Mucosal Immunity and Trained Innate Immunity of the Gut
Abstract
1. Introduction
2. Search Strategy
3. Brief Overview of the Gastrointestinal (GI) Immune System
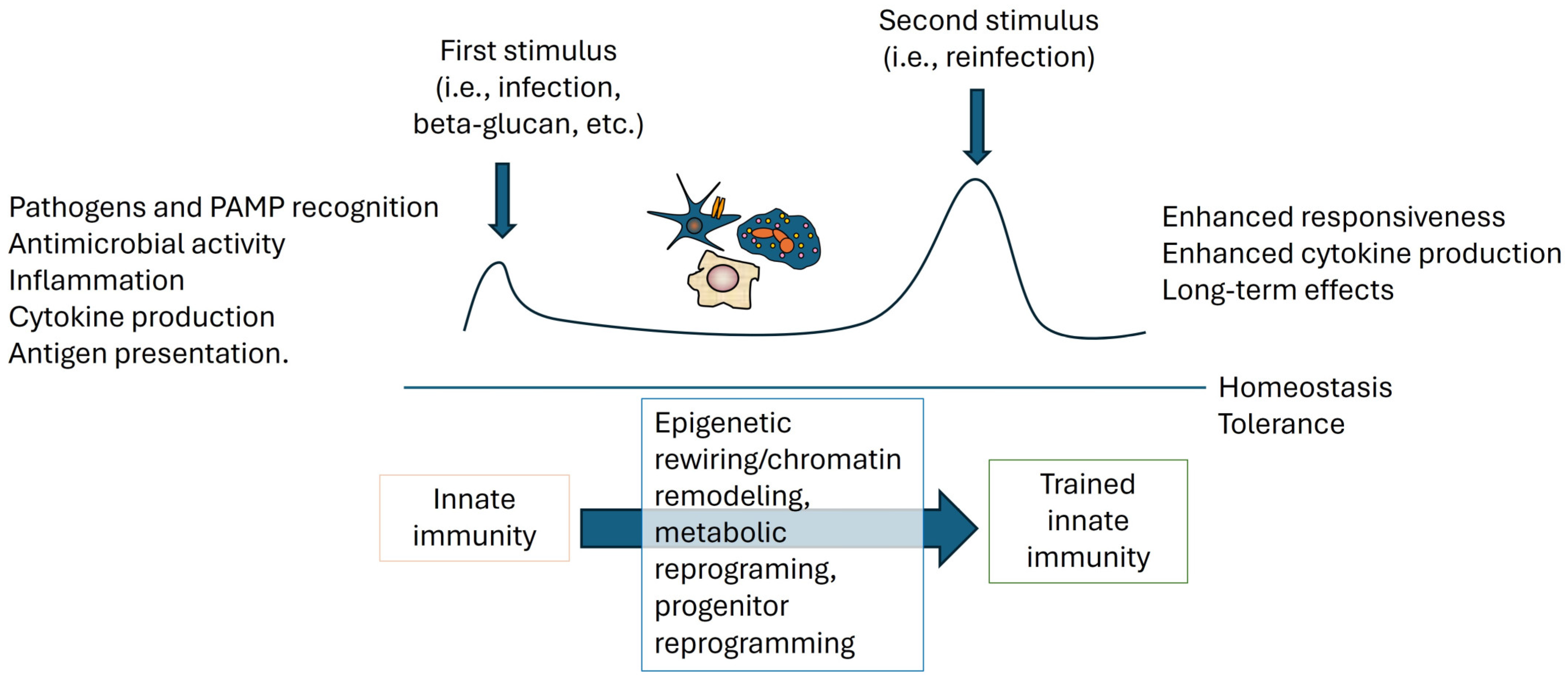
4. Mucosal Immune Responses in the Gut and the Novel Concept of Trained Immunity
5. Mechanisms of Trained Immunity
6. Disorders Affecting Gastrointestinal Immunity with a Focus on Mucosal Immunity
6.1. Inflammatory Bowel Disease (IBD)
6.2. Infectious Diseases and Mucosal Immunity
6.3. Influence of Dysregulated Immunity on Gut Disorders
6.4. Gut Disorders and Trained Innate Immunity
7. Therapeutic Implications for Gut Mucosal Immunity Modulation
8. Challenges and Future Perspectives in Mucosal Immunity and Trained Innate Immunity
8.1. Current Challenges in Understanding and Investigating Gut Immunity
8.2. Emerging Trends and Future Directions in Research
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berin, M.C.; Chehade, M. Pediatric Allergy: Principles and Practice, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- van der Heijden, P.J.; Bianchi, A.T. Mucosale immuniteit [Mucosal immunity]. Tijdschr. Diergeneeskd. 1990, 115, 1058–1064. (In Dutch) [Google Scholar] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; de Moreno de LeBlanc, A.; Gomez-Gutierrez, J.G.; LeBlanc, J.G.; Bermúdez-Humarán, L.G. Probiotics and trained immunity. Biomolecules 2021, 11, 1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nasr, R.; Shamseddine, A.; Mukherji, D.; Nassar, F.; Temraz, S. The crosstalk between microbiome and immune response in gastric cancer. Int. J. Mol. Sci. 2020, 21, 6586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsay, G.J.; Zouali, M. The interplay between innate-like b cells and other cell types in autoimmunity. Front. Immunol. 2018, 9, 1064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milner, E.C.; Anolik, J.; Cappione, A.; Sanz, I. Human innate B cells: A link between host defense and autoimmunity? Springer Semin. Immunopathol. 2005, 26, 433–452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity—Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Mills, K.H.G.; Basdeo, S.A. The effects of trained innate immunity on t cell responses; clinical implications and knowledge gaps for future research. Front. Immunol. 2021, 12, 706583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arneth, B. Trained innate immunity. Immunol. Res. 2021, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xiang, D.; Zhang, X.; Zhang, L.; Wang, S.; Jin, K.; You, L.; Huang, J. The mechanisms and cross-protection of trained innate immunity. Virol. J. 2022, 19, 210. [Google Scholar] [CrossRef]
- Wershil, B.K.; Furuta, G.T. 4. Gastrointestinal mucosal immunity. J. Allergy Clin. Immunol. 2008, 121 (Suppl. 2), S380–S383. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Langkamp-Henken, B.; Glezer, J.A.; Kudsk, K.A. Immunologic structure and function of the gastrointestinal tract. Nutr. Clin. Pract. 1992, 7, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef]
- Murphy, B.R. Chapter 43—Mucosal Immunity to Viruses. In Mucosal Immunology, 3rd ed.; Mestecky, J., Lamm, M.E., McGhee, J.R., Bienenstock, J., Mayer, L., Strober, W., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 799–813. ISBN 9780124915435. [Google Scholar]
- Boyaka, P.N.; Mestecky, J. Chapter 47—Mucosal Vaccines: An Overview. In Mucosal Immunology, 3rd ed.; 2005. Mestecky, J., Lamm, M.E., McGhee, J.R., Bienenstock, J., Mayer, L., Strober, W., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 855–873. ISBN 9780124915435. [Google Scholar]
- Holmgren, J.; Svennerholm, A.-M. Chapter 42—Mucosal immunity to bacteria. In Mucosal Immunology, 3rd ed.; Mestecky, J., Lamm, M.E., McGhee, J.R., Bienenstock, J., Mayer, L., Strober, W., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 783–797. ISBN 9780124915435. [Google Scholar]
- Liang, B.; Hyland, L.; Hou, S. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 2001, 75, 5416–5420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, L.; Saif, L.J. Induction of mucosal immune responses and protection against enteric viruses: Rotavirus infection of gnotobiotic pigs as a model. Vet. Immunol. Immunopathol. 2002, 87, 147–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheroutre, H. Starting at the beginning: New perspectives on the biology of mucosal T cells. Annu. Rev. Immunol. 2004, 22, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Honjo, T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat. Rev. Immunol. 2003, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X.; Chaplin, D.D. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 1999, 17, 399–433. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, B.; Wu, Z.; Lu, W.; Lehrer, R.I. Antibacterial activity and specificity of the six human {alpha}-defensins. Antimicrob Agents Chemother. 2005, 49, 269–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahan, S.; Roth-Walter, F.; Arnaboldi, P.; Agarwal, S.; Mayer, L. Epithelia: Lymphocyte interactions in the gut. Immunol. Rev. 2007, 215, 243–253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, L.C.; Perdue, M.H. Role of mast cells in intestinal mucosal function: Studies in models of hypersensitivity and stress. Immunol. Rev. 2001, 179, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; Monahan, R.A.; Osage, J.E.; Dickersin, G.R. Crohn’s disease: Transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum. Pathol. 1980, 11, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the mucosal immune system. Biomed. Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestecky, J.; Russell, M.W.; Elson, C.O. Intestinal IgA: Novel views on its function in the defence of the largest mucosal surface. Gut 1999, 44, 2–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fadlallah, J.; El Kafsi, H.; Sterlin, D.; Juste, C.; Parizot, C.; Dorgham, K.; Autaa, G.; Gouas, D.; Almeida, M.; Lepage, P.; et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 2018, 10, eaan1217. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, J.; Sterlin, D.; Fieschi, C.; Parizot, C.; Dorgham, K.; El Kafsi, H.; Autaa, G.; Ghillani-Dalbin, P.; Juste, C.; Lepage, P.; et al. Synergistic convergence of microbiota-specific systemic IgG and secretory IgA. J. Allergy Clin. Immunol. 2019, 143, 1575–1585.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.; Franz, K. Innate defence: Evidence for memory in invertebrate immunity. Nature 2003, 425, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 2018, 172, 176–190.e119. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Li, X.; Mitroulis, I.; Chavakis, T. Trained innate immunity and its implications for mucosal immunity and inflammation. Adv. Exp. Med. Biol. 2019, 1197, 11–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dagenais, A.; Villalba-Guerrero, C.; Olivier, M. Trained immunity: A “new” weapon in the fight against infectious diseases. Front. Immunol. 2023, 14, 1147476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.C.C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef]
- Arts, R.J.; Joosten, L.A.; Netea, M.G. Immunometabolic circuits in trained immunity. Semin. Immunol. 2016, 28, 425–430. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Caligiuri, G.; Chavakis, T.; Matarese, G.; Netea, M.G.; Nicoletti, A.; O’Neill, L.A.; Marelli-Berg, F.M. The cellular and molecular basis of translational immunometabolism. Immunity 2015, 43, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Penkov, S.; Mitroulis, I.; Hajishengallis, G.; Chavakis, T. Immunometabolic crosstalk: An ancestral principle of trained immunity? Trends Immunol. 2019, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Dos Santos, J.C.; Bekkering, S.; Mulder, W.J.M.; van der Meer, J.W.M.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Trained immunity: Adaptation within innate immune mechanisms. Physiol. Rev. 2023, 103, 313–346. [Google Scholar] [CrossRef] [PubMed]
- Nankabirwa, V.; Tumwine, J.K.; Mugaba, P.M.; Tylleskär, T.; Sommerfelt, H.; PROMISE-EBF Study Group. Child survival and BCG vaccination: A community based prospective cohort study in Uganda. BMC Public Health 2015, 15, 175. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Dominguez-Andres, J.; Netea, M.G. Long-term reprogramming of the innate immune system. J. Leukoc. Biol. 2018, 105, 329–338. [Google Scholar] [CrossRef]
- Berendsen, M.L.T.; Øland, C.B.; Bles, P.; Jensen, A.K.G.; Kofoed, P.E.; Whittle, H.; de Bree, L.C.J.; Netea, M.G.; Martins, C.; Benn, C.S.; et al. Maternal priming: Bacillus Calmette-Guérin (BCG) vaccine scarring in mothers enhances the survival of their child with a BCG vaccine scar. J. Pediatric Infect. Dis. Soc. 2020, 9, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.S.; Kaletsky, R.; Murphy, C.T. Piwi/PRG-1 argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell 2019, 177, 1827–1841. [Google Scholar] [CrossRef]
- de Mattos, B.R.; Garcia, M.P.; Nogueira, J.B.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.; Tamashiro, W.M.; Simioni, P.U. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediat. Inflamm. 2015, 2015, 493012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.H.; Kwon, J.E.; Cho, M.L. Immunological pathogenesis of inflammatory bowel disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghadimi, D.; Helwig, U.; Schrezenmeir, J.; Heller, K.J.; de Vrese, M. Epigenetic imprinting by commensal probiotics inhibits the IL-23/IL-17 axis in an in vitro intestinal mucosal immune system model. J. Leukoc. Biol. 2012, 92, 895–911. [Google Scholar] [CrossRef]
- Palkumbura, P.G.A.S.; Mahakapuge, T.A.N.; Wijesundera, R.R.M.K.K.; Wijewardana, V.; Kangethe, R.T.; Rajapakse, R.P.V.J. Mucosal Immunity of Major Gastrointestinal Nematode Infections in Small Ruminants Can Be Harnessed to Develop New Prevention Strategies. Int. J. Mol. Sci. 2024, 25, 1409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nie, S.; Yuan, Y. The Role of Gastric Mucosal Immunity in Gastric Diseases. J. Immunol. Res. 2020, 2020, 7927054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rose, M.A. Mucosal Immunity and acute viral gastroenteritis. Hum. Vaccin. Immunother. 2014, 10, 2112–2114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacinto, R.; Hartung, T.; McCall, C.; Li, L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: Distinct alterations in IL-1 receptor-associated kinase. J. Immunol. 2002, 168, 6136–6141. [Google Scholar] [CrossRef] [PubMed]
- Levine, J. The Impact of Immune Dysregulation on the Development of Autoimmune Gastrointestinal and Liver Disease. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Sterling, K.G.; Dodd, G.K.; Alhamdi, S.; Asimenios, P.G.; Dagda, R.K.; De Meirleir, K.L.; Hudig, D.; Lombardi, V.C. Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int. J. Mol. Sci. 2022, 23, 13328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Yang, F.; Eslava, S.; Cai, Z. Treatment pathways leading to biologic therapies for ulcerative colitis and Crohn’s disease in the United States. Clin. Transl. Gastroenterol. 2020, 11, e00128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinisch, W.; Gecse, K.; Halfvarson, J.; Irving, P.M.; Jahnsen, J.; Peyrin-Biroulet, L.; Rogler, G.; Schreiber, S.; Danese, S. Clinical practice of adalimumab and infliximab biosimilar treatment in adult patients with Crohn’s disease. Inflamm. Bowel Dis. 2021, 27, 106–122, Erratum in Inflamm. Bowel Dis. 2021, 27, 1367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, J.H.; Kang, E.A.; Park, S.K.; Hong, S.N.; Kim, Y.S.; Bang, K.B.; Kim, K.O.; Lee, H.S.; Kang, S.B.; Shin, S.Y.; et al. Long-term outcomes after the discontinuation of anti-tumor necrosis factor-α therapy in patients with inflammatory bowel disease under clinical remission: A korean association for the study of intestinal disease multicenter study. Gut Liver 2021, 15, 752–762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.; Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 2007, 56, 1333–1336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khorrami, S.; Ginard, D.; Marín-Jiménez, I.; Chaparro, M.; Sierra, M.; Aguas, M.; Sicilia, B.; García-Sánchez, V.; Suarez, C.; Villor ia, A.; et al. Ustekinumab for the treatment of refractory Crohn’s disease: The Spanish Experience in a Large Multicentre Open-label Cohort. Inflamm. Bowel Dis. 2016, 22, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory bowel disease treatments and predictive biomarkers of therapeutic response. Int. J. Mol. Sci. 2022, 23, 6966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrera-deGuise, C.; Serra-Ruiz, X.; Lastiri, E.; Borruel, N. JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases. Front. Med. 2023, 10, 1089099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weisshof, R.; Golan, M.A.; Yvellez, O.V.; Rubin, D.T. The use of tofacitinib in the treatment of inflammatory bowel disease. Immunotherapy 2018, 10, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Jeen, Y.T. Anti-integrin therapy for inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1868–1880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kleinschmidt-DeMasters, B.K.; Tyler, K.L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N. Engl. J. Med. 2005, 353, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Raine, T. Vedolizumab for inflammatory bowel disease: Changing the game, or more of the same? United Eur. Gastroenterol. J. 2014, 2, 333–344, Erratum in United Eur. Gastroenterol. J. 2014, 2, 551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shim, J.A.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell immunity and immune-mediated disorders. Int. J. Biol. Sci. 2023, 19, 1178–1191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Model | Characteristics | Advantages | Limitations |
|---|---|---|---|
| Murine Models | Inbred mice strains (e.g., BALB/c, C57BL/6); genetically modified mice | Controlled genetic background; extensive immunological tools | Differences from human immunity; ethical concerns |
| Gnotobiotic Mice | Germ-free or defined microbial communities | Allows study of host-microbiota interactions in a controlled setting | Technically challenging; expensive; limited availability |
| Humanized Mouse Models | Mice engrafted with human immune cells/tissues | Reflects human immune response; useful for studying human-specific pathogens | High cost; limited lifespan of human cells in mice; complex to establish |
| Organoid Cultures | 3D culture systems derived from human or animal gut stem cells | Mimics human gut tissue architecture and function; high throughput | Lack of complete immune system interaction; variability in protocols |
| In vitro Cell Culture | Epithelial cell lines, immune cells (e.g., dendritic cells, macrophages) | Controlled environment; cost-effective; high reproducibility | Simplistic; lacks tissue complexity and systemic immune interactions |
| Zebrafish Models | Transparent larvae; genetic manipulation possible | Visualize immune responses in real-time; cost-effective | Differences with mammalian immune system; limited antibodies/tools |
| Porcine Models | Pigs with similar gastrointestinal physiology to humans | Relevant to human gut physiology; size allows surgical techniques | High maintenance cost; ethical concerns; less developed genetic tools |
| Non-Human Primates | Species closely related to humans | Closest physiological and immunological model to humans | Ethical concerns; high cost; limited availability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikova, T.; Kaouri, I.E.; Bakopoulou, K.; Gulinac, M.; Naydenova, K.; Dimitrov, M.; Peruhova, M.; Lazova, S. Mucosal Immunity and Trained Innate Immunity of the Gut. Gastroenterol. Insights 2024, 15, 661-675. https://doi.org/10.3390/gastroent15030048
Velikova T, Kaouri IE, Bakopoulou K, Gulinac M, Naydenova K, Dimitrov M, Peruhova M, Lazova S. Mucosal Immunity and Trained Innate Immunity of the Gut. Gastroenterology Insights. 2024; 15(3):661-675. https://doi.org/10.3390/gastroent15030048
Chicago/Turabian StyleVelikova, Tsvetelina, Issa El Kaouri, Konstantina Bakopoulou, Milena Gulinac, Kremena Naydenova, Martin Dimitrov, Milena Peruhova, and Snezhina Lazova. 2024. "Mucosal Immunity and Trained Innate Immunity of the Gut" Gastroenterology Insights 15, no. 3: 661-675. https://doi.org/10.3390/gastroent15030048
APA StyleVelikova, T., Kaouri, I. E., Bakopoulou, K., Gulinac, M., Naydenova, K., Dimitrov, M., Peruhova, M., & Lazova, S. (2024). Mucosal Immunity and Trained Innate Immunity of the Gut. Gastroenterology Insights, 15(3), 661-675. https://doi.org/10.3390/gastroent15030048








