The Relationship between Serum Zonulin and Innate Immunity in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Examination
2.2. Statistical Analysis
3. Results
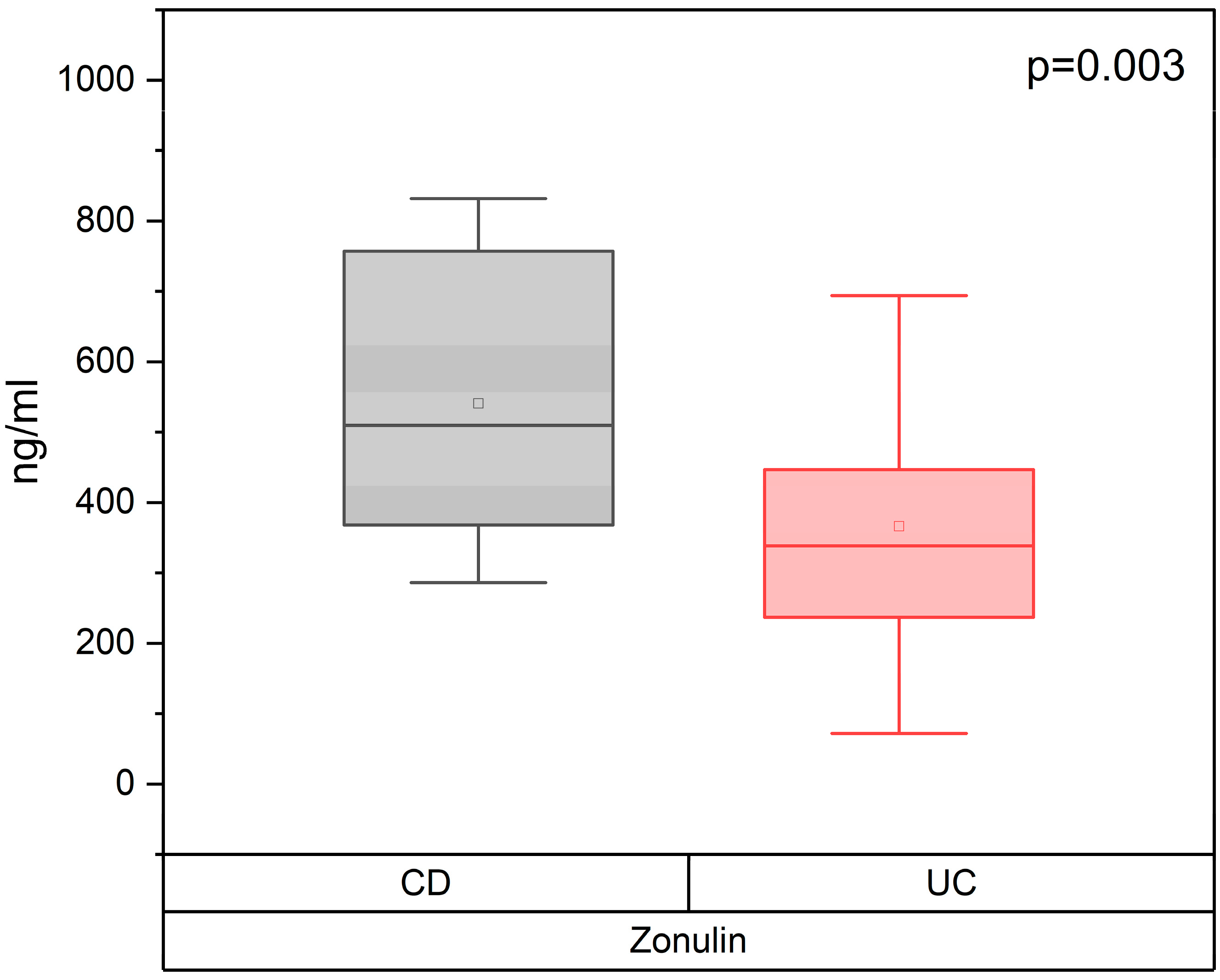
3.1. Concentration of the Serum Zonulin in Patients with CD and UC, Depending on the Severity of IBD
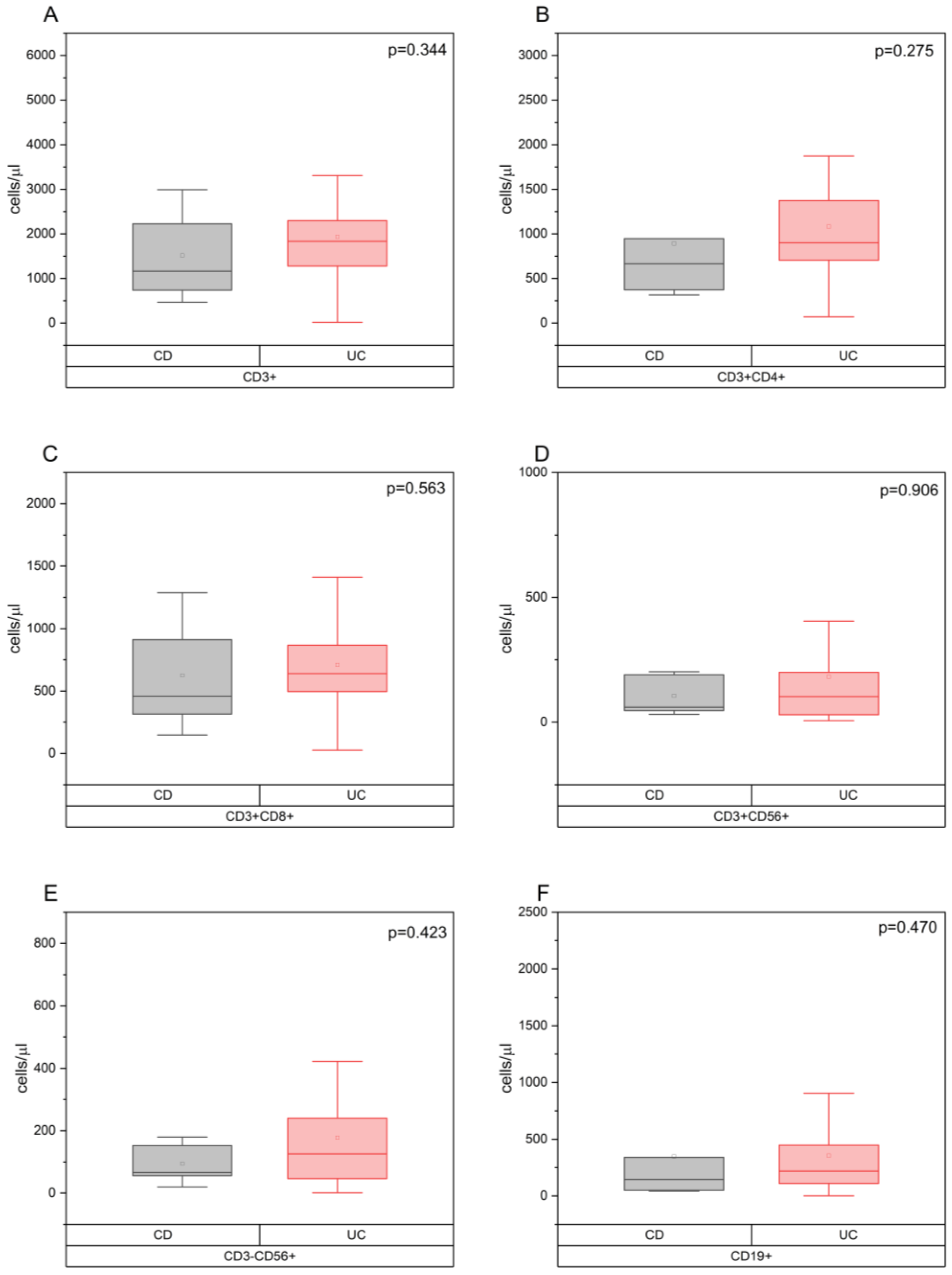
3.2. The Relationship between the Concentration of Zonulin in the Blood Serum and the Indicators of Cellular Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769. [Google Scholar] [CrossRef] [PubMed]
- Zybina, N.N.; Nikonov, E.L.; Gershtein, E.S.; Memdli, Z.Z.; Stilidi, I.S.; Kushlinskii, N.E. Zonulin is a marker of epithelial and endothelial barrier functions in non-communicable diseases (narrative review). Russ. J. Evid.-Based Gastroenterol. 2022, 11, 28–44. (In Russia) [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, F1000 Faculty Rev-69. [Google Scholar] [CrossRef]
- Ivashkin, V.T.; Shelygin, Y.A.; Khalif, I.L.; Belousova, E.A.; Shifrin, O.S.; Abdulganieva, D.I.; Abdulkhakov, R.A.; Alekseeva, O.P.; Alekseenko, S.A.; Achkasov, S.I.; et al. Clinical guide of russian association of gastroenterology and russian association of coloproctology on diagnostics and treatment of ulcerative colitis. Koloproktologia 2017, 1, 6–30. (In Russia) [Google Scholar]
- Ivashkin, V.T.; Shelygin, Y.A.; Khalif, I.L.; Belousova, E.A.; Shifrin, O.S.; Abdulganieva, D.I.; Abdulkhakov, R.A.; Alekseeva, O.P.; Alekseenko, S.A.; Achkasov, S.I.; et al. Clinical guide of russian association of gastroenterology and russian association of coloproctology on diagnostics and treatment of Crohn’s disease. Koloproktologia 2017, 2, 7–29. (In Russia) [Google Scholar]
- Dolgushin, I.I.; Savochkina, A.Y.; Pykhova, L.R.; Abramovskihk, O.S.; Chetvernina, E.A.; Poltorak, A.E. Comparative analysis of the indicators of the functional activity of neutrophils peripheral blood in patients with severe sepsis and septic shock. Russ. Immunol. J. 2019, 22, 236–238. (In Russia) [Google Scholar]
- Malíčková, K.; Francová, I.; Lukáš, M.; Kolář, M.; Králíková, E.; Bortlík, M.; Ďuricová, D.; Štěpánková, L.; Zvolská, K.; Pánková, A.; et al. Fecal zonulin is elevated in Crohn’s disease and in cigarette smokers. Pract. Lab. Med. 2017, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Buhner, S.; Buning, C.; Genschel, J.; Kling, K.; Herrmann, D.; Dignass, A.; Kuechler, I.; Krueger, S.; Schmidt, H.H.; Lochs, H. Genetic basis for increased intestinal permeability in families with Crohn’s disease: Role of CARD15 3020insC mutation? Gut 2006, 55, 342–347. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum zonulin in patients with inflammatory bowel disease: A pilot study. Minerva Med. 2019, 110, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Memon, A.A.; Palmér, K.; Hedelius, A.; Sundquist, J.; Sundquist, K. The association of zonulin-related proteins with prevalent and incident inflammatory bowel disease. BMC Gastroenterol. 2022, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Wood Heickman, L.K.; DeBoer, M.D.; Fasano, A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes Metab. Res. Rev. 2020, 36, e3309. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.C. Intestinal permeability and autoimmune diseases. Biosci. Horiz. Int. J. Stud. Res. 2017, 10, hzx015. [Google Scholar] [CrossRef]
- Gokulakrishnan, K.; Nikhil, J.; Vs, S.; Holla, B.; Thirumoorthy, C.; Sandhya, N.; Nichenametla, S.; Pathak, H.; Shivakumar, V.; Debnath, M.; et al. Altered Intestinal Permeability Biomarkers in Schizophrenia: A Possible Link with Subclinical Inflammation. Ann. Neurosci. 2022, 29, 151–158. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; He, D.M.; Shu, G.F.; Cao, B.; Xia, Y.Q.; Xing, Y.; Ni, M.; Chen, J.F.; Shi, S.L.; Gu, H.F.; et al. Increased formation of neutrophil extracellular traps is associated with gut leakage in patients with type 1 but not type 2 diabetes. J. Diabetes 2019, 11, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; An, L.L.; Chaerkady, R.; Mittereder, N.; Clarke, L.; Cohen, T.S.; Chen, B.; Hess, S.; Sims, G.P.; Mustelin, T. Evidence for a direct link between PAD4-mediated citrullination and the oxidative burst in human neutrophils. Sci. Rep. 2018, 8, 15228. [Google Scholar] [CrossRef]
- Ansari, J.; Vital, S.A.; Yadav, S.; Gavins, F.N.E. Regulating Neutrophil PAD4/NOX-Dependent Cerebrovasular Thromboinflammation. Int. J. Biol. Sci. 2023, 19, 852–864. [Google Scholar] [CrossRef]
- Tkachev, A.V.; Mkrtchyan, L.S.; Belovolova, R.A.; Devlikamova, T.A. Diagnostic significance of immunological markers with various forms of ulcerative colitis. Med. News North Cauc. 2019, 14, 334–338. (In Russia) [Google Scholar] [CrossRef]
- Long, Y.; Xia, C.; Sun, Y.; Ma, Y.; Xu, L.; Song, Y.; Liu, C. Increased circulating PD-1hiCXCR5- peripheral helper T cells are associated with disease severity of active ulcerative colitis patients. Immunol. Lett. 2021, 233, 2–10. [Google Scholar] [CrossRef]
- Geng, B.; Ding, X.; Li, X.; Liu, H.; Zhao, W.; Gong, H.; Tian, Z.; Guo, J. Peripheral blood T-lymphocyte subsets are potential biomarkers of disease severity and clinical outcomes in patients with ulcerative colitis: A retrospective study. BMC Gastroenterol. 2023, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Dąbek-Drobny, A.; Kaczmarczyk, O.; Piątek-Guziewicz, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Zagrodzki, P.; et al. Application of the Clustering Technique to Multiple Nutritional Factors Related to Inflammation and Disease Progression in Patients with Inflammatory Bowel Disease. Nutrients 2022, 14, 3960. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Altunisik, N.; Turkmen, D.; Uremis, M.M.; Sener, S.; Turkoz, Y. Evaluation of plasma zonulin level and its relationship with inflammatory cytokines in patients with vitiligo. J. Cosmet. Dermatol. 2023, 22, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, L.A.C.; Matiollo, C.; Rosa, J.S.D.; Felisberto, M.; Dalmarco, E.M.; Schiavon, L.L. Factors associated with circulating zonulin in inflammatory bowel disease. Arq. Gastroenterol. 2022, 59, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, X.; Shi, S.; Liu, L.; Lv, J.; Zhu, L.; Zhang, H. Zonulin, as a marker of intestinal permeability, is elevated in IgA nephropathy and IgA vasculitis with nephritis. Clin. Kidney J. 2022, 16, 184–191. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Wakita, D.; Franklin, M.K.; Carvalho, T.T.; Abolhesn, A.; Gomez, A.C.; Fishbein, M.C.; Chen, S.; Lehman, T.J.; Sato, K.; et al. Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation. Immunity 2019, 51, 508–521.e6. [Google Scholar] [CrossRef]




| Characteristics | CD (n = 13) | UC (n = 84) | Total (n = 97) | p |
|---|---|---|---|---|
| Male (n, %)/Female (n, %) | 2 (15.4%)/11 (84.6%) | 41 (48.8%)/43 (51.2%) | 43 (44.3%)/54 (55.7%) | 0.024 |
| Age, years (Me, [LQ; UQ]) | 40 (32.5; 64.0) | 45 (34.3; 56.8) | 45 (34.5; 57.0) | 0.987 |
| Age of IBD onset, years (Me, [LQ; UQ]) | 33.0 (29.0; 54.5) | 37.0 (28.0; 47.5) | 36.0 (28.0; 48.0) | 0.983 |
| IBD severity: | ||||
| Mild (n, %) | 2 (15.4%) | 19 (22.6%) | 21 (21.6%) | 0.556 |
| Moderate (n, %) | 3 (23.1%) | 24 (28.6%) | 27 (27.8%) | 0.681 |
| Severe (n, %) | 8 (61.5%) | 41 (48.8%) | 49 (50.6%) | 0.393 |
| CD localiztion: | ||||
| Isolated upper disease (n, %) | 1 (7.7%) | |||
| Ileal (n, %) | 2 (15.4%) | |||
| Ileocolonic (n, %) | 6 (46.2%) | |||
| Colonic (n, %) | 5 (38.4%) | |||
| UC localization: | ||||
| Proctitis (n, %) | 4 (4.8%) | |||
| Left-sided colitis (n, %) | 19 (22.6%) | |||
| Pancolitis (n, %) | 61 (72.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khusainova, G.; Genkel, V.; Kuznetsova, A.; Nikushkina, K.; Saenko, A.; Abramovskikh, O.; Dolgushina, A. The Relationship between Serum Zonulin and Innate Immunity in Patients with Inflammatory Bowel Disease. Gastroenterol. Insights 2024, 15, 179-190. https://doi.org/10.3390/gastroent15010013
Khusainova G, Genkel V, Kuznetsova A, Nikushkina K, Saenko A, Abramovskikh O, Dolgushina A. The Relationship between Serum Zonulin and Innate Immunity in Patients with Inflammatory Bowel Disease. Gastroenterology Insights. 2024; 15(1):179-190. https://doi.org/10.3390/gastroent15010013
Chicago/Turabian StyleKhusainova, Gusel, Vadim Genkel, Alla Kuznetsova, Karina Nikushkina, Anna Saenko, Olga Abramovskikh, and Anastasiya Dolgushina. 2024. "The Relationship between Serum Zonulin and Innate Immunity in Patients with Inflammatory Bowel Disease" Gastroenterology Insights 15, no. 1: 179-190. https://doi.org/10.3390/gastroent15010013
APA StyleKhusainova, G., Genkel, V., Kuznetsova, A., Nikushkina, K., Saenko, A., Abramovskikh, O., & Dolgushina, A. (2024). The Relationship between Serum Zonulin and Innate Immunity in Patients with Inflammatory Bowel Disease. Gastroenterology Insights, 15(1), 179-190. https://doi.org/10.3390/gastroent15010013








