Value of Some Scoring Systems for the Prognosis of Rebleeding and In-Hospital Mortality in Liver Cirrhosis with Acute Variceal Bleeding
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Methods
2.3. Data Collection and Statistical Analysis: Using the Statistical Software SPSS 25.0 to Draw Charts on Excel 2016
3. Results
3.1. Baseline Characteristic, Endoscopy and Clinical Progress
3.2. Characteristics of Scoring Systems
3.3. Value of Predictive Scores for Early Rebleeding and Mortality
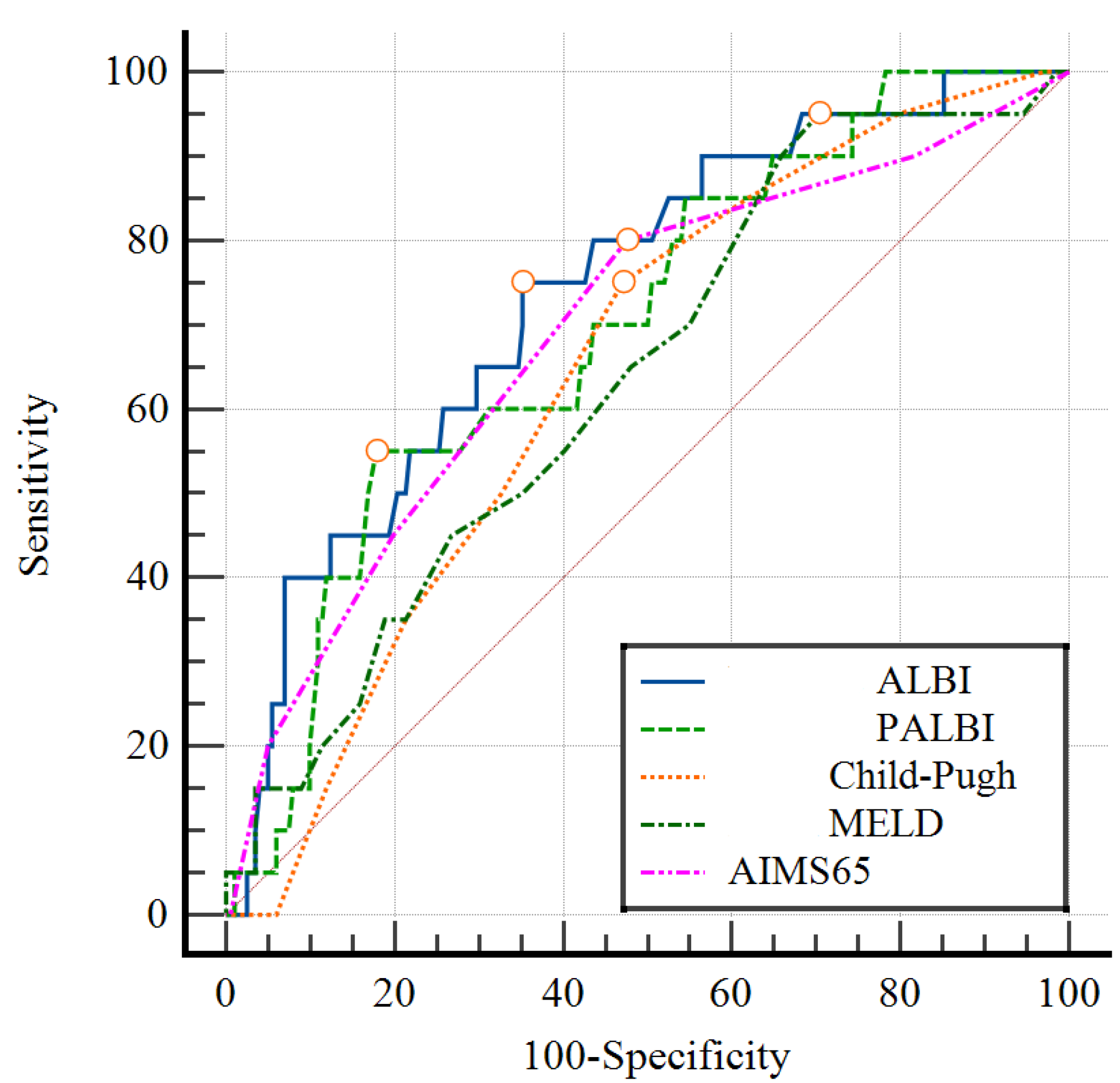
3.4. Comparison of the Area under the ROC Curve in the Prognosis of Early Rebleeding
3.5. Comparison of the Area under the ROC Curve in the Prognosis of Early Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Pagán, J.C.; Reverter, E.; Abraldes, J.G.; Bosch, J. Acute variceal bleeding. Semin. Respir. Crit. Care Med. 2012, 33, 46–54. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Stanley, A.J.; Hayes, P.C.; Patch, D.; Millson, C.; Mehrzad, H.; Austin, A.; Ferguson, J.W.; Olliff, S.P.; Hudson, M.; et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015, 64, 1680–1704. [Google Scholar] [CrossRef] [PubMed]
- Reiberger, T.; Püspök, A.; Schoder, M.; Baumann-Durchschein, F.; Bucsics, T.; Datz, C.; Dolak, W.; Ferlitsch, A.; Finkenstedt, A.; Graziadei, I.; et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien. Klin. Wochenschr. 2017, 129, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Sharara, A.I.; Rockey, D.C. Gastroesophageal variceal hemorrhage. N. Engl. J. Med. 2001, 345, 669–681. [Google Scholar] [CrossRef]
- Motola-Kuba, M.; Escobedo-Arzate, A.; Tellez-Avila, F.; Altamirano, J.; Aguilar-Olivos, N.; González-Angulo, A.; Zamarripa-Dorsey, F.; Uribe, M.; Chávez-Tapia, N.C. Validation of prognostic scores for clinical outcomes in cirrhotic patients with acute variceal bleeding. Ann. Hepatol. 2016, 15, 895–901. [Google Scholar]
- Hassanien, M.; EL-Ghannam, M.; EL-Talkawy, M.D.; Abdelrahman, Y.; El Attar, G.; Abu Taleb, H. Risk scoring systems to predict in-hospital mortality in patients with acute variceal bleeding due to hepatitis C virus-induced liver cirrhosis. Gastroenterol. Insights 2018, 9, 7629. [Google Scholar] [CrossRef]
- Yang, L.; Sun, R.; Wei, N.; Chen, H. Systematic review and meta-analysis of risk scores in prediction for the clinical outcomes in patients with acute variceal bleeding. Ann. Med. 2021, 53, 1806–1815. [Google Scholar] [CrossRef]
- Aluizio, C.L.d.S.; Montes, C.G.; Reis, G.F.S.R.; Nagasako, C.K. Risk stratification in acute variceal bleeding: Far from an ideal score. Clinics 2021, 76, e2921. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of Liver Function in Patients with Hepatocellular Carcinoma: A New Evidence-Based Approach—The ALBI Grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Berhane, S.; Tabriz-ian, P.; Park, J.W.; Yang, J.; Yan, L.; Han, G.; Izzo, F.; Chen, M.; et al. PALBI-An Objective Score Based on Platelets, Albumin Bilirubin Stratifies HCC Patients Undergoing Resection & Ablation Better than Child’s Classification. Hepatology 2015, 62, 624A–690A. [Google Scholar]
- Elshaarawy, O.; Allam, N.; Abdelsameea, E.; Gomaa, A.; Waked, I. Platelet-albumin-bilirubin score—A predictor of outcome of acute variceal bleeding in patients with cirrhosis. World J. Hepatol. 2020, 12, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Qi, X.; Zhu, C.; Ning, Z.; Hou, F.; Zhao, J.; Peng, Y.; Li, J.; Deng, H.; Guo, X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk. J. Gastroenterol. 2016, 27, 180–186. [Google Scholar] [CrossRef]
- Dunford, L.; Carr, M.; Dean, J.; Nguyen, L.T.; Thi, T.H.T.; Nguyen, B.T.; Connell, J.; Coughlan, S.; Nguyen, H.T.; Hall, W.W.; et al. A Multicentre Molecular Analysis of Hepatitis B and Blood-Borne Virus Coinfections in Viet Nam. PLoS ONE 2012, 7, e39027. [Google Scholar] [CrossRef]
- Flower, B.; Du Hong, D.; Kim, H.V.T.; Minh, K.P.; Geskus, R.B.; Day, J.; Cooke, G.S. Seroprevalence of Hepatitis B, C and D in Vietnam: A systematic review and meta-analysis. Lancet Reg. Health West Pac. 2022, 24. [Google Scholar] [CrossRef]
- Hanh, H.T.M.; Assanangkornchai, S.; Geater, A.F.; Hanh, V.T.M. Socioeconomic inequalities in alcohol use and some related consequences from a household perspective in Vietnam. Drug Alcohol Rev. 2018, 38, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gish, R.G.; Bui, T.D.; Nguyen, C.T.K.; Nguyen, D.T.; Tran, H.V.; Tran, D.M.T.; Trinh, H.N.; International Group for Liver Health in Viet. Nam Liver disease in Viet Nam: Screening, surveillance, management and education: A 5-year plan and call to action. J. Gastroenterol. Hepatol. 2012, 27, 238–247. [Google Scholar] [CrossRef]
- Nguyen-Dinh, S.-H.; Do, A.; Pham, T.N.D.; Dao, D.Y.; Nguy, T.N.; Chen, M.S. High burden of hepatocellular carcinoma and viral hepatitis in Southern and Central Vietnam: Experience of a large tertiary referral center, 2010 to 2016. World J. Hepatol. 2018, 10, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2016, 65, 310–335. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, J.; Berg, T. The etiology, diagnosis and prevention of liver cirrhosis: Part 1 of a series on liver cirrhosis. Dtsch Arztebl Int. 2013, 110, 85–91. [Google Scholar] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, J.R.; Tabak, Y.P.; Hyett, B.H.; Sun, X.; Travis, A.C.; Johannes, R.S. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest. Endosc. 2011, 74, 1215–1224. [Google Scholar] [CrossRef]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Aithal, G.P.; Palaniyappan, N.; China, L.; Härmälä, S.; Macken, L.; Ryan, J.M.; Wilkes, E.A.; Moore, K.; Leithead, J.A.; Hayes, P.C.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29. [Google Scholar] [CrossRef]
- Tajiri, T.; Yoshida, H.; Obara, K.; Onji, M.; Kage, M.; Kitano, S.; Kokudo, N.; Kokubu, S.; Sakaida, I.; Sata, M.; et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig. Endosc. 2009, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C. Baveno VII Faculty. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Thong, V.; Anh, H. Prediction of Esophageal Varices Based on Serum-Ascites Albumin Gradient in Cirrhotic Patients. Gastroenterol. Insights 2021, 12, 270–277. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Lo, T.T.-B.; La, H.D.; Doan, H.T.-N.; Le, N.T. Clinical, Laboratory and Bacterial Profile of Spontaneous Bacterial Peritonitis in Vietnamese Patients with Liver Cirrhosis. Hepatic Med. Évid. Res. 2022, 14, 101–109. [Google Scholar] [CrossRef]
- Elsafty, R.E.; Elsawy, A.A.; Selim, A.F.; Taha, A.M. Performance of albumin-bilirubin score in prediction of hepatic encephalopathy in cirrhotic patients with acute variceal bleeding. Egypt. Liver J. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Faisal, M.S.; Singh, T.; Amin, H.; Esfeh, J.M. Role of platelet-albumin-bilirubin score in predicting re-bleeding after band ligation for acute variceal hemorrhage. World J. Hepatol. 2020, 12, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Tantai, X.X.; Liu, N.; Yang, L.B.; Wei, Z.C.; Xiao, C.L.; Song, Y.H.; Wang, J.H. Prognostic value of risk scoring systems for cirrhotic patients with variceal bleeding. World J. Gastroenterol. 2019, 25, 6668–6680. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, B.S.; Madhumati, R.; Umesh, K.J.; Rashwith, U. A comparative study of albumin-bilirubin score, MELD and Child Pugh scores for predicting the in-hospital mortality in cirrhotic patients complicated with Upper GI bleeding in a Tertiary care hospital. Asian J. Med. Sci. 2019, 10, 61–65. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, M. Value of platelet-albumin-bilirubin score in predicting the short-term prognosis of patients with liver cirrhosis and acute upper gastrointestinal bleeding. J. Clin. Hepatol. 2021, 37, 1578–1581. [Google Scholar]

| Variables | X ± SD or n (%) | |
|---|---|---|
| Mean age | 54.7 ± 10.4 | |
| Gender | Male (n, %) | 209 (94.1) |
| Female (n, %) | 13 (5.9) | |
| Clinical manifestation at admision | Hematemesis (n, %) | 48 (21.6) |
| Melena (n, %) | 54 (24.3) | |
| Hematemesis and melena (n, %) | 120 (54.1) | |
| Cirrhosis history | Yes (n, %) | 201 (90.5) |
| No (n, %) | 21 (9.5) | |
| Acute variceal bleeding history | Yes (n, %) | 143 (64.4) |
| No (n, %) | 79 (35.6) | |
| Cirrhosis causes | Alcohol (n, %) | 158 (71.2) |
| HBV (n, %) | 29 (13.1) | |
| HCV (n, %) | 5 (2.2) | |
| Alcohol plus hepatic virus (n, %) | 28 (12.6) | |
| N/A (n, %) | 2 (0.9) | |
| Ascites grade | None (n, %) | 124 (55.9) |
| Mild/Moderate (n, %) | 75 (33.8) | |
| Severe (n, %) | 23 (10.3) | |
| Child–Pugh classification | Child–Pugh A (n, %) | 42 (18.9) |
| Child–Pugh B (n, %) | 104 (46.8) | |
| Child–Pugh C (n, %) | 76 (34.3) | |
| Esophageal varices grade | I (n, %) | 6 (2.7) |
| II (n, %) | 37(16.7) | |
| III (n, %) | 179 (80.6) | |
| Bleeding status | Active bleeding (n, %) | 28 (12.6) |
| Stable (n, %) | 194 (87.4) | |
| Clinical progress | Early rebleeding (n, %) | 20 (9.0) |
| In-hospital mortality (n, %) | 15 (6.8) | |
| Scoring Systems | Value | |
|---|---|---|
| CPS | X ± SD | 8.2 ± 2.2 |
| Min–Max | 5–14 | |
| A/B/C n (%) | 42 (18.9)/104 (46.8)/76 (34.3) | |
| MELD | X ± SD | 15.4 ± 5.7 |
| Min–Max | 7–42 | |
| AIMS65 | X ± SD | 1.62 ± 1.15 |
| Min–Max | 0–5 | |
| AIMS65 ≥ 2 n (%) | 112 (50.5) | |
| ALBI | X ± SD | −1.26 ± 0.63 |
| Min–Max | −2.75–0.58 | |
| ALBI-1/ALBI-2/ALBI-3 n (%) | 2 (0.9)/ 92 (41.4)/128 (57.7) | |
| PALBI | X ± SD | −1.78 ± 0.42 |
| Min–Max | −3.0–(−0.76) | |
| PALBI-1/PALBI-2/PALBI-3 n (%) | 8 (3.6)/45 (10.3)/169 (76.1) | |
| Scores | Clinical Progress | Cut Off Value | AUROC (95% CI) | Se (%) | Sp (%) | PPV (%) | NPV (%) | p |
|---|---|---|---|---|---|---|---|---|
| CPS | Early rebleeding | 9 | 0.64 (0.54–0.75) | 75 | 53 | 13.6 | 95.5 | <0.05 |
| Mortality | 11 | 0.79 (0.66–0.92) | 73.3 | 81.2 | 22 | 97.7 | <0.05 | |
| MELD | Early rebleeding | 12 | 0.64 (0.52–0.76) | 95 | 29.7 | 11.8 | 98.4 | <0.05 |
| Mortality | 18 | 0.83 (0.72–0.93) | 80 | 75.4 | 19 | 98.1 | <0.001 | |
| AIMS65 | Early rebleeding | 2 | 0.69 (0.63–0.75) | 80.0 | 52.5 | 14.3 | 96.4 | <0.001 |
| Mortality | 3 | 0.82 (0.76–0.87) | 73.3 | 81.6 | 22.4 | 97.7 | <0.001 | |
| ALBI | Early rebleeding | −1.16 | 0.74 (0.63–0.85) | 75.0 | 65.0 | 17.4 | 96.3 | <0.001 |
| Mortality | −0.97 | 0.81 (0.68–0.93) | 80.0 | 75.0 | 18.8 | 98.1 | <0.001 | |
| PALBI | Early rebleeding | −1.45 | 0.70 (0.59–0.81) | 55.0 | 82.2 | 23.4 | 94.9 | 0.004 |
| Mortality | −1.63 | 0.80 (0.69–0.91) | 86.7 | 63.3 | 14.6 | 98.5 | <0.001 |
| Child–Pugh | MELD | AIMS65 | ||
|---|---|---|---|---|
| ALBI | ΔAUROC | 0.1 | 0.1 | 0.05 |
| z statistic | 1.73 | 1.83 | 1.08 | |
| p | 0.084 | 0.067 | 0.279 | |
| PALBI | ΔAUROC | 0.06 | 0.06 | 0.01 |
| z statistic | 1.00 | 1.54 | 0.129 | |
| p | 0.318 | 0.123 | 0.897 | |
| Child–Pugh | MELD | AIMS65 | ||
|---|---|---|---|---|
| ALBI | ΔAUROC | 0.02 | −0.02 | −0.01 |
| z statistic | 0.44 | 0.64 | 0.269 | |
| p | 0.658 | 0.524 | 0.788 | |
| PALBI | ΔAUROC | 0.01 | −0.03 | −0.02 |
| z statistic | 0.24 | 1.04 | 0.371 | |
| p | 0.815 | 0.300 | 0.711 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huy, D.Q.; Chung, N.V.; Dong, D.T. Value of Some Scoring Systems for the Prognosis of Rebleeding and In-Hospital Mortality in Liver Cirrhosis with Acute Variceal Bleeding. Gastroenterol. Insights 2023, 14, 144-155. https://doi.org/10.3390/gastroent14020011
Huy DQ, Chung NV, Dong DT. Value of Some Scoring Systems for the Prognosis of Rebleeding and In-Hospital Mortality in Liver Cirrhosis with Acute Variceal Bleeding. Gastroenterology Insights. 2023; 14(2):144-155. https://doi.org/10.3390/gastroent14020011
Chicago/Turabian StyleHuy, Duong Quang, Nguyen Van Chung, and Dinh Tien Dong. 2023. "Value of Some Scoring Systems for the Prognosis of Rebleeding and In-Hospital Mortality in Liver Cirrhosis with Acute Variceal Bleeding" Gastroenterology Insights 14, no. 2: 144-155. https://doi.org/10.3390/gastroent14020011
APA StyleHuy, D. Q., Chung, N. V., & Dong, D. T. (2023). Value of Some Scoring Systems for the Prognosis of Rebleeding and In-Hospital Mortality in Liver Cirrhosis with Acute Variceal Bleeding. Gastroenterology Insights, 14(2), 144-155. https://doi.org/10.3390/gastroent14020011





