Abstract
Introduction. Biliary obstruction is a common manifestation of biliopancreatic malignancies, and its relief is an essential part of the treatment algorithm. Currently, there are three techniques to manage malignant biliary obstruction—endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic biliary drainage (PTBD), and endoscopic ultrasound-guided biliary drainage (EUS-BD). ERCP has been adopted as a first-line treatment modality but EUS-BD is gradually emerging as a viable alternative. The aim of the current article is to assess the clinical outcomes of the three nonsurgical biliary drainage procedures. Materials and methods. A total of 102 consecutive patients with unresectable biliopancreatic malignancy inducing biliary obstruction and subjected to palliative treatment by means of ERCP, EUS-BD, or PTBD were retrospectively included in the study. Results. No difference in clinical and technical success of the procedures was found: ERCP—97.2% technical; 88.9% clinical; PTBD—94.4% technical, 72.2% clinical; EUS-BD—90% technical; 83.3% clinical. Adverse events (AEs) and reinterventions were significantly more common in PTBD (38.9% and 52.8%) and ERCP (27.9% and 25%) compared to EUS-BD (10% and 3.3%). Total duration of hospital stay and number of hospitalizations were lower in the EUS-BD compared to PTBD and ERCP groups. Conclusions. In the presence of adequate expertise, EUS-BD may be superior to PTBD and ERCP in achieving and sustaining biliary drainage in the setting of unresectable malignancy.
1. Introduction
Obstructive jaundice represents a clinical entity commonly diagnosed in gastroenterological practice. The etiological spectrum of biliary obstruction varies widely, including either benign (most commonly choledocholithiasis) or malignant causes. The health burden of malignant biliary obstruction (MBO) is outlined by real-life data, suggesting that it is an underlying cause in more than 60% of obstructive jaundice patients [1]. Pancreatic cancer is the leading cause of malignant stenosis, being responsible for as much as 66% of the cases [2]. Its incidence is expected to rise by 50% by 2035 [3]. Cholangiocarcinoma, ampullary cancer, gall bladder cancer, metastatic liver disease, and hilar lymphadenopathy are other commonly established etiologies of biliary obstruction [4].
Historically, surgical treatment was the initial modality to alleviate malignant obstructive jaundice. While being highly effective for resolving obstruction and associated with low rate of recurrence (2–5%), the appreciable risk of morbidity and mortality of up to 25% has always been a source of concern [5,6]. In potentially resectable cases, surgery is still considered the first-choice therapeutic approach, with preoperative drainage generally deemed unnecessary and associated with poorer clinical outcomes. Unfortunately, at the time of diagnosis, merely 20% of pancreatic cancer patients are considered eligible for surgical treatment. In hilar cholangiocarcinoma cases, 50% are considered as good surgical candidates. Upon exploration, however, 50–60% of them are found to have inoperable disease, reducing the resectability rate to about 20–25% [7]. For all the remaining patients with radically inoperable disease, less invasive therapeutic modalities have been developed in the last 60 years to manage obstructive jaundice.
Currently, there are three pivotal techniques for the alleviation of MBO:
- Endoscopic retrograde cholangiopancreatography (ERCP). Since the initial description of the technique by McCune et al. in 1968, ERCP has gradually evolved and been established as the first-line treatment of obstructive jaundice, particularly in benign indications or in the setting of unresectable malignancy [8]. Achieving high success (90–95%) and low adverse event (AE) (5.18–9.8%) rates, while preserving the anatomy and physiology of the gastrointestinal tract, its value in the management of a broad spectrum of biliary disorders is enormous [9]. Even in expert hands, however, in about 3–10% of the patients, drainage through ERCP is impossible due to a number of factors, including an inaccessible or grossly infiltrated papilla, failure to cannulate, altered anatomy, etc. Additionally, post-ERCP pancreatitis (PEP) remains a source of grave concern. Despite the introduction of various techniques to reduce its incidence (guidewire cannulation, rectal nonsteroid anti-inflammatory drugs, prophylactic pancreatic stenting), PEP occurs in about 10% of all procedures and in up to 30% in high-risk patients [10].
- Percutaneous transhepatic biliary drainage (PTBD). The development of PTBD techniques is largely parallel to the development of ERCP. Its adoption has been slightly slower compared to ERCP (first ultrasound-guided percutaneous cholangiography performed by Makuuchi in 1977 [11]). However, for decades, PTBD was considered the only viable alternative to ERCP and even a first-choice modality in the setting of complex hilar obstruction. While being acceptably effective in terms of technical and clinical success, of 90–100% and 77–98%, respectively, percutaneous biliary intervention’s main disadvantage is the potential for AEs ranging between 8–30% in most studies, but up to 62% according to more recent large observational studies [12,13]. There is an uptrend in the incidence of AEs associated with PTBD, which probably reflects certain aspects of the patient selection process. Other factors that are worth considering include the persistent need for external drainage catheters, which worsen patients’ quality of life, impair normal intestinal absorption and integrity, and lead to loss of fluid and electrolytes.
- Endoscopic ultrasound-guided biliary drainage (EUS-BD). Initially described by Giovannini et al. in the form of endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) in 2001 and hepaticogastrostomy (HGS) in 2003, EUS-BD emerged as a viable alternative to conventional endoscopic and percutaneous drainage procedures [14,15]. While initially performed only in high-volume expert centers, the gradual increase in expertise and development of new devices and stents led to broader adoption of the technique. In 2022 and 2023 European Society of Gastrointestinal Endoscopy (ESGE) and Society of Gastrointestinal endoscopy of India (SGEI) published guidelines on the application of EUS for biliopancreatic drainage [16,17]. These guidelines outline EUS-BD as a first-choice alternative to ERCP, mainly in the setting of malignant biliary obstruction. Multiple studies, however, comparing the clinical outcomes of primary EUS-BD to ERCP state that EUS-BD has noninferior clinical and technical success rate and fewer AEs (PEP in particular) [18,19,20]. Additionally, EUS-BD has certain theoretical advantages over ERCP in terms of lower stent occlusion rate [18]. Those results raise the question of whether EUS-BD could be regarded as primary therapeutic modality at least in selected patients with anticipated difficult transpapillary biliary access.
The advent of EUS-BD techniques questions the established paradigm of how to manage MBO in palliative settings. The impaired quality of life and AEs rate attributed to PBD narrow its application worldwide. ERCP is still a first-line treatment considering its widespread availability, but whether its performance is superior to EUS-BD is yet to be determined. The presence of adequate expertise is probably the most important factor in the decision-making process.
2. Aim
The primary endpoint of the current study is to assess the clinical outcomes (defined as clinical and technical success rate and AEs rate) of ERCP, EUS-BD, and PTBD for alleviation of malignant obstructive jaundice. A comparison between the methods based on the aforementioned criteria is subsequently performed. As a secondary endpoint, we set out to analyze the influence of certain variables, including type of procedure, etiology and level of stenosis, type of stent, and patient’s performance status (PS) (see Appendix A, Table A1), on the obtained result. Eventually, we aimed to evaluate the long-term impact of the procedures on patient’s life quality based on the total number of reinterventions, hospitalizations, and duration of hospital in-stay needed to sustain adequate biliary drainage.
3. Ethics
An oral and written informed consent was obtained prior the procedures, with the patients and their relatives being thoroughly informed of the possible clinical outcomes, adverse events, and complications, as well as of the valid alternatives. The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee for studies involving humans.
4. Materials and Methods
A total of 102 consecutive patients with unresectable biliopancreatic malignancy inducing biliary obstruction and subjected to palliative treatment by means of ERCP, EUS-BD, or PTBD were retrospectively included in the study. All of them were managed in University hospital “Kaspela”, Plovdiv, Bulgaria from June2022 to June 2023. Design: Retrospective single-center comparative study.
4.1. Patient Selection
- Inclusion criteria.
- -
- Age ≥ 18 years
- -
- Histologically confirmed biliopancreatic malignancy inducing obstructive jaundice.
- -
- Imaging data (computer tomography (CT), magnetic resonance cholangiopancreatography (MRCP), or EUS) suggestive of nonresectability (locally advanced or metastatic disease) or poor general condition precluding major surgery.
- -
- Interventional treatment by means of ERCP, EUS-BD, or PTBD was considered the chief inclusion criteria.
- -
- Follow-up of at least 6 months post-procedure or until death.
- -
- Competence to give informed consent.
- Exclusion criteria.
- -
- Benign etiology of biliary obstruction.
- -
- Patients subjected to subsequent surgery with curative intent.
- -
- Modifications of the percutaneous biliary interventions including antegrade stenting, rendezvous technique, hybrid techniques (ERCP + PTBD), etc.
- -
- Patients lost to follow-up.
- -
- Refusal to participate in the study.
4.2. Methods
All procedures were performed by a single gastroenterologist conducting 300 ERCP, 400 EUS (diagnostic and therapeutic), and 50 PBI per year.
Histological confirmation and staging of the malignancy in accordance with the existing guidelines were performed prior to (or, in case of biliary tract tumors, during) the definitive treatment.
Standard preprocedural assessment was performed in all patients. It included thorough lab evaluation and abdominal ultrasonography (US). Additional advanced imaging techniques (contrast-enhanced computer tomography (CECT), MRCP, and EUS) were utilized on an individual basis when deemed necessary.
Abdominal ultrasonography was performed the day before and immediately prior to the procedure and the findings were thoroughly recorded. Preprocedural US was used to select the optimal therapeutic approach and as a baseline technique to screen for subsequent complications. The US machine used was Hitachi Aloka alpha 7 (Tokyo, Japan) combined with standard convex transducer UST-9123.
All procedures were executed under general anesthesia controlled by a certified specialist. All patients received prophylactic antibiotics (Ceftriaxone 2.0 g i.v. and Metronidazol 2 × 500 mg i.v. prior to the procedure and at least 3 days after). In the case of an established infectious agent, treatment according to antibiotic sensitivity was initiated. In ERCP, insufflation with CO2 or ambient air was used at the discretion of the endoscopist, while for EUS-BD, CO2 usage was mandatory. In PTBD, the patient’s position was determined by the optimal approach to the desired duct, with supine position being generally preferable to left lateral position. For ERCP, the patient’s position was left lateral or supine depending on the level of obstruction, with changes in position commonly performed during the procedure. In all EUS-BD procedures, the patient was set in supine position.
4.2.1. ERCP
A therapeutic duodenoscope Olympus TJF-160VR (Olympus, Hamburg, Germany) was introduced and placed at the second portion of the duodenum “en face” with the major duodenal papilla. A short scope position was generally pursued, with the endoscope withdrawn to about 60 cm from the incisors. Once stable position was achieved, standard guidewire-navigated cannulation of the common bile duct was attempted using a sphincterotome (TrueTomeTM; Boston Scientific, Marlborough, MA, USA) and a 0.025 inch guidewire (VisiGlide 2 straight type, Olympus, Hamburg, Germany). In case of difficult cannulation, a “pre-cut” technique was utilized using a needle knife (MicroknifeTM XL, Boston Scientific, Marlborough, MA, USA). Once deep biliary access was achieved, a cholangiogram was obtained to assess ductal anatomy and level of obstruction. A small amount of bile was aspirated and sent for microbiological testing. In the setting of hilar strictures, contrast injection was minimal and confined only to bile ducts already negotiated with a guidewire to reduce the risk of cholangitis. Sphincterotomy was performed in all patients prior to stent insertion. This was perceived to reduce the risk of post-ERCP pancreatitis especially when self-expandable metals stents (SEMSs) or multiple plastic stents were to be inserted.
After sufficient assessment of biliary anatomy and level and severity of stenosis, one or more plastic or SEMSs (with or without prior mechanical dilation) were introduced over the guidewire proximal to the level of obstruction. The choice of stent was determined on a case-by-case basis based on the type of stenosis, discretion of the endoscopist, patient’s general condition, and preference. In patients with hilar stenosis, drainage of at least 50% of liver volume was pursued, while bilateral stenting was generally not considered mandatory. In case of distal stenosis, fully covered SEMS was preferred. Whenever possible, the proximal end of the stent was positioned distally to the cystic/common hepatic duct confluence to reduce the risk of cholecystitis.
4.2.2. EUS-BD
Two variations of the procedure were utilized—EUS-CDS and EUS-HGS, which are described separately.
4.2.3. EUS-CDS
A curvilinear echoendoscope (Olympus GF-UCT180, Olympus, Hamburg Germany) was introduced then and placed at the first part of the duodenum. Insufflation with CO2 was utilized instead of ambient air (Olympus UCR, Hamburg, Germany). Once in the bulbar part of the duodenum, the common bile duct was identified endosonographically. Color Doppler was used to delineate the surrounding vascular structures and to exclude the presence of major interposing vessels. A crucial step was to ensure that the endoscope was in such a position so that the needle would be oriented towards the liver hilum. This was achieved by further adjusting the position of the endoscope using fluoroscoping guidance (Philips BV Pulsera C-arm, Philips, Best, The Netherlands). Generally, if on fluoroscopy the endoscope was in a long position and with the tip pointing upwards and facing the insertion tube, it was considered the optimal position to access the common bile duct. A 19Ga fine needle aspiration (FNA) needle (ExpectTM needle; Boston Scientific; Marlborough, MA, USA) was used to puncture the common bile duct. To verify the correct position, bile was initially aspirated, followed by contrast injection (Iopamidol 370 mg/mL) to obtain cholangiogram. Slight irrigation with saline was then performed, followed by insertion of 0.025 inch guidewire (JagWire RevolutionTM; Boston Scientific; Marlborough, MA, USA), which was then placed in as deep as possible in the intrahepatic bile ducts. Slight withdrawal of the needle was used to avoid “sheering” in case of advancement of the guidewire in the correct direction was cumbersome. Once stable position of the guidewire was achieved, dilation of the fistulous tract was performed using a cystotome (10fr in 1 case, 6fr in 6 cases; Endo-flex GmbH, Voerde, Germany) paired with electrosurgical unit ERBE Vio 300D (Erbe Elektromedicin Gmbh, Tübingen, Germany) set at Endocut I (effect 2, cut duration 3, cut interval 3). Eventually, FC-SEMS (WallFlexTM; Boston Scientific; Marlborough, MA, USA) was inserted—60/10 mm in six cases and 80/10 mm in one case. Every effort was made to place the stent just below the confluence to avoid inadvertent segmental obstruction of the intrahepatic bile ducts. An SEMS was intended to extend about 3 cm in the duodenum to reduce the risk of stent migration. After successful positioning of the stent, endoscopic and fluoroscopic evaluation were performed to verify the presence of bile flow and evacuation of contrast media from the bile ducts and to exclude hemorrhage. The described steps of EUS-CDS are depicted in Figure 1.
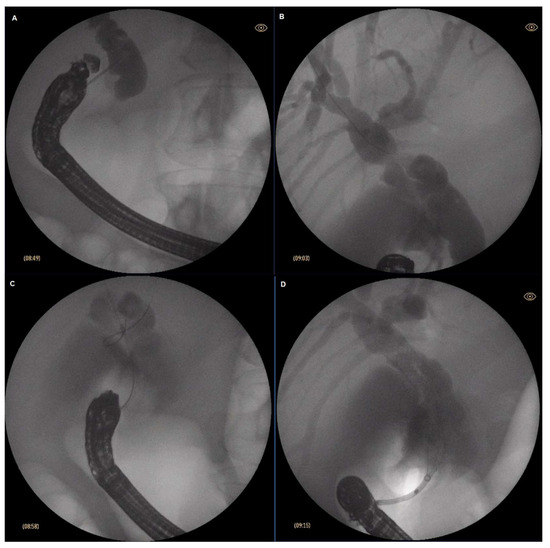
Figure 1.
Steps of EUS-CDS: (A) Puncture of the common bile duct; (B) guidewire insertion towards the hilum; (C) tract dilation with a 6fr cystotome; (D) stent insertion.
4.2.4. EUS-HGS
The echoendoscope was advanced to the corpus of the stomach. Slowly withdrawing the endoscope the left lobe of the liver was visualized. A dilated bile duct measuring at least 5 mm was considered eligible for puncture. The third segmental bile ducts we considered the optimal target, but second segmental ducts could also be utilized provided that the puncture site was not too high in the stomach. In general, every effort was made not to puncture through the esophago-gastric junction or the esophagus to avoid mediastinitis. The orientation of the scope towards the liver hilum was also a crucial step of the procedure facilitating the subsequent advancement of the guidewire in the desired direction. This was achieved under fluoroscopic guidance, flexing the tip of the endoscope upwards as much as possible and rotating it to the left on the fluoroscopy screen.
Once adequate position of the scope was achieved, the target bile duct in the left lobe of the liver was punctured with a 19Ga FNA needle (ExpectTM needle; Boston Scientific; Marlborough, MA, USA). A small amount of bile was aspirated to confirm position, followed by contrast injection (Iopamidol 370 mg/mL) to obtain cholangiogram. After slight irrigation with saline to facilitate guidewire insertion, a 0.025 inch angled type guidewire (VisiGlide 2, Olympus, Hamburg, Germany) was inserted through the needle into the bile ducts. If the needle orientation was accurate, the guidewire could be easily advanced along the intrahepatic bile duct to the liver hilum. In case of difficult cannulation, very careful manipulation of the guidewire was mandatory. First, the needle was withdrawn slowly in the liver parenchyma to reduce the risk of sheering. If withdrawal of the guidewire was needed, it was performed with extra caution. Eventually, if the guidewire was stuck in the needle or could not be positioned deeply in the bile ducts, the needle was withdrawn and the entire procedure was repeated. The goal was to position the guidewire as deep as possible in the bile ducts, preferably with loop formation to ensure adequate stability for subsequent interventions.
Once stable position with the guidewire was achieved, the next step was dilation of the fistulous tract. A 6fr cystotome (Endo-flex GmbH, Voerde, Germany) paired with electrosurgical unit ERBE Vio 300D (Erbe Elektromedicin Gmbh, Tübingen, Germany) set at Endocut I (effect 2, cut duration 3, cut interval 3) was used. With the cystotome in the bile ducts, further contrast injections or guidewire manipulations could be easily performed if necessary. Eventually, an SEMS was inserted to create a transmural tract between the left hepatic bile ducts and the stomach. Two type of SEMSs were used. Initially fully covered-SEMSs (FC-SEMS) (WallFlexTM; Boston Scientific; Marlborough, MA, USA) 10/80 mm or 10/100 mm were utilized. Subsequently, a dedicated SEMS for hepatico-gastrostomy (Hanarostent BPE, M.I. Tech, Pyeongtaek, Korea) 10/80 mm was used as standard. The stent was positioned in such a way as not to obstruct side biliary branches and to protrude at least 2 cm in the stomach. Eventually, contrast injection through the stent was performed to confirm its patency and lack of leakage in the peritoneal cavity. Different stages of EUS-HGS are illustrated in Figure 2 and Figure 3.
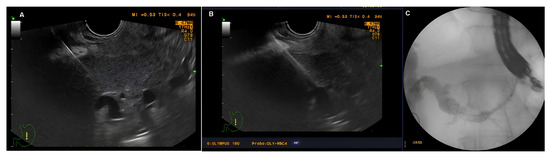
Figure 2.
Stages of EUS-HGS: (A) Puncture of a dilated left-sided bile duct; (B) tract dilation with a 6fr cystotome; (C) stent insertion under fluoroscopic guidance.
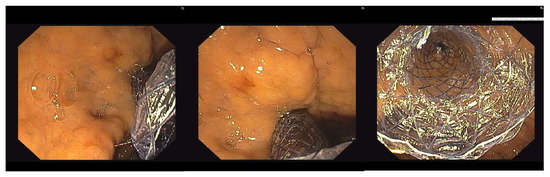
Figure 3.
Endoscopic view of the distal part of the stent in the stomach.
4.2.5. PTBD
Ultrasound (Hitachi Aloka alpha 7, Tokyo, Japan) examination was conducted and the desired duct was selected. In general, as peripheral a duct as possible was selected to reduce the risk of bile leakage. Puncture of the extrahepatic ducts was avoided. The needle direction was oriented towards the hilum of the liver to alleviate subsequent guidewire advancement and manipulation. The access point of the needle could be either transabdominal or intercostal. Transabdominal approach was the first choice and presumed safer. An 18Ga puncture needle (Urothech GmbH, Rohrdorf, Germany) was used. Upon puncturing the bile duct, the stylet was removed. Spontaneous leak of bile was awaited to confirm correct positioning; if this was not observed, then gentle suction was with a 5 mL syringe prefilled with 2 mL of 0.9% NaCl was performed. Only a small amount of bile was aspirated to avoid rapid decompression of the biliary tree and consequent loss of position. If bile leakage did not occur, cautious repositioning of the needle under US guidance was performed with repeated aspiration. In case of failure, the needle was retracted and the manipulation repeated. In rare cases with suspected purulent cholangitis, cautious irrigation with saline was performed prior to reaspiration. Once in the target duct, cholangiography was performed. A C-arm machine (Philips BV Pulsera C-arm, Philips, Best, The Netherlands) was used for fluoroscopy guidance. Only mild opacification of the bile duct was conducted to be used as a roadmap, with overextension of the biliary tree generally considered unfavorable.
Once fluoroscopic assessment of biliary anatomy was conducted, a 0.035 J-type Lunderquist guidewire (Urothech GmbH, Rohrdorf, Germany) was introduced in the biliary tree. Next, a dilation of the fistulous tract was performed with 7fr and 10fr plastic dilators (Urotech GmbH, Rohrdorf, Germany). Upon dilation, a 7fr or 10fr (Urotech GmbH, Rohrdorf, Germany) plastic “pig tail” drainage catheter was advanced along the guidewire and positioned as distally as possible in the bile ducts. Drainage catheters with locking mechanism (only 10fr available) were preferred. Eventually, the drain was fixed to the skin with two sutures. Trans-drainage cholangiography was performed at the end of the procedure to verify the position and exclude bile leakage in the peritoneal cavity or inadvertent puncture of a blood vessel.
Post-procedure in all patients, follow-up US was performed to search for complications. Provided that there were no contraindications, eating was restored the same day in the ERCP and PTBD group, while for the EUS-BD, food intake was initiated 24 h post-procedure. All patients underwent ultrasonography and lab testing on post-procedure day 1 and were discharged on day 2 if no imaging, laboratory, or clinical signs of AEs or ongoing biliary obstruction were found. Follow-up ultrasonography and lab testing were performed two and four weeks after the procedure and as per necessity thereafter.
Technical success was defined as successful completion of the planned intervention—placement of stent (above the stricture) or drain in the desired duct and flow of bile. Clinical success was defined by resolution of jaundice and pruritus and improvement of lab abnormalities (50–75% decrease in bilirubin levels on week 2). Adverse events are defined and classified according to the Clavien–Dindo system (see Appendix, Table A2).
A flow chart diagram of the study selection process is presented in Figure 4.
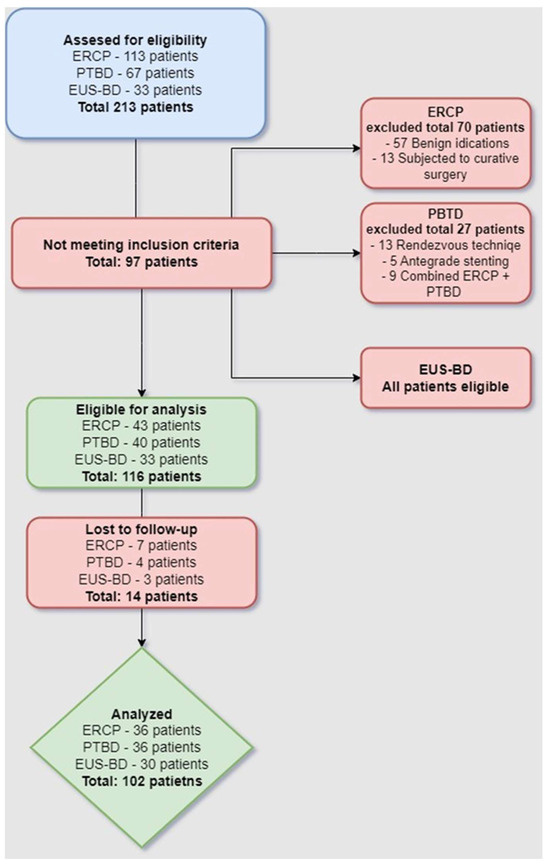
Figure 4.
Study selection process.
4.3. Statistical Methods
The SPSS statistical software version 27.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Most of the variables were categorical or ordinal and were summarized using frequencies and percentages (%). The chi-square test was used to analyze associations between target variables with more than two categories, whereas the Fisher’s exact test was used for variables with two categories. The normality of continuously measured variables (such as age and hospital stay in days) was assessed using the Shapiro–Wilk test. If normality was confirmed, the central tendencies were described through the mean values and standard deviations. Respectively, between-group comparisons were conducted using one-way ANOVAs. All statistical analyses were two-tailed, with a Type I error alpha level set at 0.05.
5. Results
5.1. Background and Clinical Data
The study comprised 102 patients, divided into three groups as follows: 36 patients who underwent endoscopic retrograde cholangiopancreatography (ERCP), 36 patients treated with percutaneous transhepatic biliary drainage (PTBD), and 30 patients who underwent endoscopic ultrasound-guided biliary drainage (EUS-BD) (Figure 5).
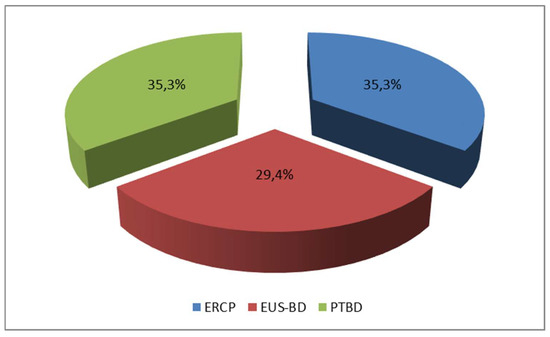
Figure 5.
Distribution of patients based on the selected procedure.
There were no significant age differences between the study groups (p = 0.577). The gender distribution showed a significantly higher proportion of males in the studied groups (p = 0.047).
Prior interventions occurred in a significantly greater proportion of patients in the PTBD group (63.90%) and the EUS-BD group (50.00%) than in the ERCP group (2.80%), p < 0.001. The PTBD group showed the highest proportion of reinterventions (52.80%) compared to the ERCP group (25.00%) and the EUS-BD group (3.30%), with significant differences between the groups (p < 0.001). Results are summarized in Figure 6.
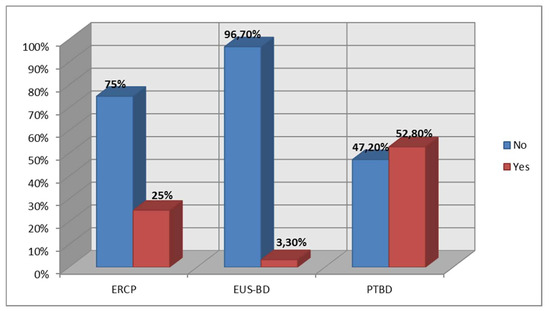
Figure 6.
Reintervention rate based on procedure.
Pancreatic cancer was present in a significantly higher percentage of patients in the ERCP treatment group (52.8%) compared to the EUS-BD group (43.30%) and the PTBD group (22.20%) (p = 0.014). The incidence of cholangiocarcinoma was found to be considerably greater in the PTBD group (41.70%) compared to the EUS-BD group (23.30%) and the ERCP group (13.90%) (p = 0.017).
The frequency of the remaining types was relatively low, with no significant variations among the groups. A significant distinction among the treatment groups was the incidence of stenosis in the distal region. The ERCP group had the highest incidence of distal stenosis (66.70%), the EUS-BD group came in second (50.00%), and the PTBD group had a significantly lower incidence of 25.00% (p = 0.010). In all treatment groups, the performance status revealed that the majority of the patients were classified into levels 3 and 4, with no significant variations among the groups. Some significant differences in performance status were observed with respect to the initial two levels (1 and 2).
The types of stents that were used differed for the different treatments. In the ERCP group, FC-SEMSs were utilized most frequently (61.10%); in the PTBD group, pigtail plastic drains were used in 97.20% of the cases; and in the EUS-BD group, HGS-SEMSs and FC-SEMSs were typical. A summary of the clinical data is presented in Table 1.

Table 1.
Clinical information about the patients.
The decision for PTBD and EUS-BD was primary or determined by various factors such as altered anatomy, inaccessible papilla, failed cannulation, etc. A summary of the decision-making process is presented in Figure 7.
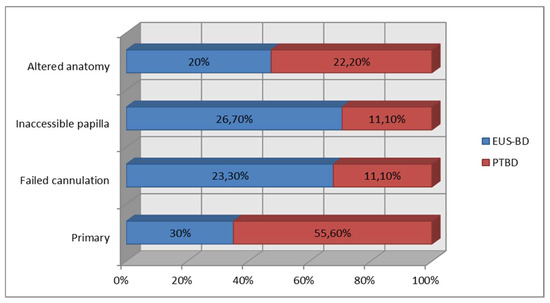
Figure 7.
Decision for EUS-BD/PTBD.
5.2. Technical and Clinical Success Rate
The technical success rate was not significantly associated with the type of treatment (p = 0.460), showing the following rates in descending order: 97.20% ERCP group (n = 35); 94.40% PTBD group (n = 34); and 90.00% EUS-BD group (n = 27).
Similarly, the clinical success rate did not show significant differences that could be associated with the type of treatment (p = 0.182); however, the highest rate was achieved in the ERCP group and the lowest in the PTBD group: 88.90% ERCP group (n = 32); 83.30% EUS-BD group (n = 25); and 72.20% PTBD group (n = 26) (Figure 8).
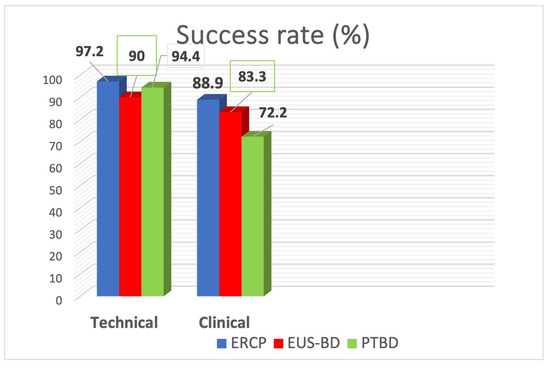
Figure 8.
Technical and clinical success rate.
In all three treatments, each analyzed separately, the technical and clinical success rates were not significantly associated with the etiology of the obstruction, the localization of the obstruction, or the performance status of the patient, with p-values > 0.05 for all 18 chi-square tests that were performed to examine these associations.
5.3. Adverse Events
The AEs, including deaths related to the procedures, are illustrated in Figure 9. The lowest rate of adverse reactions occurred in the EUS-BD group (10%, n = 3), compared to 27.90% (n = 10) in the ERCP group and 38.90% (n = 12 + 2 deaths) in the PTBD group. The difference of 28.90% between the EUS-BD treatment and the PTBD treatment was significant (p = 0.010). The two deaths occurred in the PTBD group (5.60%).
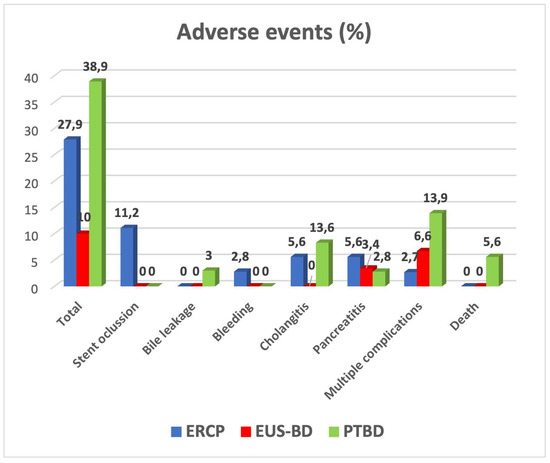
Figure 9.
Adverse events.
In any of the treatment groups, there were no significant associations between the incidence of AEs, the etiology and localization of the obstruction, and the performance status of the patients: ERCP group (etiology p = 0.541; localization p = 0.221; performance status p = 0.070); PTBD group (etiology p = 0.747; localization p = 0.504; performance status p = 0.640); EUS-BD group (etiology p = 0.449; localization p = 0.811; performance status p = 0.577). An overview of the AEs incidence and distribution is provided in Figure 10.
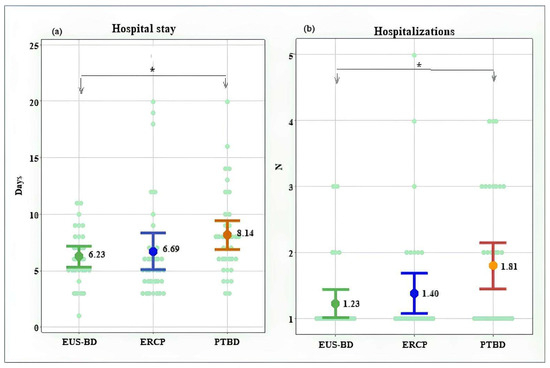
Figure 10.
Length of hospital stay (a); number of hospitalizations (b). *—Significant difference at p < 0.05.
The distribution of AEs based on the Clavien–Dindo system is presented in Table 2.

Table 2.
Distribution of AEs based on the Clavien–Dindo system.
5.4. Length of Hospital Stay and Number of Hospitalizations
The mean duration of hospitalization for the patients in the EUS-BD treatment group was 6.23 ± 2.48 days, compared to 6.69 ± 3.81 days in the ERCP group and 8.14 ± 3.77 days in the PTBD group. A statistically significant difference in hospital stay was observed between the EUS-BD and PTBD groups (p = 0.017). The individual length of hospitalization ranged between 1 and 11 days in the EUS-BD group, compared to a wider range (3 to 20 days) in the other two groups (Figure 10a and Figure 11).
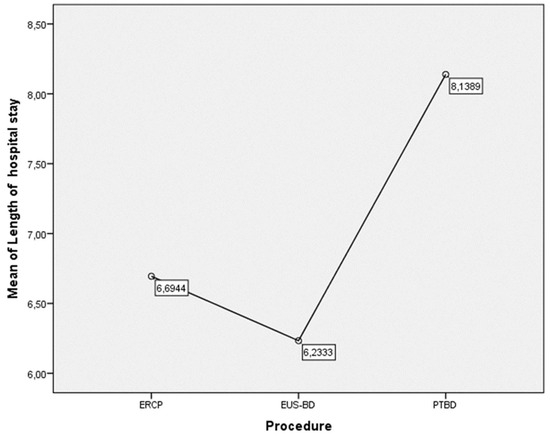
Figure 11.
Mean hospital stay (days) depending on the type of procedure.
The EUS-BD treatment was associated with fewer hospitalizations (1.23 ± 0.56) in comparison to 1.40 ± 0.90 in the ERCP group and 1.81 ± 1.03 in the PTBD group, with a significant difference to the PTBD group (p = 0.024). The maximum number of hospitalizations was three in the EUS-BD group, four in the PTBD group, and five in the ERCP group (Figure 10b and Figure 12).
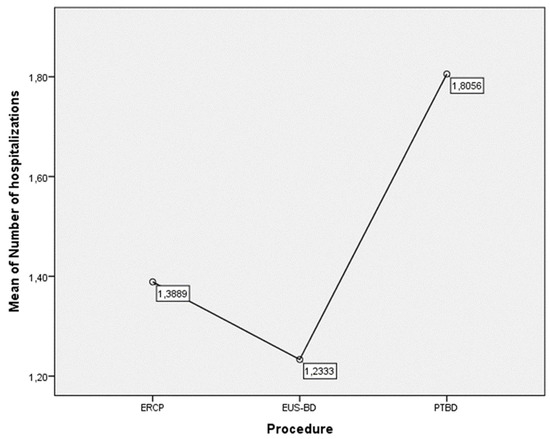
Figure 12.
Mean number of hospitalizations based on the type of procedure.
6. Discussion
Relieving obstructive jaundice is the cornerstone in the complex management of biliopancreatic malignancies, giving their low resectability rate. The constant development of interventional gastroenterology and endoscopy resulted in a marked transition from surgical to mini-invasive techniques for biliary drainage, especially in palliative settings. ERCP and PTBD have long been recognized as viable therapeutic options, with ERCP generally being considered as a first-choice treatment, with certain exceptions based on level of obstruction and local expertise. The advent of EUS-BD questioned the established paradigm in the last 50 years, providing an alternative to both methods with excellent clinical outcomes in the presence of adequate expertise. The current article aimed to provide a comparative evaluation of all procedures, selecting similar patient cohorts.
Similar numbers of patients were included in all three groups. While ERCP procedures are exceedingly more common, we included a relatively small sample size to be comparable to PTBD and EUS-BD. Modifications of PBI were deliberately excluded from analysis to achieve more homogenous results in the PTBD group. There were no age differences in the investigated groups. In all cohorts, male sex was predominant, which is to be expected, taking into consideration the higher incidence of malignant biliary obstruction in males. In all groups were included only patients with unresectable malignancy, which we consider valuable in enhancing comparability of results. Understandably, there was discrepancy in the etiology of obstruction, with pancreatic cancer (distal stenosis) being the most common indication for ERCP and cholangiocarcinoma (proximal stenosis) for PTBD. This finding is generally in line with current recommendations stating that ERCP is the first-line treatment in malignant obstruction, while PTBD might be considered as a first-choice treatment in proximal biliary stenosis, especially complex ones (Bismuth-Corlette III and IV; see Appendix Table A3) [21]. EUS-BD had equal distribution of indications between proximal and biliary stenoses. In the evaluated group were included both EUS-CDS (used in distal stenoses) and EUS-HGS (predominantly used for proximal stenoses) procedures, which explains this finding. In the current research, we found no difference in terms of clinical outcomes between the two variations of EUS-BD, but the sample size (especially EUS-CDS group) was too small to derive a statistically significant conclusion.
The primary endpoint of the current paper was to evaluate the clinical outcomes of ERCP, PTBD, and EUS-BD in terms of technical and clinical success and AEs rate. Overall, we found no statistically significant difference between the procedures’ technical (p = 0.460) and clinical (p = 0.182) success. Those results are identical to the ones obtained in other studies. For ERCP, clinical and technical success of 96.0% and 94.8%, respectively, have been established [22]. Notably, those results reflect cumulative success rates of all ERCP procedures irrespective of indication. Considering that in the current cohort were included more complex cases (defined as grade II and III according to the ASGE grading system, see Appendix Table A4), the lower clinical success rate is understandable. The outcomes of EUS-BD (94–96% technical and 87–88% clinical success) and PTBD (75–100% technical and 82.4% clinical success) are also similar to the ones obtained in our study [23,24]. None of the factors investigated (etiology and level of stenosis and performance status) had significant influence on the clinical outcomes as a whole or in any group in particular. This fact contradicts the general statement claiming that proximal strictures are more challenging and associated with less favorable outcomes.
While ERCP is obviously superior to EUS-BD and PTBD in terms of clinical and technical success, a few factors should be taken into consideration. Firstly, ERCP is almost exclusively a first-choice procedure. Our research found that in merely 30% of EUS-BD and 55.5% of PTBD groups, the treatment decision was primary. In all other cases, they were used as salvage techniques after failed ERCP. This theoretically includes patients with more complex strictures or advanced disease, which understandably are more challenging to manage. Furthermore, current results do not account for the patients ineligible for ERCP (inaccessible or grossly infiltrated papilla or surgically altered anatomy). One might speculate that if used as a primary procedure, EUS-BD (at least in the presence of adequate expertise) might provide better clinical outcomes compared to ERCP. Further prospective randomized studies are needed to verify such a statement.
Another chief factor when assessing the performance of interventional procedures on the biliary tree is the AEs rate. Our study found that EUS-BD had statistically significant fewer immediate and delayed AEs (10%) compared to ERCP (27.9%) and PTBD (38.9% including two deaths). In the literature, a higher incidence of AEs after EUS-BD (17–23%) is reported, but those results derive mainly from older studies, while newer research establishes a significantly better safety profile. Andreloni et al., for instance, reported an 11.6% AE rate when using lumen apposing metal stents for EUS-CDS [25]. Complication rates of PTBD reported in the literature vary greatly, but a more recent study reported a 50% AE rate with an 8.8% mortality directly associated with the procedure [13]. These data are largely attributable to our results. For ERCP, we found a higher incidence of AEs compared to that in the literature. However, we should emphasize that a significant proportion of those events were delayed (11.7% stent occlusion) and not directly associated with the procedure.
Another finding in the current paper that we find essential when assessing the performance of interventional procedures on the biliary tree is the need for reinterventions. Reintervention was needed in only 3.3% of EUS-BD patients, compared to 25% of ERCP and up to 52.8% of the patients in the PTBD group (p < 0.001). Existing data support our finding, claiming that EUS-BD is associated with a higher stent patency rate [19]. We found no difference between the three procedures based on the severity of AEs according to the Clavien–Dindo system. None of the evaluated factors (etiology and level of obstruction and PS) showed a statistically significant correlation with the AEs incidence.
Considering life quality in the current study, we evaluated the number of hospital admissions and the mean hospital stay for the patients with biliary tract interventions in palliative settings. In our opinion, the need for multiple readmissions and continuous hospital in-stays has a profound negative impact on the patients’ life quality, especially in those with advanced malignant disease. We found that EUS-BD was associated with statistically significant shorter hospital stay (6.23 d vs. 8.12 d) and fewer hospitalizations (1.23 vs. 1.80) compared PTBD (p < 0.05). These data reflect the general opinion that PTBD (even when clinically successful) is impairing patients’ life quality. Comparing EUS-BD and ERCP, we found a similar trend towards shorter hospital stay and fewer hospitalizations for the EUS-BD group, albeit not reaching statistical significance. Of note, all of the readmissions were procedure-related (need for reintervention or conservative management of a complication), since patients needing merely supportive and/or symptomatic treatment were managed in oncological departments.
The current study has certain limitations. While including consecutive patients, its retrospective nature obviously increases the risk of bias. The sample size is still too small to derive firm conclusions on certain aspects. On the other hand, it is our opinion that using hospital data as the main source of information makes the results attributable to real clinical practice.
7. Conclusions
Management strategies for obstructive jaundice are constantly evolving. ERCP is still the first-line treatment of malignant biliary obstruction in palliative settings. PTBD is a valid alternative, showing acceptable technical and clinical success. EUS-BD is comparable to ERCP and superior to PTBD for resolution of biliary obstruction in terms of technical and clinical success. In the presence of adequate expertise, EUS-BD is associated with fewer AEs and reinterventions compared to ERCP and PTBD, resulting in fewer hospitalizations and overall hospital stay. Our study suggests that EUS-BD may be considered as a primary therapeutic modality in malignant biliary obstruction, especially in selected patients with anticipated high risk of treatment failure by ERCP. Well-designed prospective comparative trials are needed to verify such a statement.
Author Contributions
Conceptualization, V.A. and D.C.; methodology, D.D.; formal analysis, M.S. and K.K.; investigation, B.H. and R.D.; resources, D.D.; data curation, K.K. and S.V.; writing—original draft preparation, B.H. and S.V.; writing—review and editing, G.K. and P.U.; visualization, K.D.; supervision, V.A. and R.D.; project administration, M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of University hospital “Kaspela” (protocol number 2/11.01.2024) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AE | Adverse event |
| ASGE | American Society of Gastrointestinal Endoscopy |
| CT | Computer tomography |
| CECT | Contrast-enhanced computer tomography |
| ECOG | Eastern Cooperative Oncology Group |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| ESGE | European Society of Gastrointestinal Endoscopy |
| EUS | Endoscopic ultrasonography |
| EUS-BD | Endoscopic ultrasound-guided biliary drainage |
| EUS-CDS | Endoscopic ultrasound-guided choledochoduodenostomy |
| EUS-HGS | Endoscopic ultrasound-guided hepaticogastrostomy |
| FC-SEMS | Fully covered self-expandable metal stent |
| FNA | Fine needle aspiration |
| HGS-SEMS | Hepatico-gastrostomy SEMS |
| ICU | Intensive care unit |
| MBO | Malignant biliary obstruction |
| MRCP | Magnetic resonance cholangiopancreatography |
| PBI | Percutaneous biliary interventions |
| PEP | Post-ERCP pancreatitis |
| PS | Performance status |
| PTBD | Percutaneous transhepatic biliary drainage |
| SEMS | Self-expandable metal stent |
| SGEI | Society of Gastrointestinal Endoscopy of India |
| US | Ultrasound |
Appendix A

Table A1.
ECOG Performance status scale.
Table A1.
ECOG Performance status scale.
| Grade | ECOG Performance Status |
|---|---|
| 0 | Fully active, able to carry on all predisease performance without restriction. |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work. |
| 2 | Ambulatory and capable of all selfcare but unable to carry out any work activities; up and about more than 50% of waking hours. |
| 3 | Capable of only limited selfcare; confined to bed or chair more than 50% of waking hours. |
| 4 | Completely disabled; cannot carry on any selfcare; totally confined to bed or chair. |
| 5 | Dead. |
ECOG—Eastern Cooperative Oncology Group.

Table A2.
Clavien–Dindo classification of AE.
Table A2.
Clavien–Dindo classification of AE.
| Grade | Definition of Grades | Modes of Therapy |
|---|---|---|
| Grade I | Any deviation from the normal postoperative course. | No pharmacological or surgical treatment, endoscopic, or radiological interventions were required. Acceptable therapeutic regimens are drugs such as antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy. Wound infections or small abscess requiring incision at bedside are within this category. |
| Grade II | Normal course altered. | Pharmacological management other than Grade I. Blood transfusions and total parenteral nutrition are also included. |
| Grade III | Complications that require intervention of various degrees. | Grade IIIa—complications that require an intervention performed under local anesthesia. |
| Grade IIIb—interventions that require general or epidural anesthesia. | ||
| Grade IV | Complications threatening life of patients (including CNS complications), requiring ICU support. | Grade IVa—single organ dysfunction (including dialysis). |
| Grade IVb—multiorgan dysfunction. | ||
| Grade V | Death of patient. |
AE—adverse event; ICU—intensive care unit.

Table A3.
Bismuth–Corlette classification.
Table A3.
Bismuth–Corlette classification.
| Type I | Tumor involves the common hepatic duct below the confluence of the left and right hepatic ducts. |
| Type II | Tumor involves the confluence of the left and right hepatic ducts. |
| Type IIIa | Tumor involves the confluence and right hepatic duct. |
| Type IIIb | Tumor involves the confluence and left hepatic duct. |
| Type IVa | Tumor involves the confluence and both left and right hepatic duct. |
| Type IVb | Multicentric tumors. |

Table A4.
ASGE degrees of complexity of ERCP [26].
Table A4.
ASGE degrees of complexity of ERCP [26].
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
ASGE—American Society of Gastrointestinal Endoscopy; FNA—fine needle aspiration; ERCP—endoscopic retrograde cholangiopancreatography.
References
- Björnsson, E.; Gustafsson, J.; Borkman, J.; Kilander, A. Fate of patients with obstructive jaundice. J. Hosp. Med. 2008, 3, 117–123. [Google Scholar] [CrossRef]
- Carriaga, M.T.; Henson, D.E. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 1995, 75, 171–190. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Parasher, G.; Lee, J.G. The role of ERCP in pancreato-biliary malignancies. Adv. Dig. Endosc. ERCP 2006, 6, 120–141. [Google Scholar]
- Ho, C.S.; E Warkentin, A. Evidence-Based Decompression in Malignant Biliary Obstruction. Korean J. Radiol. 2012, 13 (Suppl. 1), S56–S61. [Google Scholar] [CrossRef]
- Distler, M.; Kersting, S.; Rückert, F.; Dobrowolski, F.; Miehlke, S.; Grützmann, R.; Saeger, H.-D. Palliative treatment of obstructive jaundice in patients with carcinoma of the pancreatic head or distal biliary tree. Endoscopic stent placement vs. hepaticojejunostomy. J. Pancreas 2010, 11, 568–574. [Google Scholar] [CrossRef]
- Soares, K.C.; Kamel, I.; Cosgrove, D.P.; Herman, J.M.; Pawlik, T.M. Hilar cholangiocarcinoma: Diagnosis, treatment options, and management. Hepatobiliary Surg. Nutr. 2014, 3, 18–34. [Google Scholar]
- McCune, W.S. ERCP at thirty years: An interview with Dr. William S. McCune (1909–1998). Gastrointest. Endosc. 1998, 48, 643–644. [Google Scholar] [CrossRef]
- Szary, N.M.; Al-Kawas, F.H. Complications of endoscopic retrograde cholangiopancreatography: How to avoid and manage them. Gastroenterol. Hepatol. 2013, 9, 496–504. [Google Scholar]
- Ribeiro, I.B.; Junior, E.S.D.M.; Neto, A.A.M.; Proença, I.M.; de Moura, D.T.H.; Minata, M.K.; Ide, E.; dos Santos, M.E.L.; Luz, G.d.O.; Matuguma, S.E.; et al. Pancreatitis after endoscopic retrograde cholangiopancreatography: A narrative review. World J. Gastroenterol. 2021, 27, 2495–2506. [Google Scholar] [CrossRef]
- Makuuchi, M.; Beppu, T.; Kamiya, K.; Futagawa, S.; Sugiura, M.; Wada, T.; Muroi, T. Echo guide percutaneous transhepatic cholangiography. Jpn. J. Surg. 1977, 8, 165–175. [Google Scholar] [CrossRef]
- Van Delden, O.M.; Laméris, J.S. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur. Radiol. 2007, 18, 448–456. [Google Scholar] [CrossRef]
- Turan, A.S.; Jenniskens, S.; Martens, J.M.; Rutten, M.J.C.M.; Yo, L.S.F.; van Strijen, M.J.L.; Drenth, J.P.H.; Siersema, P.D.; van Geenen, E.J.M. Complications of percutaneous transhepatic cholangiography and biliary drainage, a multicenter observational study. Abdom. Radiol. 2022, 47, 3338–3344. [Google Scholar] [CrossRef]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J. Endoscopic Ultrasound-Guided Bilioduodenal Anastomosis: A New Technique for Biliary Drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef]
- Giovannini, M.; Dotti, M.; Bories, E.; Moutardier, V.; Pesenti, C.; Danisi, C.; Delpero, J. Hepaticogastrostomy by Echo-Endoscopy as a Palliative Treatment in a Patient with Metastatic Biliary Obstruction. Endoscopy 2003, 35, 1076–1078. [Google Scholar] [CrossRef]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Rai, P.; Udawat, P.; Chowdhary, S.D.; Gunjan, D.; Samanta, J.; Bhatia, V.; Club, I.E. Society of Gastrointestinal Endoscopy of India Consensus Guidelines on Endoscopic Ultrasound-Guided Biliary Drainage: Part I (Indications, Outcomes, Comparative Evaluations, Training). J. Digest. Endosc. 2023, 14, 30–40. [Google Scholar] [CrossRef]
- Tanikawa, T.; Ishii, K.; Katsumata, R.; Urata, N.; Nishino, K.; Suehiro, M.; Kawanaka, M.; Haruma, K.; Kawamoto, H. Efficacy of primary drainage by endoscopic ultrasound-guided biliary drainage for unresectable pancreatic adenocarcinoma. JGH Open 2022, 6, 251–256. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.-H.; Kim, S.-O.; Jang, S.; Kim, D.U. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997, Erratum in Am. J. Gastroenterol. 2018, 113, 1566. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, S.-O.; So, H.; Shin, E.; Kim, D.U.; Park, D.H. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16551. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; van Hooft, J.E. Endoscopic biliary Stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Demir, T.; Ustaoglu, M. Evaluation of the success and complication rates of endoscopic retrograde cholangiography according to the difficulty of the procedure. Precis. Med. Sci. 2023, 12, 4–9. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, J.; Xing, L.; Wang, Y.; Jin, Z.; Li, Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest. Endosc. 2016, 83, 1218–1227. [Google Scholar] [CrossRef]
- Zerem, E.; Imširović, B.; Kunosić, S.; Zerem, D.; Zerem, O. Percutaneous biliary drainage for obstructive jaundice in patients with inoperable, malignant biliary obstruction. Clin. Exp. Hepatol. 2022, 8, 70–77. [Google Scholar] [CrossRef]
- Anderloni, A.; Fugazza, A.; Troncone, E.; Auriemma, F.; Carrara, S.; Semeraro, R.; Maselli, R.; Di Leo, M.; D’Amico, F.; Sethi, A.; et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest. Endosc. 2018, 89, 69–76. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.; Romagnuolo, J.; Vargo, J.; Baron, T.; Tarnasky, P.; Petersen, B. Grading the complexity of endoscopic procedures: Results of an ASGE working party. Gastrointest. Endosc. 2011, 73, 868–874. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).