. A qualitative analysis of the reported dietary data from 24hR was also performed to describe a typical diet in the sample.
For the FFQ analyses, the frequency of consumption of all foods belonging to a category was summed up, and the percentage of each category from the total intake was calculated. The proportions of each food group’s intake were compared with ANOVA test or Kruskal–Wallis for BMI/age.
Finally, the intestinal permeability was compared between age groups by ANOVA test. A significant p-value was considered when <0.05.
3. Results
The screened sample consisted of 29 participants, of whom 5 were excluded: 1 did not meet the age range, 2 chose not to continue in the study, and 2 were unable to complete any of the measurements.
3.1. Medical History
In the initial medical history, it was noted that 9 of the 24 evaluated children were taking some form of psychopharmacotherapy, with risperidone, an atypical antipsychotic, being the most prevalent. Other psychotropic medications included antidepressants, anticonvulsants, and psychostimulants; additionally, 2 children were also taking atomoxetine, a selective norepinephrine reuptake inhibitor. Four children were taking 2 or more psychoactive drugs (Table 1).
Four children with food allergies were identified (16.7%). The reported allergies were as follows: one child had an allergy to fish (specifically tuna), another had an allergy to shellfish, and two had an allergy to strawberries. One of these children also had allergies to walnuts and almonds.
Regarding illnesses experienced in the last 2 weeks prior to the start of the study, six children reported having had a respiratory illness (25%), such as the flu (n = 4, 16.7%) and throat infection (n = 2, 8.3%). Additionally, one child experienced a mild food allergic reaction due to cross-contamination (tuna). The treatments used included anti-inflammatories, antihistamines, mucolytics, and antibiotics in the case of bacterial infection. All treatments for respiratory illness were discontinued at least one week before the study evaluation began.
3.2. Anthropometric Evaluation
All children had adequate height-for-age (H/A), and 8.3% were taller than the mean of the Z-score from the WHO growth charts. Because of their age, only 11 subjects were candidates for weight-for-age (W/A) evaluation; 72.7% had adequate weight-for-age, while 27.3% were overweight. High BMI for age was observed in 45.8% of participants, of which 20.8% (n = 5) were overweight, and 25% (n = 6) had obesity. Meanwhile, 12.5% (n = 3) were underweight. When stratified for age group, 22.2% (n = 2) of 4-to-8-year-old children were overweight, and 33.3% (n = 3) were obese; in 9-to-13-year-old children, 33.3% (n = 3) were overweight and 33.3% (n = 3) were obese; finally, among the 14-to-18-year-old adolescents, 50% of them were underweight (Figure 1).
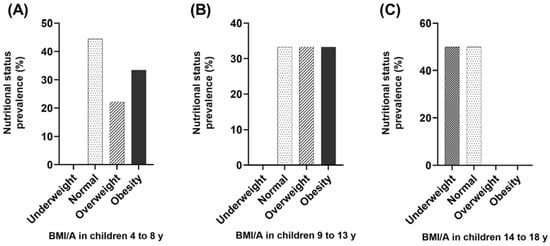
Figure 1.
Evaluation of body mass index-for-age (BMI/A) indicator in children and adolescents with ASD (Z-scores interpretation). (A) BMI/A in 4-to-8-year-old children (n = 9); (B) BMI/A in 9-to-13-year-old children (n = 9); (C) BMI/A in 14-to-18-year-old children (n = 6).
3.3. Dietary Intake
According to the 24hR, the total energy intake (TEI) distribution of macronutrients was 34.9% lipids, 49.3% carbohydrates, and 15.8% proteins. Dietary intake was compared between children from Querétaro and Aguascalientes, and there were no significant differences between diets reported in both cities (Table 2).

Table 2.
Descriptive characteristics of the study population.
Total energy and macronutrient intake were stratified by age group. According to the dietary guidelines, children aged 4 to 8 had a better macronutrient distribution (carbohydrates 55.8%, proteins 15.1%, and lipids 29.1%); as age increases, children consume more energy coming lipids (39.2% in 9-to-13-year-olds and 36.7% in 14-to-18-year-olds) and less carbohydrates (43.8% and 48.7%, respectively); the mean distribution of TEI and macronutrients can be observed in Figure 2.
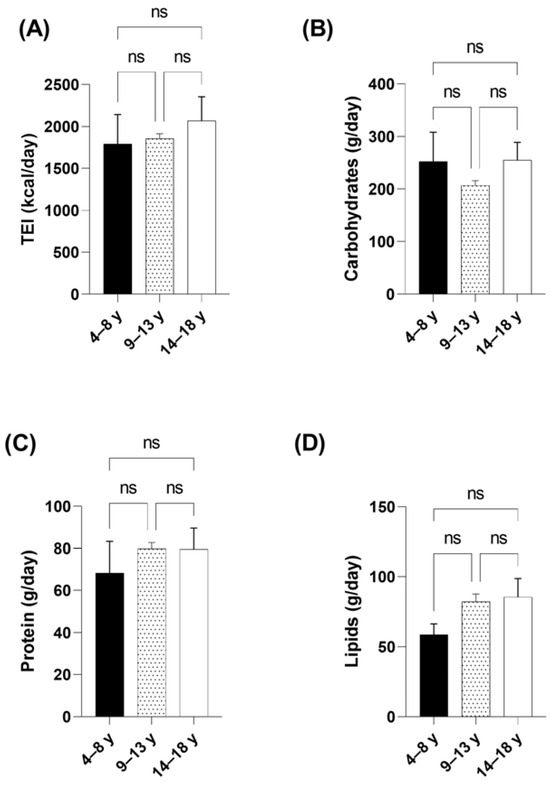
Figure 2.
Comparison of energy and macronutrient intake stratified by age group in children and adolescents with ASD. (A) Comparison of total energy intake (TEI) by age group. (B) Comparison of carbohydrate intake by age groups. (C) Comparison of protein intake by age groups. (D) Comparison of lipid intake by age groups. Data are presented as mean and SEM. Statistical test used: ANOVA, significance at p < 0.05. ns: not significant.
The mean cholesterol intake was 348.0 ± 148.5 mg/d; when stratified for age, cholesterol intake was 253.9 ± 145.4 mg/d in 4-to 8-year-old children, 408.6 ± 140.6 mg/d in 9-to-13-year-old children, while in 14-to-18-year-old adolescents cholesterol intake was 398.2 ± 102.0 mg/d. The mean saturated fat intake was 24.76 ± 11 g/d (11.65% of the total energy intake). It a significantly low consumption of fiber was observed, 13.38 ± 5 g/d based on the DRIs, and this trend remained when the data were stratified by age group (Table 3).

Table 3.
Comparison of fiber intake (g) with dietary reference intake (DRI) for Mexican population in ASD children and adolescents (n = 24).
Regarding micronutrient intake, all participants’ dietary reports indicated a deficient intake of potassium and vitamin E. Specifically, children aged 4 to 13 showed a low intake of phosphorus; children and adolescents aged 9 to 18 exhibited a low consumption of folate, zinc, and iron; adolescents had a low consumption of magnesium, showing more deficiencies as they became older. Sodium was the only mineral consumed above the recommended levels in all age groups, with significantly higher intake noted in the 9 to 13-year-old group.
It is important to note that none of the children reported taking dietary supplements. Table 4 and Table 5 present the percentage adequacy and comparisons of micronutrient intake against RDI, stratified by age group.

Table 4.
Comparison of vitamin ingestion with dietary reference intakes for Mexican population in ASD children and adolescents.

Table 5.
Comparison of mineral ingestion with the dietary reference intakes for the Mexican population in ASD children and adolescents.
According to the FFQ, the three main food groups consumed by children were as follows: (1) cereals, representing 20.8% of the total intake, including tortillas (316.9 g/week), breakfast cereals (25.0 g/week), and cookies (57.9 g/week); (2) dairy products, representing 14.8%, which included milk (3362.5 mL/week), yogurt (273.8 g/week), and Mexican melting cheese (78.0 g/week); and (3) fruits, representing 14.4%, such as apples (175.9 g/week), bananas (166.7 g/week), and lemons (16.0 g/week). Additionally, the proportions of each food group’s intake were compared among BMI/age classifications. Children with undernutrition reported a frequent ingestion of cereals, fats, and dairy foods, while children with healthy weight consumed more cereals, vegetables, and dairy. Overweight children preferred cereals, fats, and fruits, and obese children reported a higher consumption of cereals, fruits, and sugars. It is noteworthy that children with malnutrition and overweight reported significantly more frequent consumption of fats. The comparisons between proportions of food groups ingestion per BMI/age are presented in Table 6.

Table 6.
Proportions of the frequency food groups consumption by BMI/age in children and adolescents with ASD.
3.4. Intestinal Permeability
The intestinal permeability test showed that 13 out of the 24 children evaluated (54.2%) had increased intestinal permeability, with mannitol recovery of 268.11 ± 118.69 mg. The lactulose recovery was 23.91 ± 14.78 mg, and the lactulose/mannitol ratio (L/M ratio) was 0.09 ± 0.05. The remaining children evaluated did not show detectable lactulose recovery, and the mean mannitol recovery was 156.43 ± 73.40 mg, with an L/M ratio equal to zero.
When stratified by age group, children aged 9 to 13 years had the highest urine recovery of both sugars. The mean mannitol recovery was 259.04 ± 133.61 mg and lactulose recovery was 18.81 ± 18.06 mg, with no significant differences compared to other age groups (see Table 7). Similarly, when comparing recruitment cities, children from Querétaro had higher urine recoveries of mannitol (262.7 mg vs. 189.43 mg) and lactulose (15.88 mg vs. 11.2 mg) compared to children from Aguascalientes. However, these differences were not statistically significant. Also, there was no significant correlation between intestinal permeability and anthropometric measurements.

Table 7.
Evaluation of intestinal permeability in children and adolescents with autism spectrum disorder stratified by age group.
4. Discussion
Previous research has reported that children with ASD often have a high prevalence of overweight [28,29]. Our results in Mexican children with ASD confirm this finding, as nearly 50% of the sample was overweight or obese. Similar findings were reported in the Pediatric Psychopharmacology Autism Network, where the combined prevalence of overweight and obesity exceeded 60% [30]. Other studies have suggested that factors such as inadequate physical activity [31], the use of anxiolytic drugs [32], and the preference for foods high in fats and simple sugars [33] could contribute to weight gain in this population.
In our study sample, we observed a high consumption of sweetened beverages such as homemade “agua de fruta” (diluted fruit juice with added sugar), sodas, and industrialized juices among obese children. Additionally, all children evaluated showed a deficient intake of fiber, and lipid intake exceeded 30% of total energy intake in most diets. We also found that 3 children (12.5% of the sample) were underweight, possibly due to specific picky eating patterns or sensory sensitivities that predisposed them to restricted food intake [34].
Based on dietary reports, children in the sample exhibited a deficient intake of several micronutrients such as vitamin E, folic acid, potassium, zinc, and phosphorus. Vitamin C intake was notably lower among older children, although it still met the RDI. Previous studies have also documented inadequate intake of calcium, potassium, iron, vitamins A, D, E, B6, folate, and vitamin C in children with ASD [29,35,36]. A potential explanation for the insufficient intake of certain micronutrients could be the restrictive and repetitive behaviors associated with food consumption [35].
Additionally, it is common for children with ASD to avoid food groups, particularly fruits and vegetables, and prefer processed foods high in fat or simple sugars, resulting in low fiber intake [33]. Analysis of the children´s diet revealed that 50% reported consuming deep-fried foods in at least one 24hR period. Furthermore, consumption of fruits and vegetables was lower compared to cereals and sugar drinks, contributing to low fiber intake in our sample. Few children also consumed oilseeds such as walnuts, almonds, and peanuts.
In ASD, restrictive behaviors often influence food selection based on flavor, texture, color, or temperature, complicating the recognition of hunger, prolonging mealtimes, and leading to inadequate or improper food consumption [37]. A meta-analysis indicated that children with ASD are five times more likely to experience feeding problems [38]. Consequently, mothers frequently attempt to accommodate children´s food preferences to manage their disabilities or avoid tantrums [39].
Our study of Mexican children with ASD suggests that caregivers should closely monitor the intake of vitamins A, E, and C as well as minerals like iron, zinc, and magnesium. The potential benefits of supplementing these specific nutrients in children with ASD should be evaluated in controlled clinical trials.
One of the most reported nutrient deficiencies in ASD is calcium and vitamin D [35,36]. However, this deficiency was not observed in our population, which may be explained by the high intake of corn tortillas, milk, and dairy products among the sample, and the fact that none of the children or adolescents followed a casein-free diet. Corn tortillas, one of the most consumed foods, are processed with calcium during the liming process, making them a good source of calcium with good bioavailability [40]. Furthermore, vitamin D can be synthesized through the skin via sunlight exposure [41]. Due to Mexico´s geographic location, there is sufficient sunlight throughout the year, which facilitates the synthesis of this vitamin [42].
High intestinal permeability in children with ASD has been proposed as a primary cause of gastrointestinal disturbances linked to a certain dietary intake [43] and higher infection rates [44]. In our study, 13 children (54.2% of the sample) exhibited increased intestinal permeability based on criteria established by the lactulose/mannitol test. This test involves a sugar challenge followed by measuring sugar excretion in urine [45], it and was chosen for its non-invasive nature. Sugars are used in this test because they have different molecular weights and absorption pathways, low affinity for the monosaccharide transport system, passive absorption, and are excreted intact in urine once in general circulation [46].
D’Eufemia et al. (1996) were the first to compare intestinal permeability between children with ASD and neurotypical children, finding a higher prevalence of intestinal permeability in the ASD group [13]. Subsequent studies have reported similar findings. Iovene et al. (2016) analyzed intestinal permeability using the lactulose/mannitol ratio and reported increased intestinal permeability in ASD children with co-occurring gastrointestinal issues such as constipation or alternating bowel habits (constipation/diarrhea) compared to healthy children (38.8% vs. 18.8%, respectively), with these results associated with intestinal dysbiosis [15].
Fiorentino et al. (2016) examined postmortem duodenal biopsies from controls (n = 9) and ASD subjects (n = 12), revealing that 75% of the ASD samples had a reduced expression of tight junction components in the intestinal barrier (claudin-1, occludin, and tricellulin), while 66% showed increased levels of pore-forming proteins (claudin-2, -10, -15) compared to controls [47].
In contrast, other authors have reported no differences in intestinal permeability in ASD samples. Robertson et al. (2008) compared the lactulose/mannitol permeability test among 14 ASD children, 7 developmentally normal siblings, and 8 healthy unrelated children, finding no differences in the lactulose/mannitol ratio of urine excretion [48]. Another study compared intestinal permeability in ASD children following a gluten and casein-free diet versus those without dietary restrictions and found no differences in intestinal permeability between the two groups [49].
The inconsistency in intestinal permeability results among ASD subjects in previous studies could be associated with a subgroup of ASD individuals who are more susceptible to dysbiosis, gastrointestinal issues, and leaky gut. Conversely, there is a need to develop standardized techniques for evaluating intestinal permeability, including establishing uniform cutoff criteria for diagnosis and standardized solutions of lactulose/mannitol concentrations, as these currently vary among different studies [14,15,49,50,51,52]. In our study, we defined abnormal intestinal permeability using a lactulose/mannitol ratio ≥ 0.03, as previously reported [14,27], because this value is widely documented in the literature.
Among the study’s limitations, it is noteworthy that the sample size was small, partly due to the limited number of institutions providing care to children and adolescents with ASD in Mexico. Additionally, there is no national institution in which all cases of ASD in the country are registered. Conducting a national census would be advisable to determine the true prevalence of this condition in Mexico.
Another limitation is that the subject’s diets were evaluated based on maternal reports, which may lead to underreporting since these children may consume other foods during school hours or at home. To mitigate this, mothers and teachers were instructed to monitor the food intake of children and adolescents with ASD throughout the day.
Finally, we did not document whether the children had a history of frequent gastrointestinal problems prior to the start of the study. It would be valuable to assess whether children or adolescents with such histories exhibited increased intestinal permeability or if specific diets, such as those avoiding exorphins, are associated with gut health.
5. Conclusions
In this study, we found that ASD participants have inadequate diets. They exhibited a high fat and sodium intake, and a low intake of complex carbohydrates, fiber, and several micronutrients, such as vitamin E, folate, potassium, iron, zinc, and phosphorus. Obesity and overweight were present in 45.8% of the children. Additionally, intestinal permeability was observed in 54.2% of our sample of Mexican children with ASD.
It is strongly recommended that food counseling strategies be developed for children with ASD. This study will contribute to the development of programs aimed at preventing overweight and obesity in the ASD population and identifying restrictive eating behaviors that may impact patients’ nutritional status. Guiding parents on proper feeding practices is crucial as it can help prevent the onset of other comorbidities and enhance the quality of life for this population.
Author Contributions
Conceptualization, M.A.A.-L. and J.L.R.; methodology, K.A.P.-G., D.R., D.M. and J.P.-D.; formal analysis, M.d.C.C. and K.A.P.-G.; investigation, K.A.P.-G., D.R., D.M. and J.P.-D.; data curation, M.d.C.C.; writing—original draft preparation, K.A.P.-G.; writing—review and editing, M.d.C.C., M.A.A.-L. and J.L.R.; supervision, M.A.A.-L. and D.R.; project administration, M.A.A.-L. and J.L.R.; funding acquisition, M.A.A.-L. and J.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Fondo para el Fortalecimiento de la Investigación UAQ”, Universidad Autónoma de Querétaro, grant number FNN201610.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the School of Natural Sciences at the Universidad Autónoma de Querétaro (Project ID: 77FCN2015, 26 October 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ethical reasons.
Acknowledgments
We wish to thank the parents of all children in this study who generously donated their time and effort to participate in the study. We also thank the Association “Autismo Aguascalientes” and school principals from “Margarita Juárez Diez Marina I. A. P.” who collaborated in the study and for their interest in this research. The authors thank Roquette México and Latam for donating mannitol and Globe Chemicals S. A. de C. V. for donating lactulose.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-DSM-5, 5th, revised ed.; American Psychiatric Pub.: Washington, DC, USA, 2013. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E.; Marcin, C.; Manero, A.C.; Bruno, R.; Diaz, C.; Villalobos, M.; Ramsay, K.; Nealy, B. Prevalence of Autism Spectrum Disorders in Guanajuato, Mexico: The Leon survey. J. Autism Dev. Disord. 2016, 46, 1669–1685. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Figuerola, P.; Canals, J.; Fernández-Cao, J.C.; Arija Val, V. Differences in food consumption and nutritional intake between children with autism spectrum disorders and typically developing children: A meta-analysis. Autism Int. J. Res. Pract. 2019, 23, 1079–1095. [Google Scholar] [CrossRef]
- Molina-López, J.; Leiva-García, B.; Planells, E.; Planells, P. Food selectivity, nutritional inadequacies, and mealtime behavioral problems in children with autism spectrum disorder compared to neurotypical children. Int. J. Eat. Disord. 2021, 54, 2155–2166. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Flores-Rojas, K.; Torre-Aguilar, M.J.; Gomez-Fernández, A.R.; Martín-Borreguero, P.; Perez-Navero, J.L.; Gil, A.; Gil-Campos, M. Dietary Patterns, Eating Behavior, and Nutrient Intakes of Spanish Preschool Children with Autism Spectrum Disorders. Nutrients 2021, 13, 3551. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; Elbeltagi, R.; Alhawamdeh, R. Role of gastrointestinal health in managing children with autism spectrum disorder. World J. Clin. Pediatr. 2023, 12, 171–196. [Google Scholar] [CrossRef]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 501–513. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Li, H.; Huang, S.; Jing, J.; Yu, H.; Gu, T.; Ou, X.; Pan, S.; Zhu, Y.; Su, X. Dietary intake and gastrointestinal symptoms are altered in children with Autism Spectrum Disorder: The relative contribution of autism-linked traits. Nutr. J. 2024, 23, 27. [Google Scholar] [CrossRef]
- van De Sande, M.M.; van Buul, V.J.; Brouns, F.J. Autism and nutrition: The role of the gut-brain axis. Nutr. Res. Rev. 2014, 27, 199–214. [Google Scholar] [CrossRef] [PubMed]
- D’Eufemia, P.; Celli, M.; Finocchiaro, R.; Pacifico, L.; Viozzi, L.; Zaccagnini, M.; Cardi, E.; Giardini, O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996, 85, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- de Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Iovene, M.R.; Bombace, F.; Maresca, R.; Sapone, A.; Iardino, P.; Picardi, A.; Marotta, R.; Schiraldi, C.; Siniscalco, D.; Serra, N.; et al. Intestinal Dysbiosis and Yeast Isolation in Stool of Subjects with Autism Spectrum Disorders. Mycopathologia 2017, 182, 349–363. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Ianiro, G.; Vanella, G.; Bibbò, S.; Bruno, G.; Simeone, G.; Mele, G. Gut barrier in health and disease: Focus on childhood. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1077–1085. [Google Scholar]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standarization Reference Manual; Human Kinetics Book: Champaign, IL, USA, 1988. [Google Scholar]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 24 June 2020).
- Pérez Lizaur, A.B.; Palacios González, B.; Castro Becerra, A.L.; Flores-Galicia, I. Sistema Mexicano de Alimentos Equivalentes, 4th ed.; Fomento de Nutrición y Salud: Ciudad de México, Mexico, 2014. [Google Scholar]
- Bourges, E.; Casanueva, E.; Rosado, J.L. Recomendación de Ingestión de Nutrimentos para la Población Mexicana, Bases fisiológicas; Editorial Médica Panamericana: Ciudad de México, Mexico, 2008. [Google Scholar]
- García, O.P.; Ronquillo, D.; del Carmen Caamaño, M.; Martínez, G.; Camacho, M.; López, V.; Rosado, J.L. Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients 2013, 5, 5012–5030. [Google Scholar] [CrossRef]
- Caamaño, M.C.; Gutierrez, J.; García, O.P.; Ronquillo, D.; Martinez, G.; Rosado, J.L. Increased Calorie Intake at a Specific Mid-morning Meal and Increased Intake of Soft Drinks are Strongly Associated with Obesity in Mexican Rural Women. Ecol. Food Nutr. 2015, 54, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Butler, R.; Moore, D.; Davidson, G. Rapid and simultaneous quantification of rhamnose, mannitol, and lactulose in urine by HPLC for estimating intestinal permeability in pediatric practice. Clin. Chem. 1996, 42, 71–75. [Google Scholar] [CrossRef]
- de Vinck-Baroody, O.; Shui, A.; Macklin, E.A.; Hyman, S.L.; Leventhal, J.M.; Weitzman, C. Overweight and Obesity in a Sample of Children With Autism Spectrum Disorder. Acad. Pediatr. 2015, 15, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.; Faccioli, L.S.; Baronio, D.; Gottfried, C.; Perry, I.S.; Riesgo, R. Feeding behavior and dietary intake of male children and adolescents with autism spectrum disorder: A case-control study. Int. J. Dev. Neurosci. 2016, 53, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Criado, K.K.; Sharp, W.G.; McCracken, C.E.; De Vinck-Baroody, O.; Dong, L.; Aman, M.G.; McDougle, C.J.; McCracken, J.T.; Arnold, L.E.; Weitzman, C.; et al. Overweight and obese status in children with autism spectrum disorder and disruptive behavior. Autism 2018, 22, 450–459. [Google Scholar] [CrossRef]
- Memari, A.H.; Ghaheri, B.; Ziaee, V.; Kordi, R.; Hafizi, S.; Moshayedi, P. Physical activity in children and adolescents with autism assessed by triaxial accelerometry. Pediatr. Obes. 2013, 8, 150–158. [Google Scholar] [CrossRef]
- Ji, N.; Findling, R.L. An update on pharmacotherapy for autism spectrum disorder in children and adolescents. Curr. Opin. Psychiatry 2015, 28, 91–101. [Google Scholar] [CrossRef]
- Berry, R.C.; Novak, P.; Withrow, N.; Schmidt, B.; Rarback, S.; Feucht, S.; Criado, K.K.; Sharp, W.G. Nutrition Management of Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: Guideline from an Expert Panel. J. Acad. Nutr. Diet. 2015, 115, 1919–1927. [Google Scholar] [CrossRef]
- Ranjan, S.; Nasser, J.A. Nutritional status of individuals with autism spectrum disorders: Do we know enough? Adv. Nutr. 2015, 6, 397–407. [Google Scholar] [CrossRef]
- Sharp, W.G.; Postorino, V.; McCracken, C.E.; Berry, R.C.; Criado, K.K.; Burrell, T.L.; Scahill, L. Dietary Intake, Nutrient Status, and Growth Parameters in Children with Autism Spectrum Disorder and Severe Food Selectivity: An Electronic Medical Record Review. J. Acad. Nutr. Diet. 2018, 118, 1943–1950. [Google Scholar] [CrossRef]
- Hyman, S.L.; Stewart, P.A.; Schmidt, B.; Cain, U.; Lemcke, N.; Foley, J.T.; Peck, R.; Clemons, T.; Reynolds, A.; Johnson, C.; et al. Nutrient intake from food in children with autism. Pediatrics 2012, 130, S145–S153. [Google Scholar] [CrossRef]
- Malhi, P.; Venkatesh, L.; Bharti, B.; Singhi, P. Feeding Problems and Nutrient Intake in Children with and without Autism: A Comparative Study. Indian J. Pediatr. 2017, 84, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Burrell, T.L.; Jaquess, D.L. The Autism MEAL Plan: A parent-training curriculum to manage eating aversions and low intake among children with autism. Autism 2014, 18, 712–722. [Google Scholar] [CrossRef]
- Lucarelli, J.; Pappas, D.; Welchons, L.; Augustyn, M. Autism Spectrum Disorder and Avoidant/Restrictive Food Intake Disorder. J. Dev. Behav. Pediatr. 2017, 38, 79–80. [Google Scholar] [CrossRef]
- Rosado, J.L.; Díaz, M.; Rosas, A.; Griffit, I.; García, O.P. Calcium Absorption from Corn Tortilla Is Relatively High and Is Dependent upon Calcium Content and Liming in Mexican Women. J. Nutr. 2005, 135, 2578–2581. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 30 June 2024).
- The World Bank Group. Global Solar Atlas. 2024. Available online: https://globalsolaratlas.info/map?c=23.946096,-102.568359,5&r=MEX (accessed on 30 June 2024).
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Lizarraga, J.; Florens, M.V.; Viola, M.F.; Jain, P.; Decraecker, L.; Appeltans, I.; Cuende-Estevez, M.; Fabre, N.; Van Beek, K.; Perna, E.; et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature 2021, 590, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, H.J.; Verdu, E.F. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol. Motil. 2016, 28, 957–965. [Google Scholar] [CrossRef]
- Dastych, M.; Novotná, H.; Cíhalová, J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn’s disease. Dig. Dis. Sci. 2008, 53, 2789–2792. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef]
- Robertson, M.A.; Sigalet, D.L.; Holst, J.J.; Meddings, J.B.; Wood, J.; Sharkey, K.A. Intestinal permeability and glucagon-like peptide-2 in children with autism: A controlled pilot study. J. Autism Dev. Disord. 2008, 38, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Pearson, D.A.; Fatheree, N.; Mansour, R.; Hashmi, S.S.; Rhoads, J.M. Are ‘leaky gut’ and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutr. Neurosci. 2015, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Sacco, R.; Altieri, L.; Neri, C.; Urbani, A.; Bravaccio, C.; Riccio, M.P.; Iovene, M.R.; Bombace, F.; De Magistris, L.; et al. Slow intestinal transit contributes to elevate urinary p-cresol level in Italian autistic children. Autism Res. 2016, 9, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.C.; Mendonca, J.N.; Portari, G.V.; Junior, A.A.J.; Marchini, J.S.; Chiarello, P.G. Intestinal permeability and nutritional status in developmental disorders. Altern. Ther. Health Med. 2012, 18, 19–24. [Google Scholar]
- Dalton, N.; Chandler, S.; Turner, C.; Charman, T.; Pickles, A.; Loucas, T.; Simonoff, E.; Sullivan, P.; Baird, G. Gut Permeability in Autism Spectrum Disorders. Autism Res. 2014, 7, 305–313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).