Abstract
The gastrointestinal (GI) tract may be a significant entrance or interaction site for SARS-CoV-2; therefore, the gut mucosal immune system participates in virus interaction as a first-line physical and immunological defense, leading to GI involvement and symptoms. This review focuses on the GI symptoms associated with SARS-CoV-2 infection while providing specific results on variant-specific signs and syndromes related to coronavirus disease 2019 (COVID-19). The pattern of symptoms changed during the virus evolution, since the data provided a current and thorough picture of the symptoms experienced by SARS-CoV-2 infected people, and variations in symptom patterns occurred as the Alpha, Delta, and Omicron variants have spread. Since the beginning of the pandemic, GI symptoms have been linked to SARS-CoV-2 infections, even though most infected people do not report them. For example, diarrhea (28.2%) was the most frequently reported GI symptom in the early phase of the pandemic. The most observed GI tract symptoms during COVID-19 were anorexia (loss of appetite), nausea, vomiting, diarrhea, and abdominal pain, usually in at least one-third of the patients. Mesenteric ischemia and GI bleeding were less observed but more severe. While GI symptoms are not associated with increased mortality, they complicate the disease, increase the duration of the illness, and result in worse outcomes. Nevertheless, it is accepted that symptoms between variants differ significantly, i.e., the Omicron variant causes milder COVID-19 than the Delta. Still, the rate of GI symptoms has declined in the following variant-dominated phases of the pandemic (Alpha: 19.4%, Delta: 17.9%, Omicron: 13.8%), which was also demonstrated for other GI signs associated with COVID-19.
Keywords:
COVID-19; SARS-CoV-2; variant-specific; Alpha; Delta; Omicron; gastrointestinal symptoms; diarrhea; loss of taste; nausea; abdominal pain 1. Introduction
In late November 2021, South Africa reported the latest variant B.1.1.529 of severe acute respiratory syndrome virus 2 (SARS-CoV-2) to the World Health Organization (WHO). Compared to the other SARS-CoV-2 variants, the new variant has a higher risk of infection, spreads faster, and has been named “Omicron” by the WHO [1]. Since then, the omicron variant BA.1 has spread rapidly worldwide, displacing the previously dominant delta variant. In addition, other variants evolved and spread: Omicron BA.2, which has 1.4 times the effective reproduction number of the mutant BA.1, with vaccine-induced immunity being primarily ineffective, and then two other sub-variants, BA.4 and BA.5, replaced BA.2 as the dominant strains in South Africa in early May 2022, and their transmission ability increased [1,2,3].
Menni et al. demonstrated that other organ complications, such as gastrointestinal problems (GI), were uncommon in the last virus variants. However, because the GI tract may be a significant entrance or interaction site for SARS-CoV-2, the gut mucosal immune system participates in virus interaction as a first-line physical and immunological defense [2]. Additionally, GI involvement and symptoms have been associated with poorer clinical outcomes in COVID-19 patients [4].
A prominent feature of SARS-CoV-2 is that it is a mucosal pathogen that infects human respiratory epithelial cells and the GI tract by latching to angiotensin-converting enzyme 2 (ACE-2) receptors through the spike (S) receptor-binding domain. Therefore, mucosal immunity is essential for sufficient and long-term viral protection [5].
Moreover, the GI system is a common entry point for SARS-CoV-2. After exposure, the virus disturbs the mucosal immune system, but further studies are needed to understand all the intimate immunological mechanisms and processes in the gut mucosa, where the recruitment of immune cells such as neutrophils, dendritic cells, macrophages, and T cells are involved in the immune responses. The mucosal inflammatory response, on the other hand, may alter the intercellular space between enterocytes, increasing intestinal permeability and allowing numerous bacterial antigens and toxins to enter the bloodstream, aggravating COVID-19 [6]. Along with the observed gut inflammation, Ojetti et al. investigated the role of fecal calprotectin during COVID-19 [7]. The authors speculated around the hypothesis that high levels of calprotectin released by neutrophils in the gut in patients with COVID-19 pneumonia are markers for severe disease. Although fecal calprotectin is a marker useful in diagnosing mainly inflammatory bowel disease, it could also be helpful in other GI or rheumatic conditions [8,9].
However, elevated fecal and serum calprotectin in COVID-19 are inconsistent with the presented GI symptoms. A recent article mentioned a correlation between fecal calprotectin and Intensive Care Unit (ICU) admission. Although the elevation of the inflammatory marker was not associated with GI symptoms, the unwanted outcome in disease progression was reciprocal to high serum and fecal calprotectin levels. The same article implied that the negative PCR stool samples prove that direct invasion of SARS-CoV-2 was unnecessary for the appearance of GI symptoms in patients. Perhaps the elevation in fecal calprotectin levels resulted from immune response and recruitment of immunocompetent cells in the GI tract rather than marking inflammation. Fecal calprotectin could be a predictive marker for disease severity in patients with SARS-CoV-2 infection. Furthermore, its role as a marker of severity should be investigated in more studies [10].
Limited intestinal inflammation despite diarrhea, fecal viral RNA, and SARS-CoV-2-specific IgA was found in patients with acute COVID-19. On the contrary, another scientific report (2021) stated that fecal calprotectin was not associated with disease severity or stool sample viral load. However, cytokine levels in SARS-CoV-2 infected patients and healthy controls differed significantly. For example, IL-23 and IL-8 were significantly higher in severely ill patients, but the regulatory cytokine IL-10 was lower in COVID-19 patients. Again, the cytokine concentration did not correlate to GI symptoms except for increased fecal IL-6 and TNFα levels in COVID-19 patients with leading GI manifestations. No remarkable difference between the two groups was documented for IL-1β, IL-1ra, IL-6, IL-8, IL-17, and TNFα fecal levels [11].These observations also lead to the possibility of discovering mucosal COVID-19 vaccines that would be administered intranasally or orally to provide adequate immune protection and avoid severe disease and death. Specific mucosal vaccines targeting coronavirus antigens could be an option for preventing future pandemics [12]. However, the mucosal vaccine data are still scarce, and it is unknown whether they would significantly decrease GI involvement during COVID-19.
Nevertheless, considering the vulnerability of patients affected by immune-mediated and autoimmune GI and liver conditions (i.e., inflammatory bowel disease, autoimmune hepatitis, GI stromal tumors, etc.), vaccination of these populations has proven its efficacy and safety in adults and children [13,14,15].
2. COVID-19, Mucosal Involvement and GI Symptoms
Typical clinical symptoms of fever, dry cough, fatigue, sputum production, and dyspnea accompany COVID-19. Although the main symptoms that characterize the disease are respiratory, other extrapulmonary manifestations, such as GI, neurological, renal, hepatic, cardiovascular, endocrine, dermatological, etc., have been reported [16]. Among the GI symptoms, anorexia (loss of appetite), nausea, vomiting, diarrhea, and abdominal pain were the most common. Less observed but more severe were mesenteric ischemia and GI bleeding. It should be mentioned that GI symptoms complicate the disease, increase the duration of the illness, and result in worse outcomes [17].
As mentioned above, the entrance of SARS-CoV-2 in the host cells has been described in two ways. The first way is by ACE-2 receptor-mediated endocytosis, and the second is by ACE-2 receptor plus transmembrane peptidase/serine subfamily member 2/4 (TMPRSS2/4) membrane fusion [18]. The ACE-2 receptors are abundant in most GI organs [19]. Their primary function is converting Angiotensin I to Angiotensin (1–9) and Angiotensin II to Angiotensin (1–7), promoting anti-inflammation [20]. Wiese et al. hypothesized many mechanisms for how SARS-CoV-2 could affect the renin-angiotensin system (RAS) via employing ACE2 receptors. Stimulation of the classical pathway, which controls blood pressure, could explain some of the symptoms associated with COVID-19. Moreover, it is assumed that COVID-19 may suppress a protective RAS mechanism, leading to overstimulated classical renin–angiotensin–aldosterone pathways and biochemical and clinical alterations [21].
Moreover, ACE2 receptors are responsible for the uptake of dietary amino acids, thus regulating antimicrobial peptides and preserving a healthy GI microbiome [22]. Host infections usually lead to the downregulation of the ACE-2 receptor activity. Therefore, direct inflammatory damage to enterocytes and a change in the normal function of the intestinal microbiome could be observed [23,24]. The disbalance of the gut microbiome leads to further inflammation and a series of immunological processes that are not limited to the GI system but also affect the respiratory tract [25]. The GI and respiratory systems have their own microbiomes and might be linked through immunological processes in the mucosal surfaces [26]. This link is usually mentioned in the literature as the gut–lung axis, and it has shown a two-way contribution in developing diseases in both systems. Thus, enhancement of the gut microbiome with probiotics has a possible positive effect on preventing and treating COVID-19 [27].
In COVID-19, anorexia, loss of taste, fever, and sedation contribute to reducing food intake, malnutrition, muscle wasting, and sarcopenia [28]. While anorexia commonly follows infections, it can also result from nausea [29]. Furthermore, anorexia, nausea, and vomiting have mechanisms that include the area postrema. More specifically, for nausea and vomiting, two mechanisms were proposed. One is by activating vagal afferents, and one is by releasing neuroactive agents that act in the area postrema. Both mechanisms activate the nucleus tractus solitarius and cause the induction of nausea and vomiting [30]. Diarrhea could be caused by the direct virus actions on the host or by iatrogenic factors, such as medications. In each case, the progress of the disease is complicated, the microbiome is damaged, and inflammation is promoted [31].
A study by Singh et al. surprisingly reported that diarrhea was associated with a better prognosis for COVID-19 [32]. The authors screened 244 patients and included 203 in the study, 9.9% of which were with GI symptoms alone and 57.6% with GE and respiratory problems, where 30% of all GI problems were diarrhea. The outcomes demonstrated that patients with both systems affected tend to decrease mechanical ventilation and mortality, and patients with diarrhea exhibited shorter hospital stays, no mortality, and no need for mechanical ventilation [32].
Additionally, GI involvement may attenuate the immune response and decrease the risk of cytokine storm and systemic inflammation, leading to better patient outcomes and prognosis. Other investigators, such as Megyeri et al., extensively researched COVID-19-associated diarrhea, focusing on the following mechanisms: direct gut epithelium damage, gut microbiota alteration, and mucosal inflammation. However, they concluded that these changes may worsen outcomes and exacerbate the symptoms [33].
The characteristics of the COVID-19 patient with pain in the upper abdomen are prominent dyspnea, increased admission to ICU, and poor outcomes. In contrast, lower abdomen pain has less noticeable dyspnea, fewer admissions to ICU, and better results [34]. In non-COVID patients, the primary etiology of mesenteric ischemia is a mesenteric arterial embolism, mesenteric arterial thrombosis, venous thrombosis, or, less frequently, low-flow non-occlusive causes. While the pathophysiology of these alterations for the COVID-19 group is unclear and the prevalence is low, the reasons should closely mirror the non-COVID patients and be classified again as occlusive and non-occlusive. Emergent management must be performed if mesenteric ischemia is diagnosed [35]. In case of bleeding, suspicion should be raised for both the upper and lower GI tract, with a higher risk in the upper part. Causes for COVID-19-related bleeding are ulcers (peptic, duodenal, rectal), gastritis, gastroduodenitis, and varices [36].
In summary, early reports of COVID-19 GI involvement included nausea, vomiting, diarrhea, and loss of appetite in 32% of patients, sometimes preceding the respiratory symptoms [37]. Then, recent reports declared GI symptoms were prevalent, with up to one-third of COVID-19 patients presenting with GI symptoms first. Moreover, nausea and vomiting may occur in up to two-thirds of people with COVID-19. Around 40% of COVID-19 patients may experience appetite loss, and up to 50% would experience diarrhea. Abdominal discomfort was less prevalent, affecting fewer than 10% of people [38]. Nevertheless, it is accepted that the Omicron variant causes milder COVID-19 than the Delta [39].
However, in contrast to the popular belief that newer variations of SARS-CoV-2 have been milder, Omicron BA.2 was linked with more symptoms and higher interruption to daily activities than BA.1. Monitoring the shifting symptom profiles associated with SARS-CoV-2 infection and the consequences on everyday activities will become increasingly crucial as limitations are loosened, and routine testing is limited in many countries [40].
An Australian cross-sectional study demonstrated that Omicron and Delta SARS-CoV-2 infection symptom profiles differed considerably. Patients infected with the Omicron variation were less likely to be hospitalized than those infected with the Delta variant (OR 0.29, 95% CI 0.16–0.53). Additionally, people infected with Omicron were more likely to have no symptoms. However, vaccination status may have affected the outcomes in this group. One can hypothesize that COVID-19 vaccines could improve the GI involvement. However, no studies so far have shown a direct link between COVID-19 vaccine efficacy and GI improvement—vaccines are associated with decreased risk of infection, complication, and overall death, but no other mechanisms to prevent GI symptoms. Overall, Delta infection was more likely to result in hospitalization [41].
Hyams et al. reported the differences in severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalized adults in their prospective cohort study in Bristol, UK; however, they obtained limited data on GI symptoms [42]. Other investigators reported severe GI complications, hearing damage, and thrombosis due to blood clots among the severe symptoms of Delta infection [43].
3. Virus Variants Associated with Gastroenterology Syndromes
According to different data, GI involvement in SARS-CoV-2 infection has been underestimated, ranging from 3% to 79%. As stated above, over 45% of patients have diarrhea as a primary complaint [44]. An interesting observation was made by Natarajan et al. that, even after as long as 4 months after infection, viral shedding and detectable viral SARS-CoV-2 RNA were found in fecal samples of COVID-19 patients with GI symptoms [45]. That correlated with the observations made early in the pandemic.
In their systematic review and meta-analysis, Cheung et al. reported that 17.6% of COVID-19 patients experienced GI problems. Viral RNA was found in 48.1% of the patients’ stool samples, although the respiratory samples were negative. Moreover, healthcare providers should collect fecal samples or perform endoscopic procedures on COVID-19 patients cautiously, even if the patient is recovering [46].
Due to the high expression of the ACE 2 receptor in differentiated enterocytes, researchers made an experimental model that proved the possibility of viral replication in the enterocytes in the small intestine and the production of infectious viral particles [47].
Still, there is no answer as to whether the GI symptoms most patients experienced were a result of an immune response or the direct cytopathic effects of the virus. A plausible explanation was given by Lehmann et al. [48], proving the activation and recruitment of cytotoxic T lymphocytes in the intestinal mucosa. The high expression of TMPRSS2 in the mucosa of the small intestine and the even higher ACE2 expression in the gut encouraged the team to perform duodenal biopsies via esophagogastroduodenoscopy. Although there were no prominent macroscopic findings during the procedure, the histopathologic analysis revealed that CD8+ cell infiltration was an obvious sign of a local immune reaction. Further, the PCR in some samples was positive for viral RNA. Another pathological finding in COVID-19 patients was CD14+ monocyte reduction in the intestinal mucosa [48].
All of the above explains commonly observed symptoms during SARS-CoV-2 infection, such as diarrhea, nausea and vomiting, anorexia, and abdominal pain in 11.51% of all COVID-19 patients in the metanalysis by Merola et al. [49]. As previously reported, SARS-CoV-2 fecal shedding was found in 40.5% of confirmed cases in another paper [50].
Patients reported the following GI symptoms: nausea and vomiting (86/183, 47%), abdominal pain (63/183, 34%), diarrhea (61/183, 33%), GI bleeding (20/183, 11%), and anorexia (6/183, 3.3%) [51]. In addition, 17 patients presented with acute hemorrhagic colitis, 5 with drug-induced adverse events, 2 with retroperitoneal hemorrhage, 2 with appendicitis, 2 with choledocholithiasis, 2 with constipation, and 2 with anuresis, among others. All patients had acute hemorrhagic colitis in the left-sided colon. According to these findings, acute hemorrhagic colitis was seen in mild instances of the Omicron type of COVID-19 with GI bleeding. Therefore, acute hemorrhagic colitis should be considered when assessing individuals with moderate COVID-19 and GI bleeding [51]. Other GI complications associated with SARS-CoV-2 were gut ischemia [52] and surgical complications [53].
However, SARS-CoV-2 is a virus that rapidly evolved, leading to changes in the symptoms experienced by infected individuals. Therefore, Schulze et Bayer discussed the differences in signs from the first wave (Alpha) to the last waves (Omicron) regarding the symptomatic infection and symptoms from respiratory, GI, neurology, etc., and the symptoms changed evenly over time [54,55].
The widely accepted respiratory symptoms include those resembling the common cold, loss of sense of smell and/or taste (confirmed by multiple meta-analyses), fever, fatigue, headache, muscle and joint pain, sore throat, etc. The pattern of symptoms described by SARS-CoV-2-positive people varied dramatically over time, with nasal symptoms becoming more common since the development of the Alpha and Delta variants and the characteristic loss of smell and taste becoming substantially less common after the advent of the Omicron variant [54].
In addition, GI symptoms have been linked to SARS-CoV-2 infections since the outset, even though most infected people do not report them. Diarrhea (28.2%) was the most frequently reported GI symptom in the early phase of the pandemic (G614-dominated), but its rate has declined in the following variant-dominated phases (Alpha: 19.4%, Delta: 17.9%, Omicron: 13.8%) [54].
The highest frequently occurring symptoms were lost sense of smell (earlier G614, Alpha, and Delta phases at rates of 3.8 90% CI [2.5–6.1], 2.7 [1.7–4.3], and 4.5 [2.9–7.0], respectively) and altered or lost sense of taste (earlier G614, Alpha, and Delta phases at rates of 4.3 [2.8–6.8], 2.2 [1.4–3.5], and 3.6 [2.4–5.7]), with a slight decrease for the Alpha and Delta variants. However, these figures have reduced significantly to 1.0 [0.6–1.6] for the Omicron-dominated phase and 0.9 [0.6–1.5], respectively [54].
Schulze et al. also demonstrated that the reported differences are not due to vaccination—out of 428 Omicron-infected patients, 164 were fully vaccinated at the time of infection. At the same time, 264 were unvaccinated, and the frequencies of symptoms were essentially the same in both groups. Notably, only foreign body sensation in the eyes and loss of smell had lower frequencies in unvaccinated individuals than in vaccinated individuals (foreign body sensation: 4.2% vs. 9.8%; loss of smell: 20.5% vs. 32.3%) [54]. Regarding transmission of the virus and often overlooked symptoms, unilateral conjunctivitis should not be ignored. In rare cases, it can be the initial presentation of COVID-19 and even the sole sign of the disease [55]. During the initial phase of the pandemic, significant research was conducted utilizing a mobile phone app that comprised data from over 7000 individuals who tested positive for SARS-CoV-2 in a cohort of over 2 million people from the UK and the US [56,57]. In this study, the authors discovered frequencies for the symptoms “loss of smell and taste,” “fever,” “skipped meals,” and “diarrhea” that are comparable to the frequencies reported here, as well as favorable odds ratios for these symptoms, with “loss of smell and taste” having the highest odds ratio. Shi et al. reported that the Omicron variant of SARS-CoV-2 was also associated with digestive symptoms, but they were less prominent than in the previous virus variants [1].
4. Specific Digestive System Involvement in COVID-19
4.1. Pancreatic Involvement
Although SARS-CoV-2 infection was previously thought to mainly attack the pulmonary system, recent studies imply that the pancreas may be another potential target for the infection. There has been proof that, after infection, patients are at increased risk of developing diabetes mellitus. The reason behind that is the high concentration of ACE2 receptors and TMPRSS2 expression in the pancreas, especially in the Langhans islets beta cells and the pancreatic blood vessels [58]. Both exocrine and endocrine functions of the pancreas can be involved. However, a rare complication of acute pancreatitis with epigastric pain and highly elevated amylase and lipase can be one of the many presentations of coronaviral infection. Elevated pancreatic serum markers can result from multiorgan dysfunction, in severe cases, accompanied by a cytokine storm. Newly onset metabolic disorders, including hyperglycemia, are present in COVID-19 patients [58,59].
Patients often develop the so-called Post-Acute Sequelae of CoV-2 (PACS) after infection. Scherer et al. focused on the metabolic changes following SARS-CoV-2 infection. Beta-cell dysfunction, insulin resistance, and the failure of the liver to produce glucose, as well as insufficient pancreatic insulin production and chronic inflammation, are among the main reasons for postinfectious complications, which is a consecutive proof of pancreatic, liver, and GI involvement in the pathogenesis of the virus and its sequelae [60].
Additionally, Zollner et al. demonstrated that SARS-CoV-2 viral antigen persisted in the gut in patients with inflammatory bowel disease (IBD), leading to post-acute COVID-19 [61]. By performing endoscopy approximately 7 months after confirmed COVID-19, the authors found viral nucleocapsid and transcripts in 32 out of 46 patients, although the virus was not detectable in fecal samples. The investigators speculated that viral antigen persisting in the gut probably represents the immune alterations related to COVID-19.
An updated systematic review and meta-analysis by Lee et al. [62] reported the susceptibility and clinical outcomes in IBD patients infected with SARS-CoV-2. IBD conditions were not associated with an increased risk of hospital or ICU admission or death, although 5-ASA and corticosteroids were associated with an increased risk for these events.
From the gastroenterologist’s perspective, underlying conditions, such as IBD or chronic liver disease, and immunosuppressive therapy may impact the risk of developing GI complications related to SARS-CoV-2 infection and their diagnosis and treatment [63]. Bishehsari et al. reported that initial GI symptoms found in 22.4% of SARS-CoV-2-positive patients were associated with worse outcomes [64]. The authors also suggested that GI involvement in COVID-19 may define a more severe phenotype, contrary to the above-cited research of Singh et al. [32].
Adekunle et al. summarized the outcomes in the first year of the pandemic regarding GI involvement, demonstrating disease-specific hospitalization trends depending on the GI conditions [65]. For 2020 vs. 2019, all-cause inpatient mortality was higher for acute pancreatitis, diverticulitis, nonvariceal upper GI bleeding, and Crohn’s disease. However, the hospitalization rate decreased during the first year of the pandemic.
Other researchers, such as Livanos et al., performed a high-dimensional analysis of GI tissues to assess the level of inflammation, measured by cytokines and chemokines gene expression (i.e., IFNG, CXCL8, CXCL2, and IL1B) and the abundance of pro-inflammatory cells. The authors also concluded that GI symptoms were associated with reduced disease severity and mortality [66].
On the contrary, Nakhli et al. focused on the disorders of gut–brain interaction, which may develop in long-haulers and post-COVID (86% of patients with disorders of the gut–brain interaction had at least one GI sign, and, most often, accompanying depression—65%). However, GI symptoms were an independent risk factor for severe COVID-19, not always related to age above 65, diabetes, and vitamin D deficiency [67].
Among the mechanisms of pancreatic injury described by Sinagra et al. [44], drug-induced pancreatic injury should not be overlooked, as most of the antivirals used for treating the viral infection, as well as steroids and NSAIDs for control of inflammation, can be a significant cause for acute pancreatitis. In the ICU, severely ill COVID-19 patients infused with propofol can develop high triglyceride levels, a considerable cause of acute pancreatitis. Acute kidney injury is another mechanism proposed in this case. The viral translocation during infection from the duodenum to the pancreas has been suggested as the transport required for the direct cytolytic effect of SARS-CoV-2. Therefore, a multifactorial etiology is highly likely to result in pancreatic injury during COVID-19 [44].
4.2. Liver Involvement during COVID-19
Even though COVID-19 is thought to be a systemic infection targeting mainly the endothelial cells and the respiratory tract, a more in-depth overview suggests the engagement of other organs and systems such as the liver, kidney, pancreas, and the heart, which makes SARS-CoV-2 a virus with multiple targets and complicated pathogenesis resulting in multiorgan failure in susceptible populations. It is believed that most pathogens causing activation of adaptive immune response include MHC1 molecules and T-cells, which is devastating for the respiratory tract and the hepatic cells [68].
A rare complication of cytokine storm could be liver dysfunction. However, liver damage may also occur due to the treatment, since many medications used to treat the infection are hepatotoxic. Ischemia, cytokine storm, and hypoxia were the primary variables contributing to liver injury during COVID-19. Elevated liver enzymes during hospitalization can be attributable to drugs, sepsis, and shock. As a result, the proportion of hospitalized patients with COVID-19 and pathological liver biomarkers ranges from 14% to 53%. In addition, aminotransferases (i.e., aspartate aminotransferase, AST, alanine aminotransferase, ALT) and bilirubin levels are frequently elevated. Elevated gamma-glutamyltransferase, alkaline phosphatase and reduced serum albumin levels are typically seen [69].
Mao et al. reported the incidence of GI symptoms (15%) and liver injury (19%) in a review of data from the beginning of the pandemic in Hubei province. In addition, there was a correlation between the disease’s severity and the symptoms of abdominal pain and elevated liver enzymes [70].
In another meta-analysis from 2020, the rate of elevated liver enzymes was estimated at 15% in laboratory testing. GI symptoms, such as nausea and vomiting, were the highest, with a 7.8% prevalence, followed by 7.7% for diarrhea and 2.7% for abdominal pain that could also be attributed to liver injury [71]. We can conclude that the GI symptom prevalence was <10% from the early pandemic, which implied a variant-dependent change during the pandemic.
One possible explanation for these observations in the liver is that, although it is widely distributed in human tissues, ACE2 has the highest expression in the biliary epithelium. It has been proposed that SARS-CoV-2 can replicate in cholangiocytes. A rare finding in COVID-19 patients is cholangiopathy. However, a more common presentation regarding the liver is elevated levels of AST and ALT (as much as 40% in studies) and not the cholestatic GGT and AF. We may presume that the biliary system may act as a SARS-CoV-2 reservoir without causing apoptosis, but more research needs to be conducted. A correlation was found in COVID-19 patients with registered liver injury and higher levels of pro-inflammatory cytokines, especially IL-6. In addition, endothelial cell dysfunction and epitheliopathy in the liver were documented. It is still unclear whether the pro-inflammatory state is to blame for the liver injury or if there is undiagnosed chronic liver disease in some COVID-19 patients [72].
The aberrant immune response caused by SARS-CoV-2 infection in some patients, the direct injury caused by the hepatotropism of the virus, and the hepatotoxicity of some medications used during treatment are the main causes of liver damage. In patients with diagnosed liver diseases, COVID-19 may lead to acute worsening of symptoms. In cirrhotic patients, the ACE 2 expression increased, which explains the poorer outcome in that population and the less-than-desirable immune responses following vaccination [66].
Recently, we published our data on liver involvement of children with multisystem inflammatory syndrome associated with SARS-CoV-2 in children (MIS-C), in which syndrome GI involvement is among the diagnostic criteria for the condition [73,74,75]. Our observations were made on a study group of pediatric patients right after the Delta wave in Bulgaria. Interestingly, during the subsequent waves, i.e., Omicron, the cases of MIS-C dropped off significantly. Along with the increased cases of MIS-C during the Delta wave in Bulgaria, we also demonstrated that the Delta wave overburdened our country’s hospital capacity [76].
Additionally, the Shanghai experience revealed that GI symptoms caused by Omicron variants are relatively uncommon. In the intestinal model, Kei et al. discovered that the Omicron variant strain had a lower proliferation efficiency. They measured viral RNA and titer in the culture supernatant and found that the Omicron versions had limited replication capability [77]. A large retrospective study of the Omicron outbreak in South Africa found that the hospitalization rate among adolescents and children was significantly higher than in previous outbreaks. Children under 20 years old accounted for 14.3% of all hospitalized patients, and about 25.4% of infected children under 5 years old required hospitalizations [78]. The hospitalization rate of children infected with Omicron was also reported to be high in the US, with 19% requiring ICU treatment [79]. Weak immunity, low vaccination rates, and increased respiratory illness rates in adolescents and children are all plausible causes.
Recently, the UK, the US, Japan, and other countries reported unexplained acute hepatitis in children previously infected with the Omicron variant. The pathological cause could be the continuous release of viral proteins from the Omicron variant in the intestinal epithelium, producing “superantigen” mediating multisystem inflammatory syndrome in children with fever, rash, vomiting, and diarrhea. If an adenovirus is infected during this process, the “superantigen” influence becomes more apparent, resulting in an abnormal immune response and an outbreak of acute hepatitis [80]. Nishiura et al. studied Omicron infection data from 39 countries and discovered that countries with fulminant hepatitis in children also had a higher number of Omicron infections (p = 0.013). They hypothesized that prior exposure to Omicron variants raises the risk of severe hepatitis in children [81]. Although the Omicron variant is less replicable in the intestinal epithelium, infection with the Omicron variant in children should be avoided because it can lead to more severe complications. Since the COVID-19 outbreak, the Swedish Centre for Environmental Epidemiology has been testing the virus in Swedish urban wastewater to track the spread of the SARS-CoV-2 virus. Their findings in February 2022 revealed that the virus content of the Omicron variant was higher than that of the Delta variant [82].
Poor outcomes have been found in patients with hepatocellular carcinoma and chronic liver disease. The cause behind liver injury in COVID-19 patients is multifactorial. It includes the pathogenic mechanism of the direct viral cytopathic effect and the upregulated immune response, and it results in vascular endothelitis, ischemia caused by the hypercoagulable state, concomitant hypoxia caused by the respiratory failure, and iatrogenic drug-induced liver injury [83]. The use of remdesivir and monoclonal antibodies, like tocilizumab and lopinavir/ritonavir, require careful assessment of previous liver disease and frequent monitoring of transferase enzymes; drug-induced liver injury (DILI) should be taken into consideration when treating COVID-19 patients [84].
A recent article discussed the possibility of the SARS-CoV-2 virus causing liver injury. The hepatotropism of the virus is mediated mainly by the ACE2 receptor expression in the hepatic tissue. Again, like in the case of pancreatic damage, TMPRSS2 was mentioned as a target for Spike-protein binding. There is a possibility of viral replication at a lower rate that does not result in apoptosis, which may be involved in using the small intestine as a reservoir of the virus, eventually leading to elevated liver enzymes one to two times above the normal range with an incidence approximately of 30–60% of patients [85].
In the SARS-CoV-2 infected patients, elevated liver enzymes have been a non-specific marker for severity since the beginning of the pandemic. There are a few mechanisms that can explain these laboratory findings. Most authors imply that overmedication results in DILI, especially in senior patients. However, there are many other mechanisms for liver injury due to ACE2 receptor expression in the liver. More importantly, direct damage from the virus should not be overlooked when patients have elevated ASL and ALT. The unresolved inflammation, caused by an overstimulated immune system and associated with cytokine storm multiorgan failure in the end stages, is another etiological cause of liver injury in patients with COVID-19 [86].
Apart from this, the concept of DILI should be explained in more detail with examples. Idiosyncratic DILI (IDILI), by definition, is an adverse drug reaction. Although rare, it should be considered in patients with elevated liver enzymes, clinical manifestation of acute hepatitis, or liver failure after medication administration with possible hepatotoxic effects [87].
An international DILI Expert Working Group of clinicians and scientists suggested the following approach for a diagnosis of DILI: (a) increase in ALT value times five, (b) alkaline phosphatase (ALP) value times two, or (c) ALT value times three and total bilirubin times two. This could help manage COVID-19-related DILI [88].
Therefore, in conclusion, DILI should not be overlooked with its spectrum of clinical manifestations, risk of chronic liver damage with life-altering possibilities, and potentially fatal outcomes. However, DILI caused by overuse of medication during SARS-CoV-2 infections can be classified as an iatrogenic complication of the disease hand-in-hand with Clostridodes infection resulting from antibiotic treatment of bacterial superinfections. This infection will be described in the following section.
A summary of the SARS-CoV-2 impact on the GI and hepatobiliary system is shown in Figure 1.
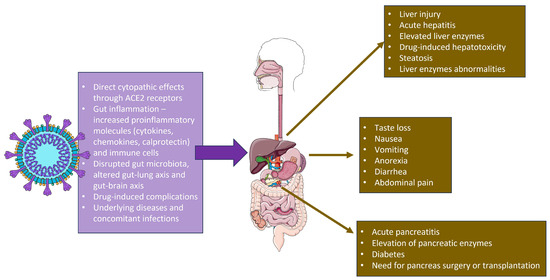
Figure 1.
SARS-CoV-2 actions in the gastrointestinal tract and the relevant clinical symptoms. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
5. Clostridoides Difficile Infection as Gi Complication during COVID-19
As previously stated, antibiotic-associated diarrhea is a significant cause of poorer outcomes in COVID-19 patients. In 2022, Maslennikov et al. analyzed data from infected individuals. They concluded that coinfection with Clostridoides—one of the culprits behind antibiotic-associated diarrhea—negatively influenced the prognosis and course in SARS-CoV-2 infected individuals. The article stated that, in COVID-19 patients with diarrhea, Clostridoides should be considered and treated accordingly [89].
It has been hypothesized that a concomitant SARS-CoV-2 infection may lead to transitory immune dysregulation, making Clostridoides infection more common in COVID-19 patients. Another mechanism explaining the high prevalence of this infection in COVID-19 patients is mentioned above (i.e., a pathogenic mechanism involving the ACE2, altered gut microbiome, and antibiotic use) [90].
Trottein et al. noted the role of gut microbiota concerning viral illnesses, immune response, and outcomes in patients with COVID-19. Altogether, hypoxia, cytokine production, inflammation, direct invasion of enterocytes, antibiotic use, and alteration in homeostasis could be considered pathophysiological mechanisms in SARS-CoV-2 infection and may be the culprit behind GI symptoms in most patients. Vice versa, dysbiosis may cause a poorer outcome in COVID-19, too [91].
Moreover, the risk for GI disorders after COVID-19 could be related to gut microbiota alterations. This topic was determined by Xu et al. in 2023. Patients infected with the SARS-CoV-2 virus presented in the post-acute phase (30 days after infection) with signs and symptoms of GERD, acute gastritis, peptic ulcers, pancreatic (acute pancreatitis), biliary (cholangitis), and hepatic disorders, as well as functional abnormalities such as motility disorders, IBS, and functional dyspepsia [92]. The evidence suggests that GI symptoms are a part of the so-called “long COVID” in hospitalized and non-hospitalized patients. A systematic review and meta-analysis of long COVID-19 caused by different SARS-CoV-2 variants showed that long COVID-19 usually represents symptoms from the respiratory, GI, cardiovascular, and nervous systems, similar to the classical COVID-19 [93]. Wild type of SARS-CoV-2 was associated with a pooled prevalence of 1.8 for more than 1 GI symptom, and the following: loss of appetite (Delta—2.2, Omicron—11.9, wild type—3.0), nausea (Delta—0.6, Omicron—15.3, wild type—1.5), diarrhea (Omicron—4.4, wild-type—2.5), abdominal pain (Omicron—3.2, wild-type—2.0), constipation (Delta—2.6, Omicron—10.6, wild—type 3.1), demonstrating that Omicron strains were associated most often with GI symptoms after infection resolution [93].
In line with this, preventing infections and reinfections decreases the risk of GI disorders. It has been hypothesized that the pathogenic mechanisms behind the symptoms are due to the viral tropism to tissues in the GI tract, induced autoimmunity, chronic inflammation due to viral persistence with residual antigenic stimulation, and dysbiosis. In addition to the pathophysiology of COVID-19 sequelae, notable changes in the gut microbiome are present. Furthermore, the appendix is a site for continuous viral replication after infection. Other findings in patients with Post-Acute-Coronavirus Sequalae are associated with the reactivation of viruses from the Herpesviridae family. All of this implies the necessity for a better understanding of the condition to discover new ways to treat and prevent the late complications of SARS-CoV-2 infection [92].
6. Conclusions
Commonly observed symptoms during SARS-CoV-2 infection were associated with GI involvement, including diarrhea, nausea, vomiting, anorexia, and abdominal pain. However, disease signs differ from the first wave (Alpha) to the last wave (Omicron) regarding the symptomatic infection and symptoms from respiratory, GI, neurology, etc. Most studies demonstrated that the Omicron variant of SARS-CoV-2 was also associated with digestive symptoms, but this was less prominent than the previous virus variants. In contrast, Delta was associated with an increased risk for hospitalization due to COVID-19, mainly due to GI complications. As other GI infections, microbiota alterations, and pancreatic and liver involvement could deteriorate the overall prognosis of COVID-19 patients, it was established that preventing infections and reinfections decreased the risk of GI disorders and death.
Author Contributions
Conceptualization: Y.S. and T.V.; resources: Y.S., S.G., M.P.-S., V.S., H.B., G.H.V., M.S. and T.V.; data curation, S.L., D.M., M.G., L.T., G.H.V. and G.V.V.; visualization, D.M. and M.P.-S.; writing—original draft preparation: Y.S., S.G. and T.V.; writing—review and editing, D.M., M.P.-S., V.S., H.B., G.V.V., M.S. and T.V.; supervision: T.V. All authors revised and approved the final version of the manuscript.
Funding
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No BG-RRP-2.004-0008-C01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0008-C01.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, Y.; Mei, Z.; Wang, H. Characteristics and implications of Omicron variant associated digestive system infections—Correspondence. Int. J. Surg. 2022, 104, 106750. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef]
- Wang, M.K.; Yue, H.Y.; Cai, J.; Zhai, Y.J.; Peng, J.H.; Hui, J.F.; Hou, D.Y.; Li, W.P.; Yang, J.S. COVID-19 and the digestive system: A comprehensive review. World J. Clin. Cases 2021, 9, 3796–3813. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.V.; Kotsev, S.V.; Georgiev, D.S.; Batselova, H.M. Immunological aspects of COVID-19: What do we know? World J. Biol. Chem. 2020, 11, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based Design of Prefusion-stabilized SARS-CoV-2 Spikes. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science (New York, N.Y.) 2020, 369, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.; Snegarova, V.; Kukov, A.; Batselova, H.; Mihova, A.; Nakov, R. Gastrointestinal mucosal immunity and COVID-19. World J. Gastroenterol. 2021, 27, 5047–5059. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Saviano, A.; Covino, M.; Acampora, N.; Troiani, E.; Franceschi, F.; Gemelli against COVID-19 Group. COVID-19 and intestinal inflammation: Role of fecal calprotectin. Dig. Liver Dis. 2020, 52, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.; Xue, X. Editorial: Recent advances and new biomarkers in ulcerative colitis. Front. Med. 2023, 10, 1214882. [Google Scholar] [CrossRef]
- Ardelean, M.V.; Kundnani, N.R.; Sharma, A.; Dumitru, M.; Buzas, R.; Rosca, C.I.; Dahdal, D.; Ottman, N.; Ardelean, O.F.; Daniel-Marius, D.S.; et al. Fecal calprotectin—A valuable predictor of microscopic colitis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9382–9392. [Google Scholar] [CrossRef]
- Shokri-Afra, H.; Alikhani, A.; Moradipoodeh, B.; Noorbakhsh, F.; Fakheri, H.; Moradi-Sardareh, H. Elevated fecal and serum calprotectin in COVID-19 are not consistent with gastrointestinal symptoms. Sci. Rep. 2021, 11, 22001. [Google Scholar] [CrossRef]
- Britton, G.J.; Chen-Liaw, A.; Cossarini, F.; Livanos, A.E.; Spindler, M.P.; Plitt, T.; Eggers, J.; Mogno, I.; Gonzalez-Reiche, A.S.; Siu, S.; et al. Limited intestinal inflammation despite diarrhea, fecal viral RNA and SARS-CoV-2-specific IgA in patients with acute COVID-19. Sci. Rep. 2021, 11, 13308. [Google Scholar] [CrossRef] [PubMed]
- Miteva, D.; Peshevska-Sekulovska, M.; Snegarova, V.; Batselova, H.; Alexandrova, R.; Velikova, T. Mucosal COVID-19 vaccines: Risks, benefits and control of the pandemic. World J. Virol. 2022, 11, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Peshevska-Sekulovska, M.; Bakalova, P.; Snegarova, V.; Lazova, S.; Velikova, T. COVID-19 Vaccines for Adults and Children with Autoimmune Gut or Liver Disease. Vaccines 2022, 10, 2075. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.; Georgiev, T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021, 41, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Snegarova, V.; Miteva, D.; Gulinac, M.; Peshevska-Sekulovska, M.; Batselova, H.; Velikova, T. COVID-19 in patients with gastrointestinal stromal tumors: Recommendations for management and vaccination. World J. Gastrointest. Pathophysiol. 2022, 13, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Berdowska, I.; Matusiewicz, M. Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis—With impact on gastrointestinal tract. World J. Gastroenterol. 2021, 27, 6590–6600. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Wiese, O.J.; Allwood, B.W.; Zemlin, A.E. COVID-19 and the renin-angiotensin system (RAS): A spark that sets the forest alight? Med. Hypotheses 2020, 144, 110231. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, B.; Dang, Q.; Chen, Z.; Zhou, Q.; Luo, H.; Yuan, W.; Sun, Z. Pathogenesis and Mechanism of Gastrointestinal Infection With COVID-19. Front. Immunol. 2021, 12, 674074. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Borsellino, C.; Sankararaman, S.; Roche, K.; Burns, J.; Landis, R.M. Impact of COVID-19 on the Intestinal Microbiome. Curr. Nutr. Rep. 2021, 10, 300–306. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut-lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.S.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef]
- Borner, T.; Shaulson, E.D.; Ghidewon, M.Y.; Barnett, A.B.; Horn, C.C.; Doyle, R.P.; Grill, H.J.; Hayes, M.R.; De Jonghe, B.C. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020, 31, 351–362.e5. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Cai, W.; Rudd, J.A.; Sanger, G.J. COVID-19, nausea, and vomiting. J. Gastroenterol. Hepatol. 2021, 36, 646–656. [Google Scholar] [CrossRef]
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Samanta, J.; Suri, V.; Bhalla, A.; Puri, G.D.; Sehgal, R.; Kochhar, R. Presence of diarrhea associated with better outcomes in patients with COVID-19—A prospective evaluation. Indian J. Med. Microbiol. 2022, 40, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, K.; Dernovics, Á.; Al-Luhaibi, Z.I.I.; Rosztóczy, A. COVID-19-associated diarrhea. World J. Gastroenterol. 2021, 27, 3208–3222. [Google Scholar] [CrossRef] [PubMed]
- Balaphas, A.; Gkoufa, K.; Colucci, N.; Perdikis, K.C.; Gaudet-Blavignac, C.; Pataky, Z.; Carballo, S.; Ris, F.; Stirnemann, J.; Lovis, C.; et al. Abdominal pain patterns during COVID-19: An observational study. Sci. Rep. 2022, 12, 14677. [Google Scholar] [CrossRef]
- Serban, D.; Tribus, L.C.; Vancea, G.; Stoian, A.P.; Dascalu, A.M.; Suceveanu, A.I.; Tanasescu, C.; Costea, A.C.; Tudosie, M.S.; Tudor, C.; et al. Acute Mesenteric Ischemia in COVID-19 Patients. J. Clin. Med. 2021, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Maida, M.; Morreale, G.C.; Licata, M.; Renzulli, M.; Cremon, C.; Stanghellini, V.; Barbara, G. Gastrointestinal Bleeding in COVID-19 Patients: A Systematic Review with Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 2534975. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57. [Google Scholar] [CrossRef]
- Wise, J. COVID-19: Symptomatic infection with omicron variant is milder and shorter than with Delta, study reports. BMJ 2022, 377, o922. [Google Scholar] [CrossRef]
- Whitaker, M.; Elliott, J.; Bodinier, B.; Barclay, W.; Ward, H.; Cooke, G.; Donnelly, C.A.; Chadeau-Hyam, M.; Elliott, P. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat. Commun. 2022, 13, 6856. [Google Scholar] [CrossRef]
- Gomez, A.; Kelly, M.; Sloan-Gardner, T.S.; Voo, T.V.; Kirk, M.D. Severity and Symptom Characteristics between Omicron and Delta SARS-CoV-2 Variant Infections in the Australian Capital Territory: A Cross-Sectional Study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Hyams, C.; Challen, R.; Marlow, R.; Nguyen, J.; Begier, E.; Southern, J.; King, J.; Morley, A.; Kinney, J.; Clout, M.; et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom. Lancet Reg. Health Eur. 2023, 25, 100556. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Bezbaruah, R.; Deka, K.; Nongrang, L.; Kalita, T. The Delta and Omicron Variants of SARS-CoV-2: What We Know So Far. Vaccines 2022, 10, 1926. [Google Scholar] [CrossRef]
- Sinagra, E.; Shahini, E.; Crispino, F.; Macaione, I.; Guarnotta, V.; Marasà, M.; Testai, S.; Pallio, S.; Albano, D.; Facciorusso, A.; et al. COVID-19 and the Pancreas: A Narrative Review. Life 2022, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef]
- Lehmann, M.; Allers, K.; Heldt, C.; Meinhardt, J.; Schmidt, F.; Rodriguez-Sillke, Y.; Kunkel, D.; Schumann, M.; Böttcher, C.; Stahl-Hennig, C.; et al. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8+ T cells. Mucosal Immunol. 2021, 14, 1381–1392. [Google Scholar] [CrossRef]
- Merola, E.; Armelao, F.; de Pretis, G. Prevalence of gastrointestinal symptoms in coronavirus disease 2019: A meta-analysis. Acta Gastroenterol. 2020, 83, 603–615. [Google Scholar]
- Parasa, S.; Desai, M.; Thoguluva Chandrasekar, V.; Patel, H.K.; Kennedy, K.F.; Roesch, T.; Spadaccini, M.; Colombo, M.; Gabbiadini, R.; Artifon, E.L.A.; et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011335. [Google Scholar] [CrossRef]
- Maruyama, S.; Wada, D.; Oishi, T.; Saito, F.; Yoshiya, K.; Nakamori, Y.; Kuwagata, Y. A descriptive study of abdominal complications in patients with mild COVID-19 presenting to the emergency department: A single-center experience in Japan during the omicron variant phase. BMC Gastroenterol. 2023, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Peshevska-Sekulovska, M.; Boeva, I.; Sekulovski, M.; Zashev, M.; Peruhova, M. Gastrointestinal Ischemia—Stumbling Stone in COVID-19 Patients. Gastroenterol. Insights 2022, 13, 206–217. [Google Scholar] [CrossRef]
- Gulinac, M.; Novakov, I.P.; Antovic, S.; Velikova, T. Surgical complications in COVID-19 patients in the setting of moderate to severe disease. World J. Gastrointest. Surg. 2021, 13, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.; Bayer, W. Changes in Symptoms Experienced by SARS-CoV-2-Infected Individuals—From the First Wave to the Omicron Variant. Front. Virol. 2022, 2, 880707. [Google Scholar] [CrossRef]
- Mocanu, V.; Bhagwani, D.; Sharma, A.; Borza, C.; Rosca, C.I.; Stelian, M.; Bhagwani, S.; Haidar, L.; Kshtriya, L.; Kundnani, N.R.; et al. COVID-19 and the Human Eye: Conjunctivitis, a Lone COVID-19 Finding—A Case-Control Study. Med. Princ. Pract. 2022, 31, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Looi, M. How are COVID-19 symptoms changing? BMJ 2023, 380, p3. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-Time Tracking of Self-Reported Symptoms to Predict Potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Thaweerat, W. Current evidence on pancreatic involvement in SARS-CoV-2 infection. Pancreatology 2020, 20, 1013–1014. [Google Scholar] [CrossRef]
- Morris, A. Effects of pancreatic SARS-CoV-2 infection identified. Nat. Rev. Endocrinol. 2021, 17, 192. [Google Scholar] [CrossRef]
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-acute sequelae of COVID-19: A metabolic perspective. eLife 2022, 11, e78200. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Li, H.J.; Wasuwanich, P.; Kim, S.E.; Kim, J.Y.; Jeong, G.H.; Park, S.; Yang, J.W.; Kim, M.S.; Yon, D.K.; et al. COVID-19 susceptibility and clinical outcomes in inflammatory bowel disease: An updated systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2414. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.H.; East, J.E.; Lanas, A.; Malfertheiner, P.; Satsangi, J.; Scarpignato, C.; Webb, G.J. COVID-19 and Gastrointestinal Disease: Implications for the Gastroenterologist. Dig. Dis. 2021, 39, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Bishehsari, F.; Adnan, D.; Deshmukh, A.; Khan, S.R.; Rempert, T.; Dhana, K.; Mahdavinia, M. Gastrointestinal Symptoms Predict the Outcomes From COVID-19 Infection. J. Clin. Gastroenterol. 2022, 56, e145–e148. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.D.; Rubens, M.; Sedarous, M.; Tariq, T.; Okafor, P.N. Trends in gastrointestinal disease hospitalizations and outcomes during the first year of the coronavirus pandemic. World J. Gastroenterol. 2023, 29, 744–757. [Google Scholar] [CrossRef]
- Livanos, A.E.; Jha, D.; Cossarini, F.; Gonzalez-Reiche, A.S.; Tokuyama, M.; Aydillo, T.; Parigi, T.L.; Ladinsky, M.S.; Ramos, I.; Dunleavy, K.; et al. Intestinal Host Response to SARS-CoV-2 Infection and COVID-19 Outcomes in Patients With Gastrointestinal Symptoms. Gastroenterology 2021, 160, 2435–2450.e34. [Google Scholar] [CrossRef]
- Ebrahim Nakhli, R.; Shanker, A.; Sarosiek, I.; Boschman, J.; Espino, K.; Sigaroodi, S.; Al Bayati, I.; Elhanafi, S.; Sadeghi, A.; Sarosiek, J.; et al. Gastrointestinal symptoms and the severity of COVID-19: Disorders of gut-brain interaction are an outcome. Neurogastroenterol. Motil. 2022, 34, e14368. [Google Scholar] [CrossRef]
- Bzeizi, K.; Abdulla, M.; Mohammed, N.; Alqamish, J.; Jamshidi, N.; Broering, D. Effect of COVID-19 on liver abnormalities: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10599. [Google Scholar] [CrossRef]
- Taneva, G.; Dimitrov, D.; Velikova, T. Liver dysfunction as a cytokine storm manifestation and prognostic factor for severe COVID-19. World J. Hepatol. 2021, 13, 2005–2012. [Google Scholar] [CrossRef]
- Mao, R.; Qiu, Y.; He, J.-S.; Tan, J.-Y.; Li, X.-H.; Liang, J.; Shen, J.; Zhu, L.-R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334.e27. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Ballester, M.P.; Soffientini, U.; Jalan, R.; Mehta, G. SARS-CoV-2 infection and liver involvement. Hepatol. Int. 2022, 16, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Lazova, S.; Gerenska, D.; Slabakova, Y.; Velikova, T. Immunological features of the multisystem inflammatory syndrome associated with SARS-CoV-2 in children. Am. J. Clin. Exp. Immunol. 2022, 11, 64–71. [Google Scholar] [PubMed]
- Lazova, S.; Alexandrova, T.; Gorelyova-Stefanova, N.; Atanasov, K.; Tzotcheva, I.; Velikova, T. Liver Involvement in Children with COVID-19 and Multisystem Inflammatory Syndrome: A Single-Center Bulgarian Observational Study. Microorganisms 2021, 9, 1958. [Google Scholar] [CrossRef] [PubMed]
- Lazova, S.; Dimitrova, Y.; Hristova, D.; Tzotcheva, I.; Velikova, T. Cellular, Antibody and Cytokine Pathways in Children with Acute SARS-CoV-2 Infection and MIS-C-Can We Match the Puzzle? Antibodies 2022, 11, 25. [Google Scholar] [CrossRef]
- Tomov, L.P.; Batselova, H.M.; Velikova, T.V. COVID-19 Delta Wave Caused Early Overburden of Hospital Capacity in the Bulgarian Healthcare System in 2021. Healthcare 2022, 10, 600. [Google Scholar] [CrossRef]
- Miyakawa, K.; Machida, M.; Kawasaki, T.; Nishi, M.; Akutsu, H.; Ryo, A. Reduced replication efficacy of SARS-CoV-2 Omicron variant in “mini-gut” organoids. Gastroenterology. 2022, 163, 514–516. [Google Scholar] [CrossRef]
- Jassat, W.; Abdool Karim, S.S.; Mudara, C.; Welch, R.; Ozougwu, L.; Groome, M.J.; Govender, N.; von Gottberg, A.; Wolter, N.; Wolmarans, M.; et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: A retrospective observational study. Lancet Glob. Health 2022, 10, e961–e969. [Google Scholar] [CrossRef]
- Shi, D.S.; Whitaker, M.; Marks, K.J.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kawasaki, B.; Meek, J.; et al. Hospitalizations of children aged 5–11 Years with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 574–581. [Google Scholar] [CrossRef]
- Brodin, P.; Arditi, M. Severe acute hepatitis in children: Investigate SARS-CoV-2 superantigens. Lancet Gastroenterol. Hepatol. 2022, 7, 594–595. [Google Scholar] [CrossRef]
- Nishiura, H.; Jung, S.M.; Hayashi, K. High population burden of Omicron variant (B.1.1.529) is associated with the emergence of severe hepatitis of unknown etiology in children. Int. J. Infect. Dis. 2022, 122, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Hubert, C.R.; Acosta, N.; Waddell, B.J.; Hasing, M.E.; Qiu, Y.; Fuzzen, M.; Harper, N.B.; Bautista, M.A.; Gao, T.; Papparis, C.; et al. Emergence and spread of the SARS-CoV-2 omicron variant in Alberta communities revealed by wastewater monitoring. medRxiv Preprint 2022. [Google Scholar] [CrossRef]
- Nasa, P.; Alexander, G. COVID-19 and the liver: What do we know so far? World J. Hepatol. 2021, 13, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Sodeifian, F.; Seyedalhosseini, Z.S.; Kian, N.; Eftekhari, M.; Najari, S.; Mirsaeidi, M.; Farsi, Y.; Nasiri, M.J. Drug-Induced Liver Injury in COVID-19 Patients: A Systematic Review. Front. Med. 2021, 8, 731436. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Webb, G.J.; Barritt, A.S., 4th; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Karlafti, E.; Paramythiotis, D.; Pantazi, K.; Georgakopoulou, V.E.; Kaiafa, G.; Papalexis, P.; Protopapas, A.A.; Ztriva, E.; Fyntanidou, V.; Savopoulos, C. Drug-Induced Liver Injury in Hospitalized Patients during SARS-CoV-2 Infection. Medicina 2022, 58, 1848. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M.; End, P.; Benesic, A.; Gerbes, A.L.; Aithal, G.P. Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017, 66, 1154–1164. [Google Scholar] [CrossRef]
- García-Cortés, M.; Ortega-Alonso, A.; Matilla-Cabello, G.; Medina-Cáliz, I.; Castiella, A.; Conde, I.; Bonilla-Toyos, E.; Pinazo-Bandera, J.; Hernández, N.; Tagle, M.; et al. Clinical presentation, causative drugs and outcome of patients with autoimmune features in two prospective DILI registries. Liver Int. 2023, 43, 1749–1760. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Ufimtseva, A.; Poluektova, E.; Ulyanin, A. Clostridioides difficile coinfection in patients with COVID-19. Future Microbiol. 2022, 17, 653–663. [Google Scholar] [CrossRef]
- Lakkasani, S.; Chan, K.; Shaaban, H.S. Clostridiodes difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2299–2300. [Google Scholar] [CrossRef]
- Trottein, F.; Sokol, H. Potential Causes and Consequences of Gastrointestinal Disorders during a SARS-CoV-2 Infection. Cell Rep. 2020, 32, 107915. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 2023, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Ma, Y.; Deng, J.; Liu, M.; Liu, J. Comparison of Long COVID-19 Caused by Different SARS-CoV-2 Strains: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 16010. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).