Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy
Abstract
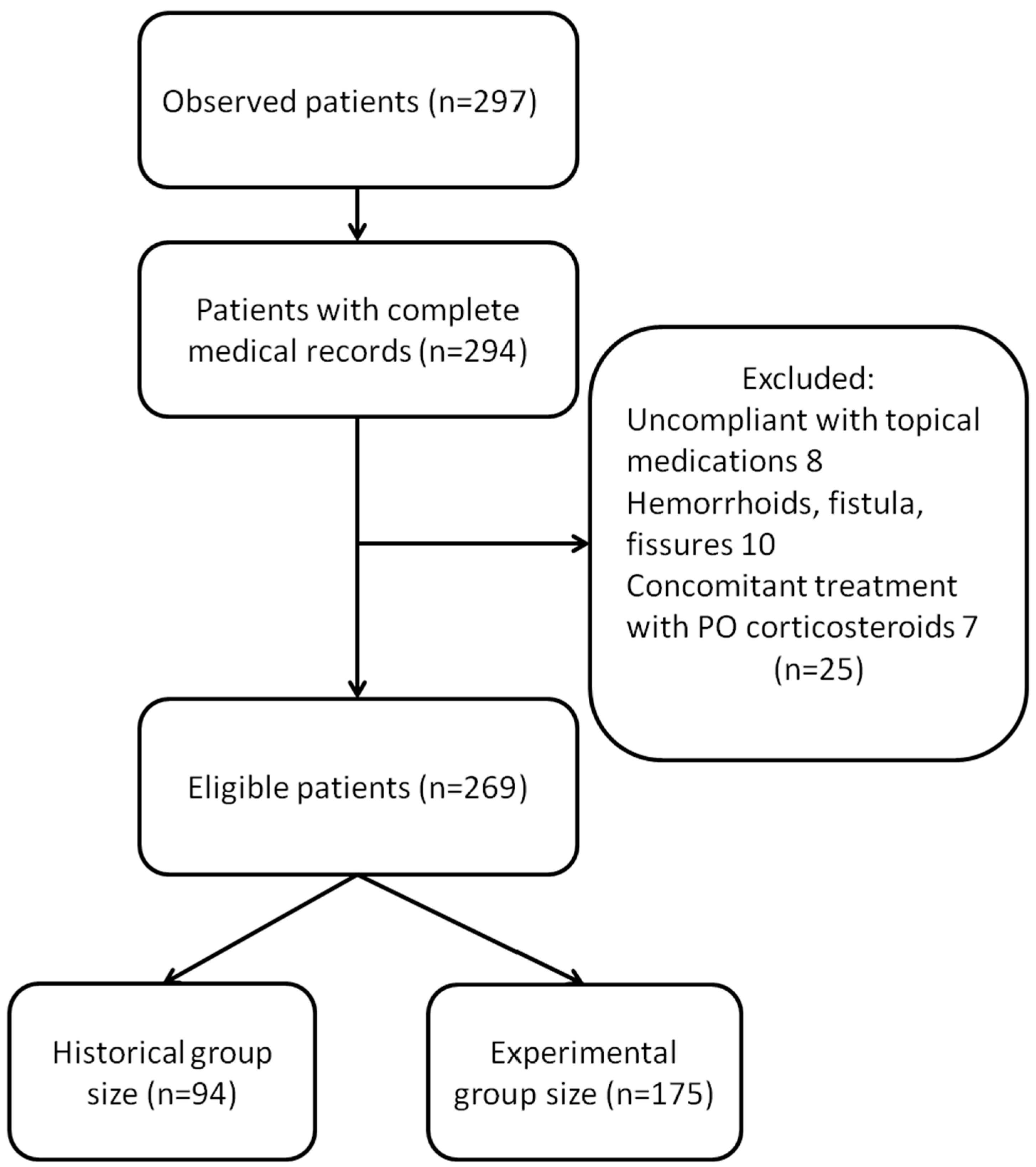
:1. Background/Aim
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barocas, D.A.; Alvarez, J.; Resnick, M.J.; Koyama, T.; Hoffman, K.E.; Tyson, M.D.; Conwill, R.; Mccollum, D.; Cooperberg, M.R.; Goodman, M.; et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017, 317, 1126–1140. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.C.; Basak, R.; Meyer, A.-M.; Kuo, T.-M.; Carpenter, W.R.; Agans, R.P.; Broughman, J.R.; Reeve, B.B.; Nielsen, M.E.; Usinger, D.S.; et al. Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men With Localized Prostate Cancer. JAMA 2017, 317, 1141–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, J.; Maatz, P.; Muck, T.; Keck, B.; Friederich, H.-C.; Herzog, W.; Ihrig, A. The effect of an online support group on patients’ treatment decisions for localized prostate cancer: An online survey. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 37.e19–37.e28. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Khoo, V.S.; Norman, A.R.; Meyer, L.; Nahum, A.; Tait, D.; Yarnold, J.; Horwich, A. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: A randomised trial. Lancet 1999, 353, 267–272. [Google Scholar] [CrossRef]
- Koper, P.C.; Stroom, J.C.; van Putten, W.L.; Korevaar, G.A.; Heijmen, B.J.; Wijnmaalen, A.; Jansen, P.P.; Hanssens, P.E.; Griep, C.; Krol, A.D.; et al. Acute morbidity reduction using 3DCRT for prostate carcinoma: A randomized study. Int. J. Radiat. Oncol. 1999, 43, 727–734. [Google Scholar] [CrossRef]
- Jani, A.B.; Su, A.; Correa, D.; Gratzle, J. Comparison of late gastrointestinal and genitourinary toxicity of prostate cancer patients undergoing intensity-modulated versus conventional radiotherapy using localized fields. Prostate Cancer Prostatic Dis. 2006, 10, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Shan, G.; Hu, W.; Chen, L.; Price, R.A.; Ma, C.-M.C. Dosimetric evaluation of image-guided radiation therapy for prostate cancer. Med Dosim. 2021, 46, 117–126. [Google Scholar] [CrossRef]
- Splinter, M.; Sachpazidis, I.; Bostel, T.; Fechter, T.; Zamboglou, C.; Thieke, C.; Jäkel, O.; Huber, P.E.; Debus, J.; Baltas, D.; et al. Dosimetric Impact of the Positional Imaging Frequency for Hypofractionated Prostate Radiotherapy—A Voxel-by-Voxel Analysis. Front. Oncol. 2020, 10, 564068. [Google Scholar] [CrossRef]
- Poźniak-Balicka, R.; Chomiak, B.; Wośkowiak, P.; Nowicki, N.; Bojarski, J.; Salagierski, M. Does the radiation approach affects acute toxicity in prostate cancer patients? A comparison of four radiation techniques. Central Eur. J. Urol. 2020, 73, 295–299. [Google Scholar] [CrossRef]
- Matta, R.; Chapple, C.R.; Fisch, M.; Heidenreich, A.; Herschorn, S.; Kodama, R.T.; Koontz, B.F.; Murphy, D.G.; Nguyen, P.L.; Nam, R.K. Pelvic Complications After Prostate Cancer Radiation Therapy and Their Management: An International Collaborative Narrative Review. Eur. Urol. 2019, 75, 464–476. [Google Scholar] [CrossRef]
- Heemsbergen, W.D.; Incrocci, L.; Pos, F.J.; Heijmen, B.J.M.; Witte, M.G. Local Dose Effects for Late Gastrointestinal Toxicity After Hypofractionated and Conventionally Fractionated Modern Radiotherapy for Prostate Cancer in the HYPRO Trial. Front. Oncol. 2020, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Lim, K.H.; Chia, D.W.; Chong, Y.L.; Png, K.S.; Chong, K.T.; Soon, Y.Y.; Tey, J.C. Clinical outcomes of external beam radiotherapy in patients with localized prostate cancer: Does dose escalation matter? Asia-Pacific J. Clin. Oncol. 2019, 15, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wortel, R.C.; Incrocci, L.; Pos, F.J.; Lebesque, J.V.; Witte, M.G.; van der Heide, U.A.; van Herk, M.; Heemsbergen, W.D. Acute Toxicity After Image-Guided Intensity Modulated Radiation Therapy Compared to 3D Conformal Radiation Therapy in Prostate Cancer Patients. Int. J. Radiat. Oncol. 2015, 91, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Maucieri, A.; Marvaso, G.; Gandini, S.; Fodor, C.I.; Zerini, D.; Riva, G.; Alessandro, O.; Surgo, A.; Volpe, S.; et al. Impact of image guidance on toxicity and tumour outcome in moderately hypofractionated external-beam radiotherapy for prostate cancer. Med. Oncol. 2018, 36, 9. [Google Scholar] [CrossRef]
- Sander, L.; Langkilde, N.C.; Holmberg, M.; Carl, J. MRI target delineation may reduce long-term toxicity after prostate radiotherapy. Acta Oncol. 2013, 53, 809–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferini, G.; Molino, L.; Tripoli, A.; Valenti, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; Borzi, G.R. Anatomical Predictors of Dosimetric Advantages for Deep-inspiration-breath-hold 3D-conformal Radiotherapy Among Women With Left Breast Cancer. Anticancer. Res. 2021, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Gay, H.; Jackson, A.; Tucker, S.L.; Deasy, J. Radiation Dose–Volume Effects in Radiation-Induced Rectal Injury. Int. J. Radiat. Oncol. 2010, 76, S123–S129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaife, J.; Harrison, K.; Romanchikova, M.; Parker, A.; Sutcliffe, M.; Bond, S.; Thomas, S.; Freeman, S.; Jena, R.; Bates, A.; et al. Random variation in rectal position during radiotherapy for prostate cancer is two to three times greater than that predicted from prostate motion. Br. J. Radiol. 2014, 87, 20140343. [Google Scholar] [CrossRef] [Green Version]
- Denton, A.S.; Forbes, A.; Andreyev, J.J.; Maher, J. Non surgical interventions for late radiation proctitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst. Rev. 2016, 4, CD003455. [Google Scholar] [CrossRef]
- Petersen, S.E.; Bentzen, L.; Emmertsen, K.J.; Laurberg, S.; Lundby, L.; Høyer, M. Development and validation of a scoring system for late anorectal side-effects in patients treated with radiotherapy for prostate cancer. Radiother. Oncol. 2014, 111, 94–99. [Google Scholar] [CrossRef]
- Ferini, G.; Pergolizzi, S. A Ten-year-long Update on Radiation Proctitis Among Prostate Cancer Patients Treated With Curative External Beam Radiotherapy. In Vivo 2021, 35, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Liguori, V.; Guillemin, C.; Pesce, G.F.; Mirimanoff, R.O.; Bernier, J. Double-blind, randomized clinical study comparing hyaluronic acid cream to placebo in patients treated with radiotherapy. Radiother. Oncol. 1997, 42, 155–161. [Google Scholar] [CrossRef]
- Ots, P.M.S.; Carrizosa, C.L.; Rodríguez, A.; Sáez, J.D.D.; Delgado, J.M.; De Miguel, M.M.; Vidal, M. Vesical instillations of hyaluronic acid to reduce the acute vesical toxicity caused by high-dose brachytherapy do not affect the survival: A five-year follow-up study. Clin. Transl. Oncol. 2009, 11, 828–834. [Google Scholar] [CrossRef]
- Laliscia, C.; Delishaj, D.; Fabrini, M.G.; Gonnelli, A.; Morganti, R.; Perrone, F.; Tana, R.; Paiar, F.; Gadducci, A. Acute and late vaginal toxicity after adjuvant high-dose-rate vaginal brachytherapy in patients with intermediate risk endometrial cancer: Is local therapy with hyaluronic acid of clinical benefit? J. Contemp. Brachytherapy 2016, 8, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Delia, P.; Sansotta, G.; Pontoriero, A.; Iati, G.; De Salvo, S.; Pisana, M.; Potami, A.; Lopes, S.; Messina, G.; Pergolizzi, S. Clinical Evaluation of Low-Molecular-Weight Hyaluronic Acid-Based Treatment on Onset of Acute Side Effects in Women Receiving Adjuvant Radiotherapy after Cervical Surgery: A Randomized Clinical Trial. Oncol. Res. Treat. 2019, 42, 217–223. [Google Scholar] [CrossRef]
- Ferini, G.; Cacciola, A.; Parisi, S.; Lillo, S.; Molino, L.; Tamburella, C.; Davi, V.; Napoli, I.; Platania, A.; Settineri, N.; et al. Curative Radiotherapy in Elderly Patients With Muscle Invasive Bladder Cancer: The Prognostic Role of Sarcopenia. In Vivo 2021, 35, 571–578. [Google Scholar] [CrossRef]
- Stefanelli, A.; Pascale, G.; Rainieri, E.; Ursino, S.; Colella, M.; Zini, G.; Berretta, M.; Fiorica, F. Can we decrease the acute proctitis in prostate cancer patients using hyaluronic acid during radiation therapy: A prospective historically controlled clinical study. Eur. Rev. Med Pharmacol. Sci. 2012, 16, 639–645. [Google Scholar] [PubMed]
- Montrone, S.; Gonnelli, A.; Cantarella, M.; Sainato, A. Use of Proktis-M suppositories in patients undergoing neoadjuvant radiochemotherapy for adenocarcinoma of the rectum. Minerva Gastroenterol Dietol. 2015, 61, 293–297. [Google Scholar]
- Prada, P.J.; Fernández, J.; Martinez, A.A.; Ángeles de la Rúa, M.D.; Gonzalez, J.M.; Juan, G. Transperineal Injection of Hyaluronic Acid in Anterior Perirectal Fat to Decrease Rectal Toxicity From Radiation Delivered With Intensity Modulated Brachytherapy or EBRT for Prostate Cancer Patients. Int. J. Radiat. Oncol. 2007, 69, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Chapet, O.; Decullier, E.; Bin, S.; Faix, A.; Ruffion, A.; Jalade, P.; Fenoglietto, P.; Udrescu, C.; Enachescu, C.; Azria, D. Prostate Hypofractionated Radiation Therapy With Injection of Hyaluronic Acid: Acute Toxicities in a Phase 2 Study. Int. J. Radiat. Oncol. 2015, 91, 730–736. [Google Scholar] [CrossRef]
- Chapet, O.; Udrescu, C.; Tanguy, R.; Ruffion, A.; Fenoglietto, P.; Sotton, M.-P.; Devonec, M.; Colombel, M.; Jalade, P.; Azria, D. Dosimetric Implications of an Injection of Hyaluronic Acid for Preserving the Rectal Wall in Prostate Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. 2014, 88, 425–432. [Google Scholar] [CrossRef]
- Wilder, R.B.; Barme, G.A.; Gilbert, R.F.; Holevas, R.E.; Kobashi, L.I.; Reed, R.R.; Solomon, R.S.; Walter, N.L.; Chittenden, L.; Mesa, A.V.; et al. Cross-Linked Hyaluronan Gel Reduces the Acute Rectal Toxicity of Radiotherapy for Prostate Cancer. Int. J. Radiat. Oncol. 2010, 77, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Boissier, R.; Udrescu, C.; Rebillard, X.; Terrier, J.-E.; Faix, A.; Chapet, O.; Azria, D.; Devonec, M.; Paparel, P.; Ruffion, A. Technique of Injection of Hyaluronic Acid as a Prostatic Spacer and Fiducials Before Hypofractionated External Beam Radiotherapy for Prostate Cancer. Urology 2017, 99, 265–269. [Google Scholar] [CrossRef]
- Trifiletti, D.M.; Garda, A.E.; Showalter, T.N. Implanted spacer approaches for pelvic radiation therapy. Expert Rev. Med Devices 2016, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; Sato, M.; Shirai, S.; Sonomura, T.; Yamama, R. Reirradiation of prostate cancer with rectum preservation: Eradicative high-dose-rate brachytherapy with natural type hyaluronate injection. Brachytherapy 2012, 11, 144–148. [Google Scholar] [CrossRef]
- Ozyigit, G.; Hurmuz, P.; Akinci, D.; Esen, S.; Yilmaz, M.; Akdogan, B.; Akyol, F. Hyaluronic acid spacer in focal prostate reirradiation: A single centre experience. Cancer/Radiothérapie 2020, 24, 805–811. [Google Scholar] [CrossRef]
- Elad, S.; Rn, K.K.F.C.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Dds, D.P.S.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Dunst, J.; Semlin, S.; Pigorsch, S.; Müller, A.-C.; Reese, T. Intermittent Use of Amifostine during Postoperative Radiochemotherapy and Acute Toxicity in Rectal Cancer Patients. Strahlentherapie und Onkologie 2000, 176, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Volpi, N.; Schiller, J.; Stern, R.; Soltés, L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef]
- DiNicola, S.; Pasta, V.; Costantino, D.; Guaraldi, C.; Bizzarri, M. Hyaluronic acid and vitamins are effective in reducing vaginal atrophy in women receiving radiotherapy. Minerva Ginecol 2015, 67, 523–531. [Google Scholar] [PubMed]
- Gacci, M.; Saleh, O.; Giannessi, C.; Detti, B.; Livi, L.; Pasquetti, E.M.; Masoni, T.; Agro, E.F.; Marzi, V.L.; Minervini, A.; et al. Sodium hyaluronate and chondroitin sulfate replenishment therapy can improve nocturia in men with post-radiation cystitis: Results of a prospective pilot study. BMC Urol. 2015, 15, 65. [Google Scholar] [CrossRef] [Green Version]
- Liem, X.; Saad, F.; Delouya, G. A Practical Approach to the Management of Radiation-Induced Hemorrhagic Cystitis. Drugs 2015, 75, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Riehl, T.E.; Foster, L.; Stenson, W.F. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am. J. Physiol. Liver Physiol. 2012, 302, G309–G316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, J.M.; Gibson, R.J.; Coller, J.K.; Blijlevens, N.; Bossi, P.; Al-Dasooqi, N.; Bateman, E.H.; Chiang, K.; de Mooij, C.; Mayo, B.; et al. Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines. Support. Care Cancer 2019, 27, 4011–4022. [Google Scholar] [CrossRef] [Green Version]
- Fuccio, L.; Guido, A.; Laterza, L.; Eusebi, L.H.; Busutti, L.; Bunkheila, F.; Barbieri, E.; Bazzoli, F. Randomised clinical trial: Preventive treatment with topical rectal beclomethasone dipropionate reduces post-radiation risk of bleeding in patients irradiated for prostate cancer. Aliment. Pharmacol. Ther. 2011, 34, 628–637. [Google Scholar] [CrossRef] [PubMed]
- D’Angelillo, R.M.; Francolini, G.; Ingrosso, G.; Ravo, V.; Triggiani, L.; Magli, A.; Mazzeo, E.; Arcangeli, S.; Alongi, F.; Jereczek-Fossa, B.A.; et al. Consensus statements on ablative radiotherapy for oligometastatic prostate cancer: A position paper of Italian Association of Radiotherapy and Clinical Oncology (AIRO). Crit. Rev. Oncol. 2019, 138, 24–28. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.B.; El-Ashry, D.; Turley, E.A. Hyaluronan, Cancer-Associated Fibroblasts and the Tumor Microenvironment in Malignant Progression. Front. Cell Dev. Biol. 2018, 6, 48. [Google Scholar] [CrossRef]
- Bharadwaj, A.G.; Kovar, J.L.; Loughman, E.; Elowsky, C.; Oakley, G.G.; Simpson, M.A. Spontaneous Metastasis of Prostate Cancer Is Promoted by Excess Hyaluronan Synthesis and Processing. Am. J. Pathol. 2009, 174, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Rizzardi, A.E.; Vogel, R.I.; Koopmeiners, J.S.; Forster, C.L.; Marston, L.O.; Rosener, N.K.; Akentieva, N.; Price, M.A.; Metzger, G.; Warlick, C.A.; et al. Elevated hyaluronan and hyaluronan-mediated motility receptor are associated with biochemical failure in patients with intermediate-grade prostate tumors. Cancer 2014, 120, 1800–1809. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, A.G.; Goodrich, N.P.; McAtee, C.O.; Haferbier, K.; Oakley, G.G.; Wahl, J.K.; Simpson, M.A. Hyaluronan suppresses prostate tumor cell proliferation through diminished expression of N-cadherin and aberrant growth factor receptor signaling. Exp. Cell Res. 2011, 317, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Shentova-Eneva, R.; Kofinova, D.; Hadzhiyski, P.; Yaneva, P.; Lazarova, E.; Baycheva, M. Anemia in Newly Diagnosed Pediatric Patients with Inflammatory Bowel Disease. Gastroenterol. Insights 2021, 12, 376–383. [Google Scholar] [CrossRef]
- Sacks, H.; Chalmers, T.C.; Smith, H. Randomized versus historical controls for clinical trials. Am. J. Med. 1982, 72, 233–240. [Google Scholar] [CrossRef]
- Sedgwick, P.; Greenwood, N. Understanding the Hawthorne effect. BMJ 2015, 351, h4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Conventional Fractionation (54–80 Gy) | Hypofractionation (62.1–70 Gy) | Total | |
|---|---|---|---|
| No of patients | 216 (80.3%) | 53(19.7%) | 269 |
| Age | |||
| Range | 52–85 | 60–92 | 52–92 |
| Median | 73 | 82 | 75 |
| Radiotherapy | |||
| Radical | 104(48.15%) | 51(96.2%) | 155(57.6%) |
| Adjuvant | 112(51.85%) | 2(3.8%) | 114(42.4%) |
| Medications | |||
| Hyaluronic acid | 129(59.7%) | 46(86.8%) | 175(65%) |
| Beclomethasone dipropionate | 87(40.3%) | 7(13.2%) | 94(35%) |
| Hyaluronic Acid Group | |||
| Proctitis grade | G0 | G1 | G2 |
| Acute | 77(44%) | 90(51.4%) | 8(4.6%) |
| Late | 150 (85.7%) | 22(12.6%) | 3(1.7%) |
| Beclomethasone Dipropionate Group | |||
| Proctitis grade | G0 | G1 | G2 |
| Acute | 32(34.1%) | 51(54.2%) | 11(11.7%) |
| Late | 72(76.6%) | 15(16%) | 7(7.4%) |
| Overall Acute Toxicity | |||||
| GRADE | Hyaluronic Acid | Beclomethasone Dipropionate | Chi-Squared Test | ||
| No Tox | 77(44%) | 32(34%) | 109(40.5%) | Chi-squared | 2.12 |
| DF | 1 | ||||
| Tox | 98(56%) | 62(66%) | 160(59.5%) | Significance level | p = 0.1454 |
| 175(65.1%) | 94(34.9%) | 269 | |||
| Overall Late Toxicity | |||||
| GRADE | Hyaluronic Acid | Beclomethasone Dipropionate | Chi-Squared Test | ||
| No Tox | 150 (85.7%) | 72 (76.6%) | 222 (82.5%) | Chi-squared | 2.922 |
| DF | 1 | ||||
| Tox | 25 (14.3%) | 22 (23.4%) | 47 (17.5%) | Significance level | p = 0.0874 |
| 175 (65.1%) | 94 (34.9%) | 269 | |||
| GRADE | Hyaluronic Acid | Beclomethasone Dipropionate | p-Value | |
|---|---|---|---|---|
| G1 | Acute | 51.4% | 54.3% | 0.750 |
| Late | 12.6% | 16.0% | 0.560 | |
| G2 | Acute | 4.6% | 11.7% | 0.046 |
| Late | 1.7% | 7.4% | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferini, G.; Tripoli, A.; Umina, V.; Borzì, G.R.; Marchese, V.A.; Illari, S.I.; Cacciola, A.; Lillo, S.; Parisi, S.; Valenti, V. Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy. Gastroenterol. Insights 2021, 12, 446-455. https://doi.org/10.3390/gastroent12040043
Ferini G, Tripoli A, Umina V, Borzì GR, Marchese VA, Illari SI, Cacciola A, Lillo S, Parisi S, Valenti V. Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy. Gastroenterology Insights. 2021; 12(4):446-455. https://doi.org/10.3390/gastroent12040043
Chicago/Turabian StyleFerini, Gianluca, Antonella Tripoli, Vincenza Umina, Giuseppina Rita Borzì, Valentina Anna Marchese, Salvatore Ivan Illari, Alberto Cacciola, Sara Lillo, Silvana Parisi, and Vito Valenti. 2021. "Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy" Gastroenterology Insights 12, no. 4: 446-455. https://doi.org/10.3390/gastroent12040043
APA StyleFerini, G., Tripoli, A., Umina, V., Borzì, G. R., Marchese, V. A., Illari, S. I., Cacciola, A., Lillo, S., Parisi, S., & Valenti, V. (2021). Radiation Proctitis: The Potential Role of Hyaluronic Acid in the Prevention and Restoration of Any Damage to the Rectal Mucosa among Prostate Cancer Patients Submitted to Curative External Beam Radiotherapy. Gastroenterology Insights, 12(4), 446-455. https://doi.org/10.3390/gastroent12040043







