Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique?
Abstract
1. Introduction
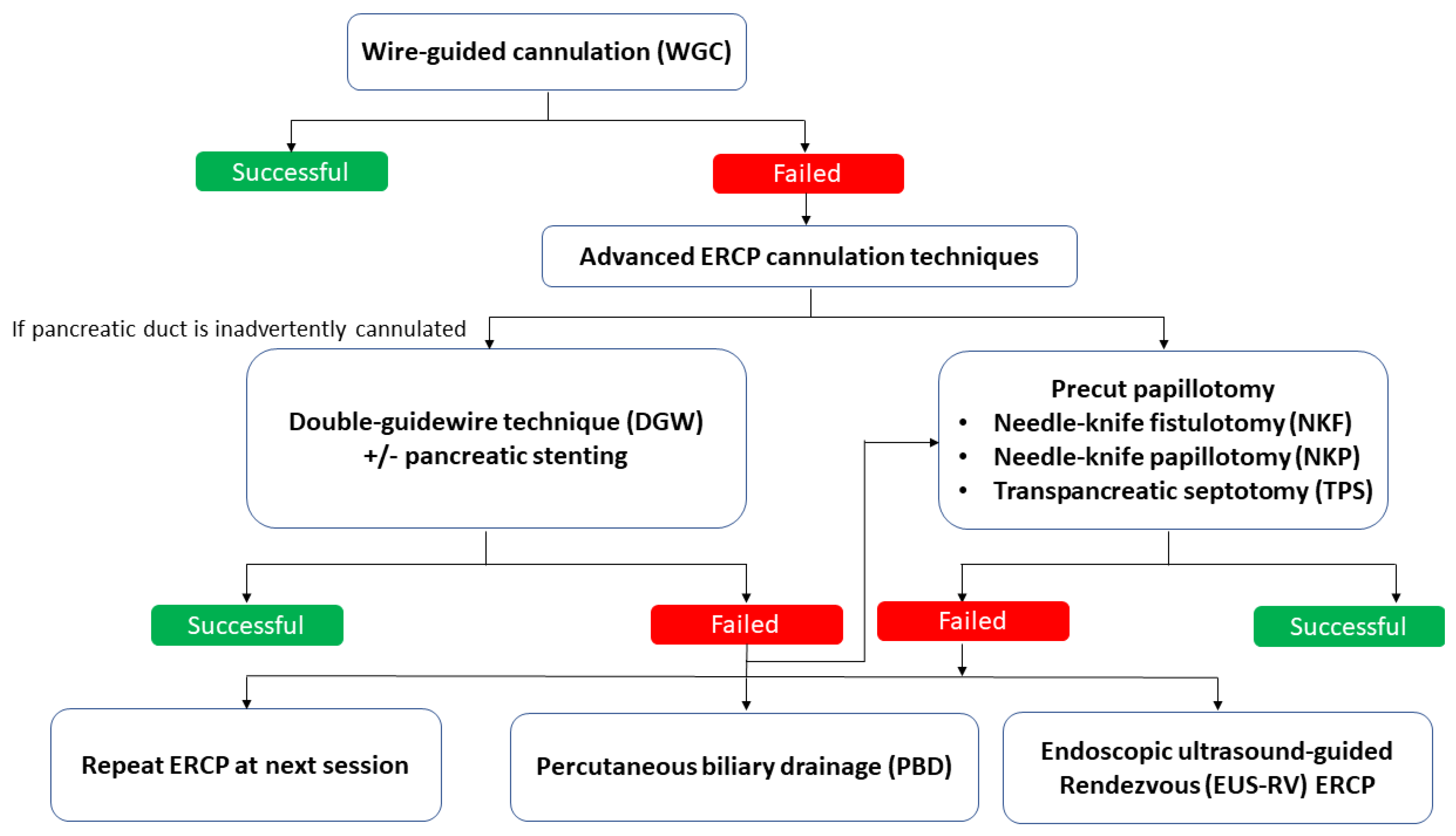
2. Advanced ERCP Cannulation Techniques
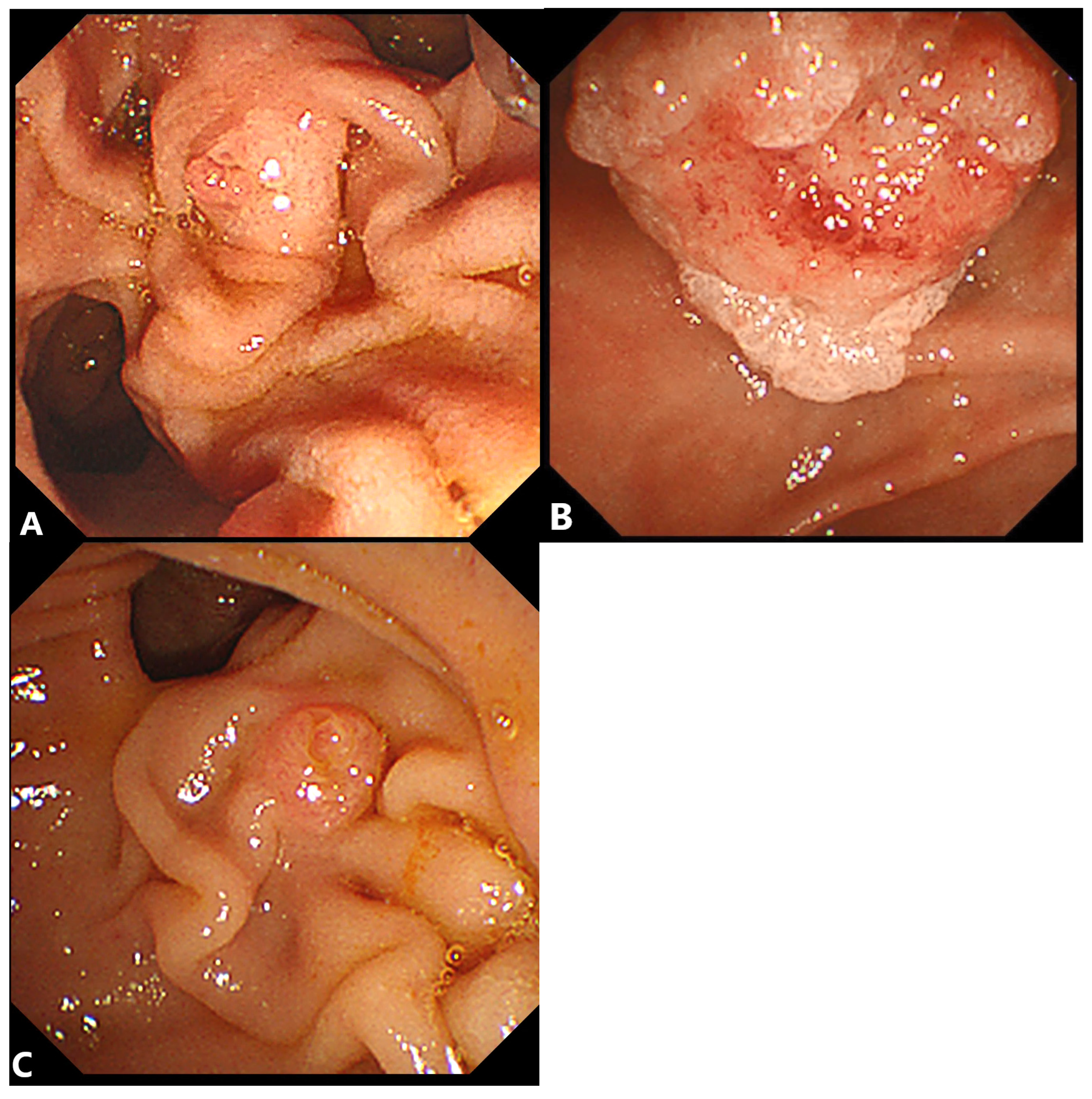
2.1. Double-Guidewire (DGW) Technique with or without Pancreatic Stenting
2.1.1. Advantages
2.1.2. Disadvantages
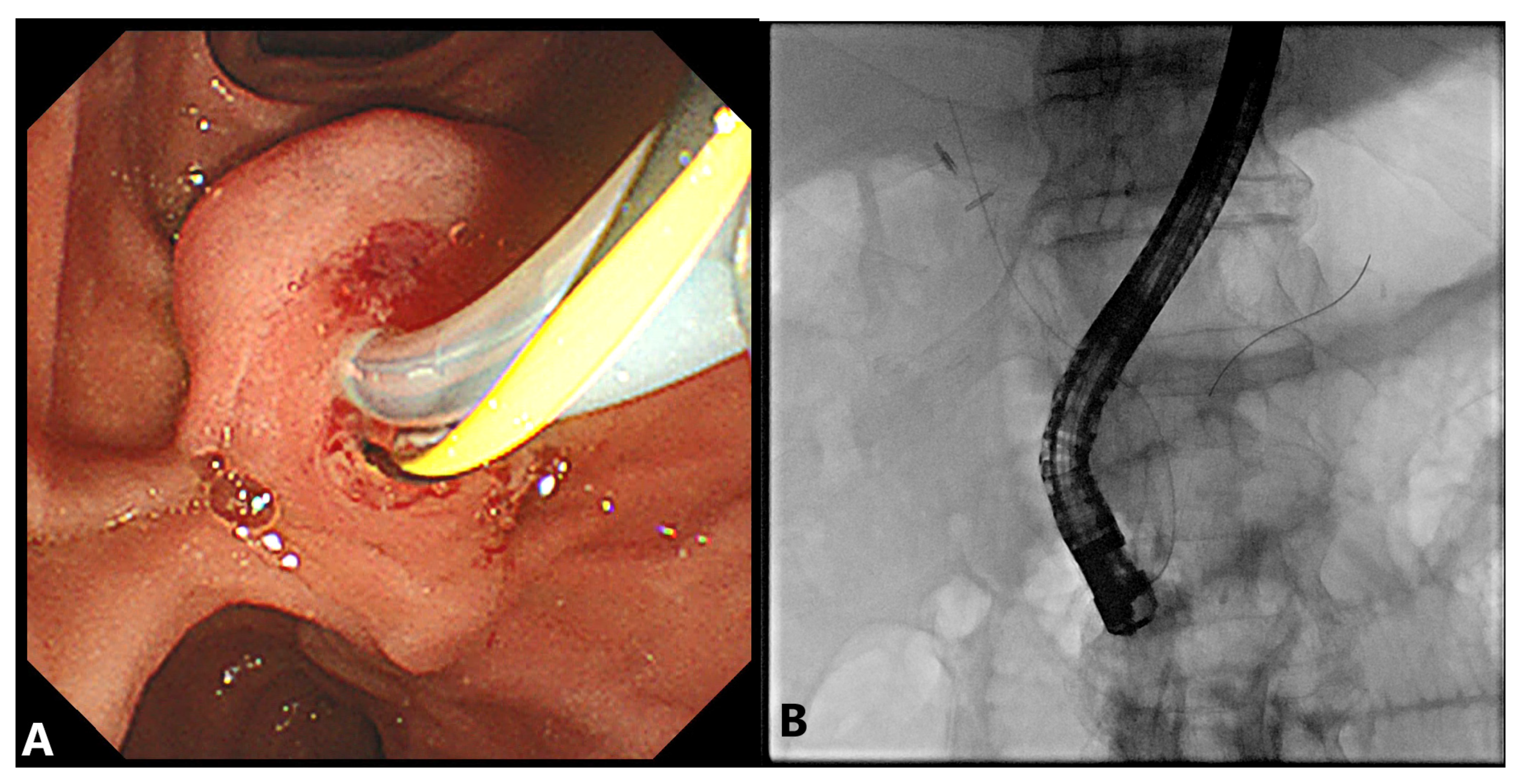
2.2. Precut Papillotomy
2.2.1. Advantages
2.2.2. Disadvantages
3. Percutaneous Biliary Drainage (PBD)
3.1. Advantages
3.2. Disadvantages
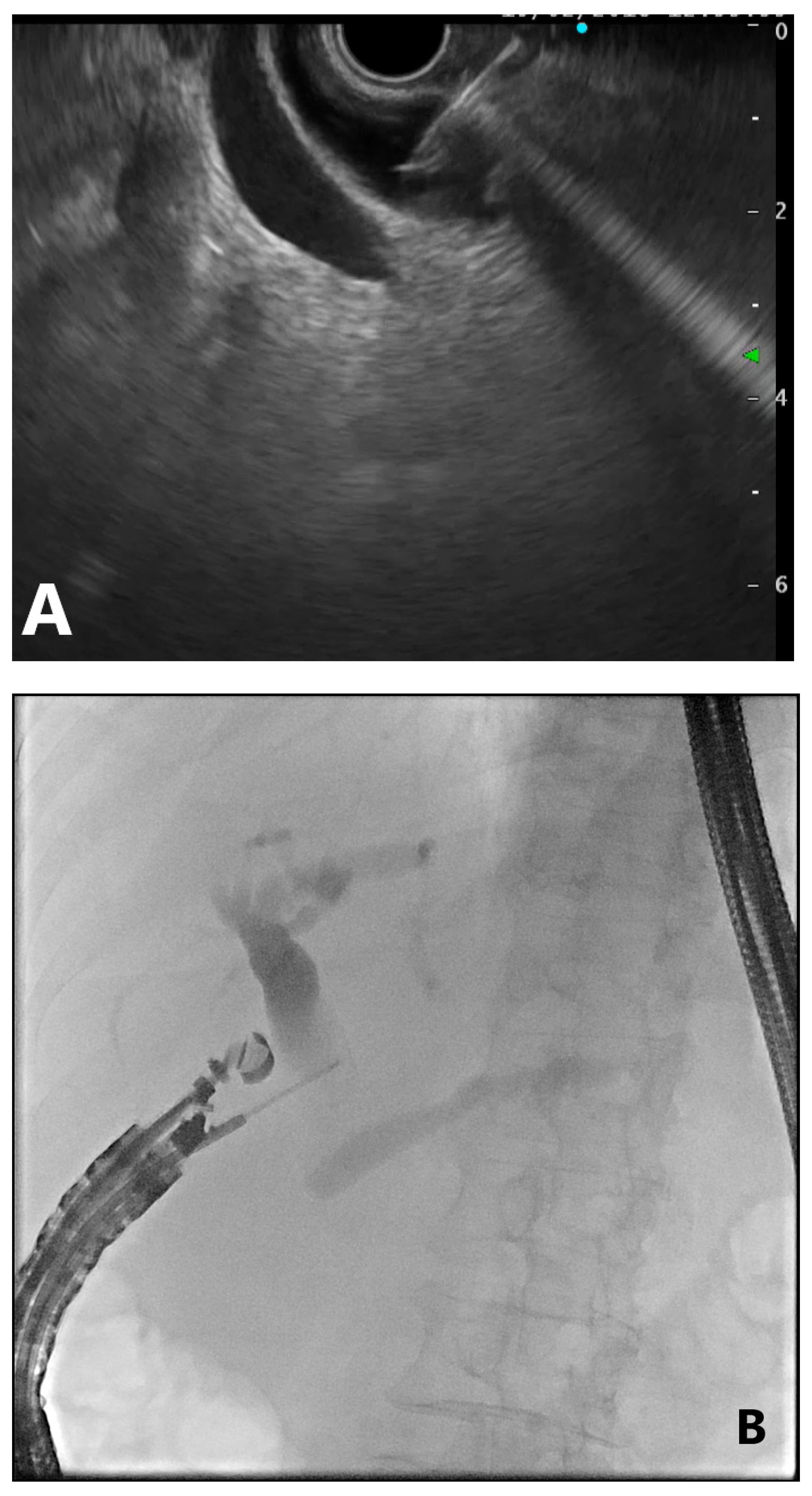
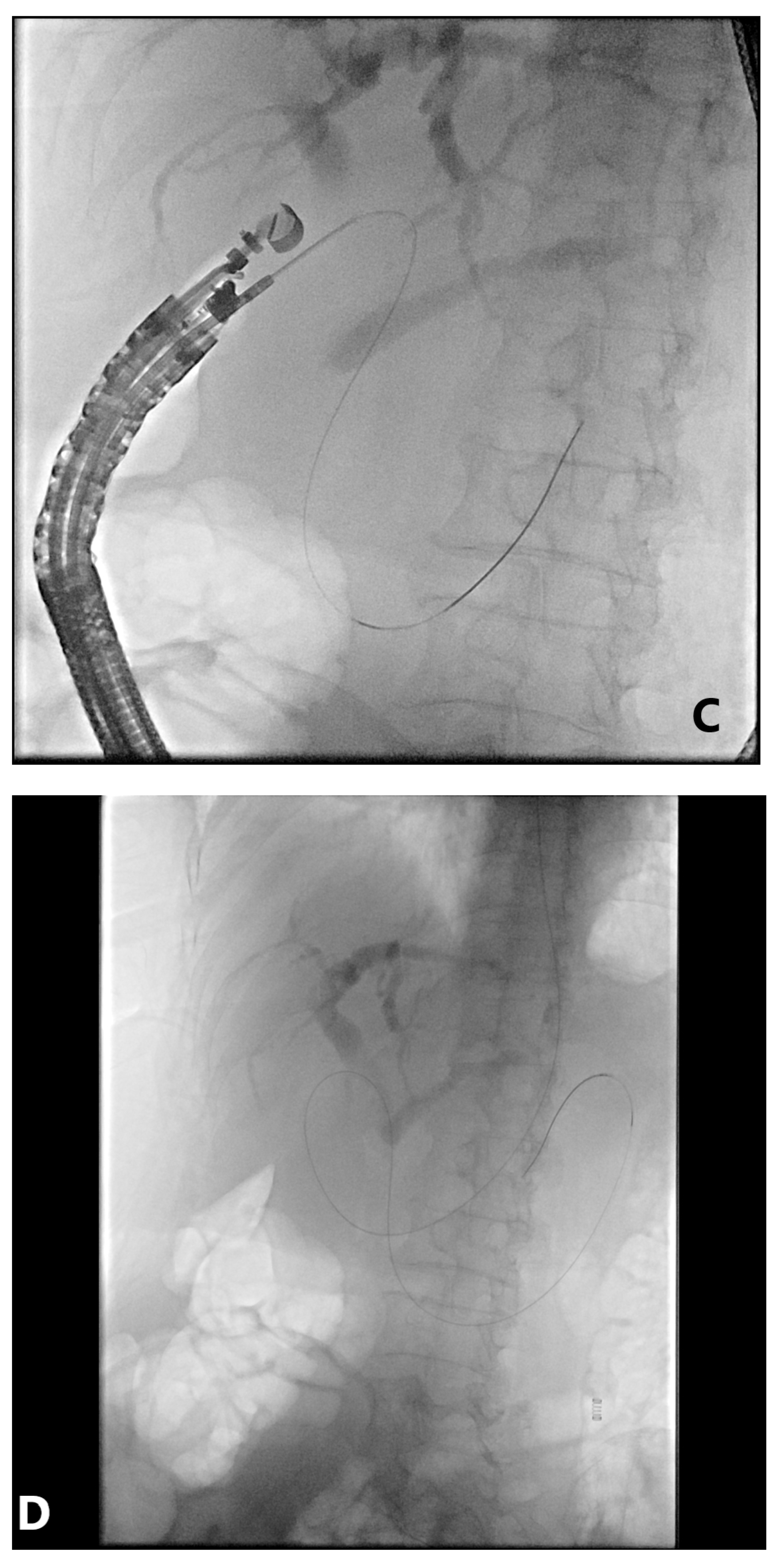
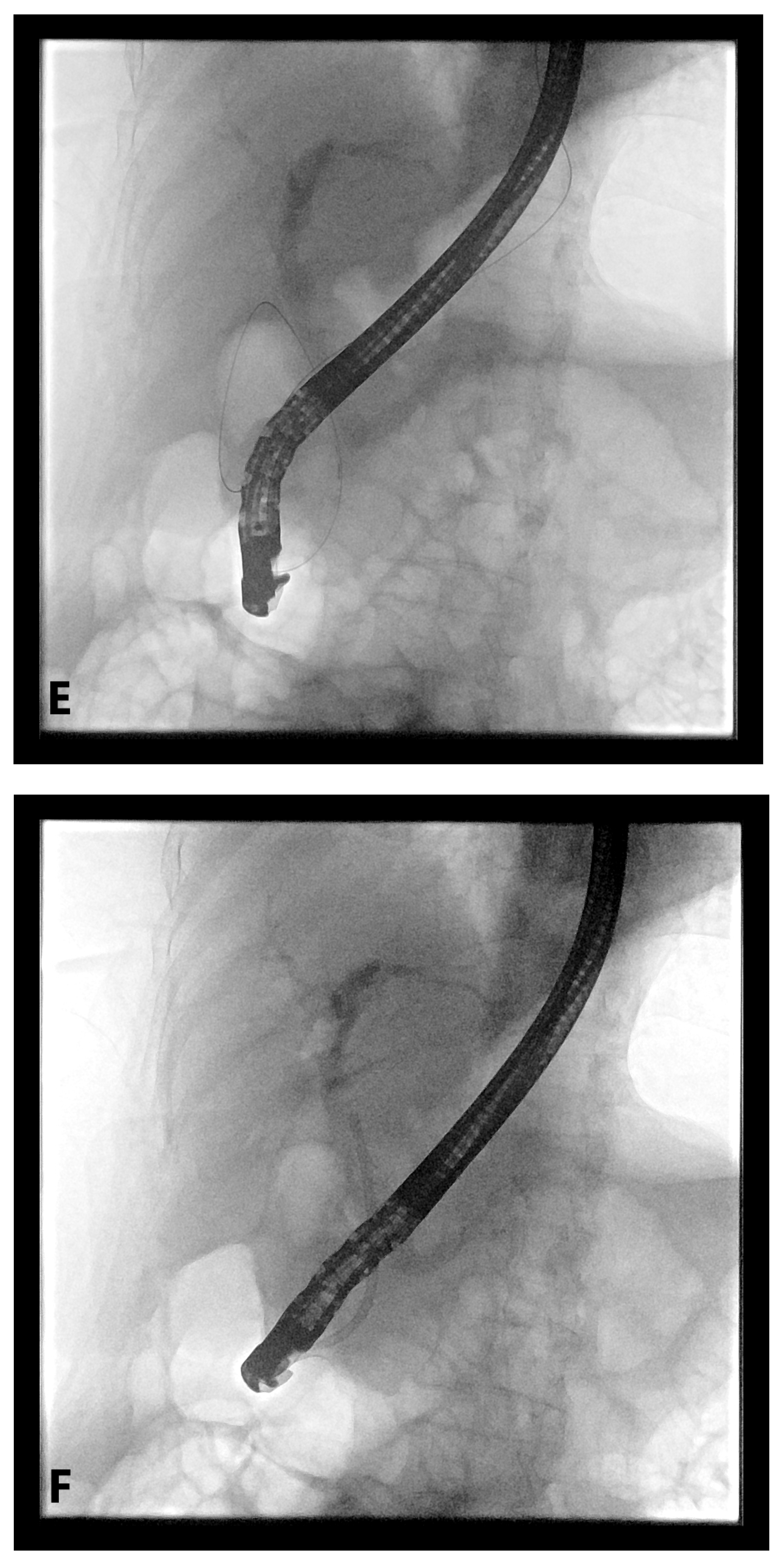
4. EUS-Guided Rendezvous (EUS-RV) Technique
4.1. Advantages
4.2. Disadvantages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ERCP | endoscopic retrograde cholangiopancreatography |
| ESGE | European Society of Gastrointestinal Endoscopy |
| WGC | wire-guided cannulation |
| DGW | double-guidewire |
| PBD | percutaneous biliary drainage |
| EUS-RV | endoscopic ultrasound-guided Rendezvous |
| PD | pancreatic duct |
| PEP | post-ERCP pancreatitis |
| NKF | needle-knife fistulotomy |
| NKP | needle-knife papillotomy |
| TPS | transpancreatic septotomy |
| PTC | percutaneous transhepatic cholecystostomy |
| PTBD | percutaneous transhepatic biliary drainage |
| EUS | endoscopic ultrasound |
| EUS-BD | EUS-guided biliary drainage |
| EUS-AG | EUS-antegrade approach |
| CDS | choledochoduodenostomy |
| HGS | hepaticogastrostomy |
| TH | transhepatic |
| EH | extrahepatic |
References
- Varadarajulu, S.; Kilgore, M.L.; Wilcox, C.M.; Eloubeidi, M.A. Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointest. Endosc. 2006, 64, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.A.; Bourke, M.J.; Williams, S.J.; Walsh, P.R.; Murray, M.A.; Lee, E.Y.; Kwan, V.; Lynch, P.M. A prospective randomized trial of cannulation technique in ERCP: Effects on technical success and post-ERCP pancreatitis. Endoscopy 2008, 40, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Testoni, P.A.; Mariani, A.; Aabakken, L.; Arvanitakis, M.; Bories, E.; Costamagna, G.; Devière, J.; Dinis-Ribeiro, M.; Dumonceau, J.M.; Giovannini, M.; et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016, 48, 657–683. [Google Scholar] [CrossRef]
- Liao, W.-C.; Angsuwatcharakon, P.; Isayama, H.; Dhir, V.; Devereaux, B.; Khor, C.J.L.; Ponnudurai, R.; Lakhtakia, S.; Lee, D.-K.; Ratanachu-ek, T.; et al. International consensus recommendations for difficult biliary access. Gastrointest. Endosc. 2017, 85, 295–304. [Google Scholar] [CrossRef]
- Swan, M.P.; Bourke, M.J.; Williams, S.J.; Alexander, S.; Moss, A.; Hope, R.; Ruppin, D. Failed biliary cannulation: Clinical and technical outcomes after tertiary referral endoscopic retrograde cholangiopancreatography. World J. Gastroenterol. 2011, 17, 4993–4998. [Google Scholar] [CrossRef]
- Pavlides, M.; Barnabas, A.; Fernandopulle, N.; Bailey, A.A.; Collier, J.; Phillips-Hughes, J.; Ellis, A.; Chapman, R.; Braden, B. Repeat endoscopic retrograde cholangiopancreaticography after failed initial precut sphincterotomy for biliary cannulation. World J. Gastroenterol. 2014, 20, 13153–13158. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, F.; Enns, R.; Kim, E.; Lam, E.; Amar, J.; Telford, J.; Byrne, M.F. Outcome of repeat ERCP after initial failed use of a needle knife for biliary access. Dig. Dis. Sci. 2012, 57, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Kevans, D.; Zeb, F.; Donnellan, F.; Courtney, G.; Aftab, A.R. Failed biliary access following needle knife fistulotomy: Is repeat interval ERCP worthwhile? Scand. J. Gastroenterol. 2010, 45, 1238–1241. [Google Scholar] [CrossRef]
- Freeman, M.L.; Guda, N.M. ERCP cannulation: A review of reported techniques. Gastrointest. Endosc. 2005, 61, 112–125. [Google Scholar] [CrossRef]
- Gyökeres, T.; Duhl, J.; Varsányi, M.; Schwab, R.; Burai, M.; Pap, Á. Double guide wire placement for endoscopic pancreaticobiliary procedures. Endoscopy 2003, 35, 95–96. [Google Scholar] [CrossRef]
- Draganov, P.; Devonshire, D.A.; Cunningham, J.T. A new technique to assist in difficult bile duct cannulation at the time of endoscopic retrograde cholangiopancreatography. JSLS 2005, 9, 218–221. [Google Scholar]
- Fry, L.C.; Barriga, J.A.; Linder, J.D.; Mönkemüller, K.E. Placement of a biliary catheter in the pancreatic duct to aid common bile duct cannulation. Endoscopy 2003, 35, 97. [Google Scholar] [CrossRef]
- Gotoh, Y.; Tamada, K.; Tomiyama, T.; Wada, S.; Ohashi, A.; Satoh, Y.; Higashizawa, T.; Miyata, T.; Ido, K.; Sugano, K. A new method for deep cannulation of the bile duct by straightening the pancreatic duct. Gastrointest. Endosc. 2001, 53, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Hayashi, H.; Hosokawa, O.; Dohden, K.; Hattori, M.; Morita, M.; Kidani, E.; Ibe, N.; Tatsumi, S. Prospective randomized pilot trial of selective biliary cannulation using pancreatic guide-wire placement. Endoscopy 2003, 35, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Obana, T.; Horaguchi, J.; Takasawa, O.; Koshita, S.; Kanno, Y. Pancreatic guidewire placement for achieving selective biliary cannulation during endoscopic retrograde cholangio-pancreatography. World J. Gastroenterol. 2008, 14, 5595–5600, discussion 5599. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Kung, J.; Liu, Y.; Tse, A.; Datta, A.; Singh, I.; Eysselein, V.E.; Reicher, S. Use of double wire-guided technique and transpancreatic papillary septotomy in difficult ERCP: 4-year experience. Endosc. Int. Open 2016, 4, E1107–E1110. [Google Scholar] [CrossRef][Green Version]
- Yang, M.J.; Hwang, J.C.; Yoo, B.M.; Kim, J.H.; Ryu, H.-K.; Kim, S.S.; Kang, J.K.; Kim, M.K. Wire-guided cannulation over a pancreatic stent versus double guidewire technique in patients with difficult biliary cannulation. BMC Gastroenterol. 2015, 15, 150. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Ren, G.; Xi, Y.; Luo, H.; Liang, S.; Wang, B.; Tao, Q.; Luo, B.; Qin, Q.; Farrell, J.J.; et al. Learning curve of double-guidewire technique by trainees during hands-on endoscopic retrograde cholangiopancreatography training. J. Gastroenterol. Hepatol. 2020, 35, 2176–2183. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Ogawa, M.; Omata, F.; Ito, H.; Shimosegawa, T.; Mine, T. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J. Gastroenterol. 2012, 18, 1635–1641. [Google Scholar] [CrossRef]
- Hakuta, R.; Hamada, T.; Nakai, Y.; Isayama, H.; Kogure, H.; Takahara, N.; Mizuno, S.; Yagioka, H.; Togawa, O.; Matsubara, S.; et al. Early pancreatic stent placement in wire-guided biliary cannulation: A multicenter retrospective study. J. Gastroenterol. Hepatol. 2019, 34, 1116–1122. [Google Scholar] [CrossRef]
- Phillip, V.; Pukitis, A.; Epstein, A.; Hapfelmeier, A.; Haf, D.; Schwab, M.; Demir, I.E.; Rosendahl, J.; Hoffmeister, A.; Schmid, R.M.; et al. Pancreatic stenting to prevent post-ERCP pancreatitis: A randomized multicenter trial. Endosc. Int. Open 2019, 7, E860–E868. [Google Scholar] [CrossRef] [PubMed]
- Hawes, R.H.; Devière, J. How I cannulate the bile duct. Endoscopy 2018, 50, 75–77. [Google Scholar]
- Masci, E.; Mangiavillano, B.; Luigiano, C.; Bizzotto, A.; Limido, E.; Cantù, P.; Manes, G.; Viaggi, P.; Spinzi, G.; Radaelli, F.; et al. Comparison between loop-tip guidewire-assisted and conventional endoscopic cannulation in high risk patients. Endosc. Int. Open 2015, 3, E464–E470. [Google Scholar] [CrossRef]
- Tse, F.; Yuan, Y.; Moayyedi, P.; Leontiadis, G.I.; Barkun, A.N. Double-guidewire technique in difficult biliary cannulation for the prevention of post-ERCP pancreatitis: A systematic review and meta-analysis. Endoscopy 2017, 49, 15–26. [Google Scholar] [CrossRef]
- Herreros de Tejada, A.; Calleja, J.L.; Díaz, G.; Pertejo, V.; Espinel, J.; Cacho, G.; Jiménez, J.; Millán, I.; García, F.; Abreu, L. Double-guidewire technique for difficult bile duct cannulation: A multicenter randomized, controlled trial. Gastrointest. Endosc. 2009, 70, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Overby, C.; Qi, D. Pancreatic stent insertion: Consequences of failure and results of a modified technique to maximize success. Gastrointest. Endosc. 2004, 59, 8–14. [Google Scholar] [CrossRef]
- Ito, K.; Fujita, N.; Noda, Y.; Kobayashi, G.; Obana, T.; Horaguchi, J.; Takasawa, O.; Koshita, S.; Kanno, Y.; Ogawa, T. Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J. Gastroenterol. 2010, 45, 1183–1191. [Google Scholar] [CrossRef]
- Ito, K.; Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Koshita, S.; Kanno, Y.; Ogawa, T.; Masu, K.; Hashimoto, S. Clinical usefulness of double-guidewire technique for difficult biliary cannulation in endoscopic retrograde cholangiopancreatography. Dig. Endosc. 2014, 26, 442–449. [Google Scholar] [CrossRef]
- Mavrogiannis, C.; Liatsos, C.; Romanos, A.; Petoumenos, C.; Nakos, A.; Karvountzis, G. Needle-knife fistulotomy versus needle-knife precut papillotomy for the treatment of common bile duct stones. Gastrointest. Endosc. 1999, 50, 334–339. [Google Scholar] [CrossRef]
- Rollhauser, C.; Johnson, M.; Al-Kawas, F.H. Needle-knife papillotomy: A helpful and safe adjunct to endoscopic retrograde cholangiopancreatography in a selected population. Endoscopy 1998, 30, 691–696. [Google Scholar] [CrossRef]
- Goff, J.S. Long-term experience with the transpancreatic sphincter pre-cut approach to biliary sphincterotomy. Gastrointest. Endosc. 1999, 50, 642–645. [Google Scholar] [CrossRef]
- Horiuchi, A.; Nakayama, Y.; Kajiyama, M.; Tanaka, N. Effect of precut sphincterotomy on biliary cannulation based on the characteristics of the major duodenal papilla. Clin. Gastroenterol. Hepatol. 2007, 5, 1113–1118. [Google Scholar] [CrossRef]
- Zang, J.; Zhang, C.; Gao, J. Guidewire-assisted transpancreatic sphincterotomy for difficult biliary cannulation: A prospective randomized controlled trial. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Gkagkalis, S.; Chatzimavroudis, G.; Beltsis, A.; Terzoudis, S.; Zavos, C.; Gatopoulou, A.; Lazaraki, G.; Vasiliadis, T.; Kountouras, J. Comparison of three types of precut technique to achieve common bile duct cannulation: A retrospective analysis of 274 cases. Dig. Dis. Sci. 2012, 57, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Winn, J.; Siddique, S.; Arif, M.; Arif, Z.; Hammoud, G.M.; Puli, S.R.; Ibdah, J.A.; Bechtold, M.L. Effect of precut sphincterotomy on post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. World J. Gastroenterol. 2014, 20, 4093–4101. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Xu, J.-H.; Dong, Z.-Q.; Gao, P.; Shen, Y.-C. Success and safety of needle knife papillotomy and fistulotomy based on papillary anatomy: A prospective controlled trial. Dig. Dis. Sci. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Kylänpää, L.; Koskensalo, V.; Saarela, A.; Ejstrud, P.; Udd, M.; Lindström, O.; Rainio, M.; Tenca, A.; Halttunen, J.; Qvigstad, G.; et al. Transpancreatic biliary sphincterotomy versus double guidewire in difficult biliary cannulation: A randomized controlled trial. Endoscopy 2021, 53, 1011–1019. [Google Scholar]
- Cennamo, V.; Fuccio, L.; Zagari, R.M.; Eusebi, L.H.; Ceroni, L.; Laterza, L.; Fabbri, C.; Bazzoli, F. Can early precut implementation reduce endoscopic retrograde cholangiopancreatography-related complication risk? Meta-analysis of randomized controlled trials. Endoscopy 2010, 42, 381–388. [Google Scholar] [CrossRef]
- Navaneethan, U.; Konjeti, R.; Venkatesh, P.G.; Sanaka, M.R.; Parsi, M.A. Early precut sphincterotomy and the risk of endoscopic retrograde cholangiopancreatography related complications: An updated meta-analysis. World J. Gastrointest. Endosc. 2014, 6, 200–208. [Google Scholar] [CrossRef]
- Gong, B.; Hao, L.; Bie, L.; Sun, B.; Wang, M. Does precut technique improve selective bile duct cannulation or increase post-ERCP pancreatitis rate? A meta-analysis of randomized controlled trials. Surg. Endosc. 2010, 24, 2670–2680. [Google Scholar] [CrossRef]
- Kaffes, A.J.; Sriram, P.V.; Rao, G.V.; Santosh, D.; Reddy, D.N. Early institution of pre-cutting for difficult biliary cannulation: A prospective study comparing conventional vs. a modified technique. Gastrointest. Endosc. 2005, 62, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Maharshi, S.; Sharma, S.S. Early precut versus primary precut sphincterotomy to reduce post-ERCP pancreatitis: Randomized controlled trial (with videos). Gastrointest. Endosc. 2021, 93, 586–593. [Google Scholar] [CrossRef]
- Davee, T.; Garcia, J.A.; Baron, T.H. Precut sphincterotomy for selective biliary duct cannulation during endoscopic retrograde cholangiopancreatography. Ann. Gastroenterol. 2012, 25, 291–302. [Google Scholar] [PubMed]
- Kubota, K.; Sato, T.; Kato, S.; Watanabe, S.; Hosono, K.; Kobayashi, N.; Hisatomi, K.; Matsuhashi, N.; Nakajima, A. Needle-knife precut papillotomy with a small incision over a pancreatic stent improves the success rate and reduces the complication rate in difficult biliary cannulations. J. Hepatobiliary Pancreat. Sci. 2013, 20, 382–388. [Google Scholar] [CrossRef]
- Cha, S.W.; Leung, W.D.; Lehman, G.A.; Watkins, J.L.; McHenry, L.; Fogel, E.L.; Sherman, S. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest. Endosc. 2013, 77, 209–216. [Google Scholar] [CrossRef]
- Loperfido, S.; Angelini, G.; Benedetti, G.; Chilovi, F.; Costan, F.; De Berardinis, F.; De Bernardin, M.; Ederle, A.; Fina, P.; Fratton, A. Major early complications from diagnostic and therapeutic ERCP: A prospective multicenter study. Gastrointest. Endosc. 1998, 48, 1–10. [Google Scholar] [CrossRef]
- Veitch, A.M.; Vanbiervliet, G.; Gershlick, A.H.; Boustiere, C.; Baglin, T.P.; Smith, L.A.; Radaelli, F.; Knight, E.; Gralnek, I.M.; Hassan, C.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut 2016, 65, 374–389. [Google Scholar] [CrossRef]
- Acosta, R.D.; Abraham, N.S.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fisher, D.A.; Fonkalsrud, L.; et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest. Endosc. 2016, 83, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Akaraviputh, T.; Lohsiriwat, V.; Swangsri, J.; Methasate, A.; Leelakusolvong, S.; Lertakayamanee, N. The learning curve for safety and success of precut sphincterotomy for therapeutic ERCP: A single endoscopist’s experience. Endoscopy 2008, 40, 513–516. [Google Scholar] [CrossRef]
- Madhusudhan, K.S.; Gamanagatti, S.; Srivastava, D.N.; Gupta, A.K. Radiological interventions in malignant biliary obstruction. World J. Radiol. 2016, 8, 518–529. [Google Scholar] [CrossRef]
- Long, W.B.; Schwarz, W.; Ring, E.J. Endoscopic sphincterotomy assisted by catheterization antegrade. Gastrointest. Endosc. 1984, 30, 36–39. [Google Scholar] [CrossRef]
- Rieber, A.; Brambs, H.J.; Friedl, P. Therapy of bile duct occlusion. Transhepatic/endoscopic “Rendezvous procedure” and percutaneous transhepatic stent placement. Fortschr. Medizin 1990, 108, 565–567. [Google Scholar]
- Calvo, M.M.; Bujanda, L.; Heras, I.; Cabriada, J.L.; Bernal, A.; Orive, V.; Miguelez, J. The rendezvous technique for the treatment of choledocholithiasis. Gastrointest. Endosc. 2001, 54, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Kim, J.H.; Hwang, J.C.; Yoo, B.M.; Kim, S.S.; Lim, S.G.; Won, J.H. Usefulness of combined percutaneous-endoscopic rendezvous techniques after failed therapeutic endoscopic retrograde cholangiography in the era of endoscopic ultrasound guided rendezvous. Medicine 2017, 96, e8991. [Google Scholar] [CrossRef] [PubMed]
- Okuno, M.; Iwashita, T.; Yasuda, I.; Mabuchi, M.; Uemura, S.; Nakashima, M.; Doi, S.; Adachi, S.; Mukai, T.; Moriwaki, H. Percutaneous transgallbladder rendezvous for enteroscopic management of choledocholithiasis in patients with surgically altered anatomy. Scand. J. Gastroenterol. 2013, 48, 974–978. [Google Scholar] [CrossRef]
- Bokemeyer, A.; Müller, F.; Niesert, H.; Brückner, M.; Bettenworth, D.; Nowacki, T.; Beyna, T.; Ullerich, H.; Lenze, F. Percutaneous-transhepatic-endoscopic rendezvous procedures are effective and safe in patients with refractory bile duct obstruction. United Eur. Gastroenterol. J. 2019, 7, 397–404. [Google Scholar] [CrossRef]
- Wayman, J.; Mansfield, J.C.; Matthewson, K.; Richardson, D.L.; Griffin, S.M. Combined percutaneous and endoscopic procedures for bile duct obstruction: Simultaneous and delayed techniques compared. Hepatogastroenterology 2003, 50, 915–918. [Google Scholar]
- Tomizawa, Y.; Di Giorgio, J.; Santos, E.; McCluskey, K.M.; Gelrud, A. Combined interventional radiology followed by endoscopic therapy as a single procedure for patients with failed initial endoscopic biliary access. Dig. Dis. Sci. 2014, 59, 451–458. [Google Scholar] [CrossRef]
- Ko, G.Y.; Sung, K.B.; Yoon, H.K.; Kim, K.R.; Gwon, D.I.; Lee, S.G. Percutaneous transhepatic treatment of hepaticojejunal anastomotic biliary strictures after living donor liver transplantation. Liver Transpl. 2008, 14, 1323–1332. [Google Scholar] [CrossRef]
- Nakai, Y.; Kogure, H.; Yamada, A.; Isayama, H.; Koike, K. Endoscopic management of bile duct stones in patients with surgically altered anatomy. Dig. Endosc. 2018, 30, 67–74. [Google Scholar] [CrossRef]
- Born, P.; Rösch, T.; Triptrap, A.; Frimberger, E.; Allescher, H.D.; Ott, R.; Weigert, N.; Lorenz, R.; Classen, M. Long-term results of percutaneous transhepatic biliary drainage for benign and malignant bile duct strictures. Scand. J. Gastroenterol. 1998, 33, 544–549. [Google Scholar]
- Saad, W.E.A.; Wallace, M.J.; Wojak, J.C.; Kundu, S.; Cardella, J.F. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J. Vasc. Interv. Radiol. 2010, 21, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Yasunaga, H.; Nakai, Y.; Isayama, H.; Horiguchi, H.; Fushimi, K.; Koike, K. Severe bleeding after percutaneous transhepatic drainage of the biliary system: Effect of antithrombotic agents—Analysis of 34,606 cases from a Japanese Nationwide Administrative Database. Radiology 2015, 274, 605–613. [Google Scholar] [CrossRef]
- Al-Bahrani, A.Z.; Holt, A.; Hamade, A.M.; Abid, G.H.; Laasch, H.U.; O’Shea, S.J.; Lee, S.H.; Ammori, B.J. Acute pancreatitis: An under-recognized risk of percutaneous transhepatic distal biliary intervention. HPB 2006, 8, 446–450. [Google Scholar] [CrossRef][Green Version]
- Iwashita, T.; Doi, S.; Yasuda, I. Endoscopic ultrasound-guided biliary drainage: A review. Clin. J. Gastroenterol. 2014, 7, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Li, T.; Cheng, Y.; Wang, B.; Cao, Y.; Wang, Y. Endoscopic ultrasound-guided vs ERCP-guided biliary drainage for malignant biliary obstruction: A up-to-date meta-analysis and systematic review. Dig. Liver Dis. 2021, 53, 1247–1253. [Google Scholar] [CrossRef]
- Miller, C.S.; Barkun, A.N.; Martel, M.; Chen, Y.I. Endoscopic ultrasound-guided biliary drainage for distal malignant obstruction: A systematic review and meta-analysis of randomized trials. Endosc. Int. Open 2019, 7, E1563–E1573. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Kim, S.-O.; So, H.; Shin, E.; Kim, D.U.; Park, D.H. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16551. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Dhir, V. EUS-guided biliary rendezvous: Slow, hesitant, baby steps forward. Gastrointest. Endosc. 2016, 83, 401–403. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Itoi, T.; Sofuni, A.; Tonozuka, R.; Mukai, S. Endoscopic ultrasonography-guided rendezvous technique. Dig. Endosc. 2016, 28, 96–101. [Google Scholar] [CrossRef]
- Giovannini, M.; Moutardier, V.; Pesenti, C.; Bories, E.; Lelong, B.; Delpero, J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001, 33, 898–900. [Google Scholar] [CrossRef]
- Dhir, V.; Kwek, B.E.; Bhandari, S.; Bapat, M.; Maydeo, A. EUS-guided biliary rendezvous using a short hydrophilic guidewire. J. Interv. Gastroenterol. 2011, 1, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakai, Y.; Isayama, H.; Matsubara, S.; Kogure, H.; Mizuno, S.; Hamada, T.; Takahara, N.; Nakamura, T.; Sato, T.; Takeda, T.; et al. A novel “hitch-and-ride” deep biliary cannulation method during rendezvous endoscopic ultrasound-guided ERCP technique. Endoscopy 2017, 49, 983–988. [Google Scholar] [CrossRef]
- Martínez, B.; Martínez, J.; Casellas, J.A.; Aparicio, J.R. Endoscopic ultrasound-guided rendezvous in benign biliary or pancreatic disorders with a 22-gauge needle and a 0.018-inch guidewire. Endosc. Int. Open 2019, 7, E1038–E1043. [Google Scholar]
- Marrache, M.K.; Al-Sabban, A.; Itani, M.; Farha, J.; Fayad, L.; Khashab, M.A.; Kumbhari, V. Endoscopic ultrasound-guided rendezvous ERCP using a steerable access device. Endoscopy 2020, 52, E355–E356. [Google Scholar] [CrossRef] [PubMed]
- Mallery, S.; Matlock, J.; Freeman, M.L. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest. Endosc. 2004, 59, 100–107. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gupta, K.; Mallery, S.; Li, R.; Kinney, T.; Freeman, M.L. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: Cumulative experience at a single center. A case series. Endoscopy 2010, 42, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, T.; Lee, J.G.; Shinoura, S.; Nakai, Y.; Park, D.H.; Muthusamy, V.R.; Chang, K.J. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy 2012, 44, 60–65. [Google Scholar] [CrossRef]
- Klair, J.S.; Zafar, Y.; Ashat, M.; Bomman, S.; Murali, A.R.; Jayaraj, M.; Law, J.; Larsen, M.; Singh, D.P.; Rustagi, T.; et al. Effectiveness and safety of EUS rendezvous after failed biliary cannulation with ERCP: A Systematic review and proportion meta-analysis. J. Clin. Gastroenterol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Iwashita, T.; Yasuda, I.; Mukai, T.; Iwata, K.; Ando, N.; Doi, S.; Nakashima, M.; Uemura, S.; Mabuchi, M.; Shimizu, M. EUS-guided rendezvous for difficult biliary cannulation using a standardized algorithm: A multicenter prospective pilot study (with videos). Gastrointest. Endosc. 2016, 83, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Bhandari, S.; Bapat, M.; Joshi, N.; Vivekanandarajah, S.; Maydeo, A. Comparison of transhepatic and extrahepatic routes for EUS-guided rendezvous procedure for distal CBD obstruction. United Eur. Gastroenterol. J. 2013, 1, 103–108. [Google Scholar] [CrossRef]
- Dhir, V.; Bhandari, S.; Bapat, M.; Maydeo, A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest. Endosc. 2012, 75, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Aditi, A.; Bhat, Y.M.; Binmoeller, K.F.; Hamerski, C.; Sendino, O.; Kane, S.; Cello, J.P.; Day, L.W.; Mohamadnejad, M.; et al. Endoscopic ultrasound-guided biliary access versus precut papillotomy in patients with failed biliary cannulation: A retrospective study. Endoscopy 2017, 49, 146–153. [Google Scholar] [CrossRef]
- Khashab, M.A.; Valeshabad, A.K.; Afghani, E.; Singh, V.K.; Kumbhari, V.; Messallam, A.; Saxena, P.; El Zein, M.; Lennon, A.M.; Canto, M.I.; et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig. Dis. Sci. 2015, 60, 557–565. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Khan, M.A.; Kamal, F.; Tyberg, A.; Tombazzi, C.R.; Ali, B.; Tombazzi, C.; Kahaleh, M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 85, 904–914. [Google Scholar] [CrossRef]
- Bill, J.G.; Darcy, M.; Fujii-Lau, L.L.; Mullady, D.K.; Gaddam, S.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M. A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction. Endosc. Int. Open 2016, 4, E980–E985. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, Y.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Nagai, K.; et al. EUS-guided hepaticoenterostomy with using a dedicated plastic stent for the benign pancreaticobiliary diseases: A single-center study of a large case series. Endosc. Ultrasound 2021, 10, 294–304. [Google Scholar] [PubMed]
- Shiomi, H.; Yamao, K.; Hoki, N.; Hisa, T.; Ogura, T.; Minaga, K.; Masuda, A.; Matsumoto, K.; Kato, H.; Kamada, H.; et al. Endoscopic ultrasound-guided rendezvous technique for failed biliary cannulation in benign and resectable malignant biliary disorders. Dig. Dis. Sci. 2018, 63, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Nakai, Y.; Kawakubo, K.; Kawakami, H.; Itoi, T.; Yamamoto, N.; Kogure, H.; Koike, K. The endoscopic ultrasonography-guided rendezvous technique for biliary cannulation: A technical review. J. Hepatobiliary Pancreat. Sci. 2013, 20, 413–420. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, J.; Xing, L.; Wang, Y.; Jin, Z.; Li, Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest. Endosc. 2016, 83, 1218–1227. [Google Scholar] [CrossRef] [PubMed]


| Techniques | Advantages | Disadvantages |
|---|---|---|
| Double-guidewire (DGW) technique with or without pancreatic stenting |
|
|
| Precut papillotomy |
|
|
| Percutaneous biliary drainage (PBD) |
|
|
| EUS-guided Rendezvous (EUS-RV) ERCP |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, T.-T.; Chew, M.C.H.; Tang, R.S.Y. Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique? Gastroenterol. Insights 2021, 12, 405-422. https://doi.org/10.3390/gastroent12040039
Chan T-T, Chew MCH, Tang RSY. Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique? Gastroenterology Insights. 2021; 12(4):405-422. https://doi.org/10.3390/gastroent12040039
Chicago/Turabian StyleChan, Ting-Ting, Marcus C. H. Chew, and Raymond S. Y. Tang. 2021. "Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique?" Gastroenterology Insights 12, no. 4: 405-422. https://doi.org/10.3390/gastroent12040039
APA StyleChan, T.-T., Chew, M. C. H., & Tang, R. S. Y. (2021). Troubleshooting Difficult Bile Duct Access: Advanced ERCP Cannulation Techniques, Percutaneous Biliary Drainage, or EUS-Guided Rendezvous Technique? Gastroenterology Insights, 12(4), 405-422. https://doi.org/10.3390/gastroent12040039








