Susceptibility Weighted Imaging in Migraine with and Without Aura: A Case–Control Study
Abstract
1. Introduction
2. Methods
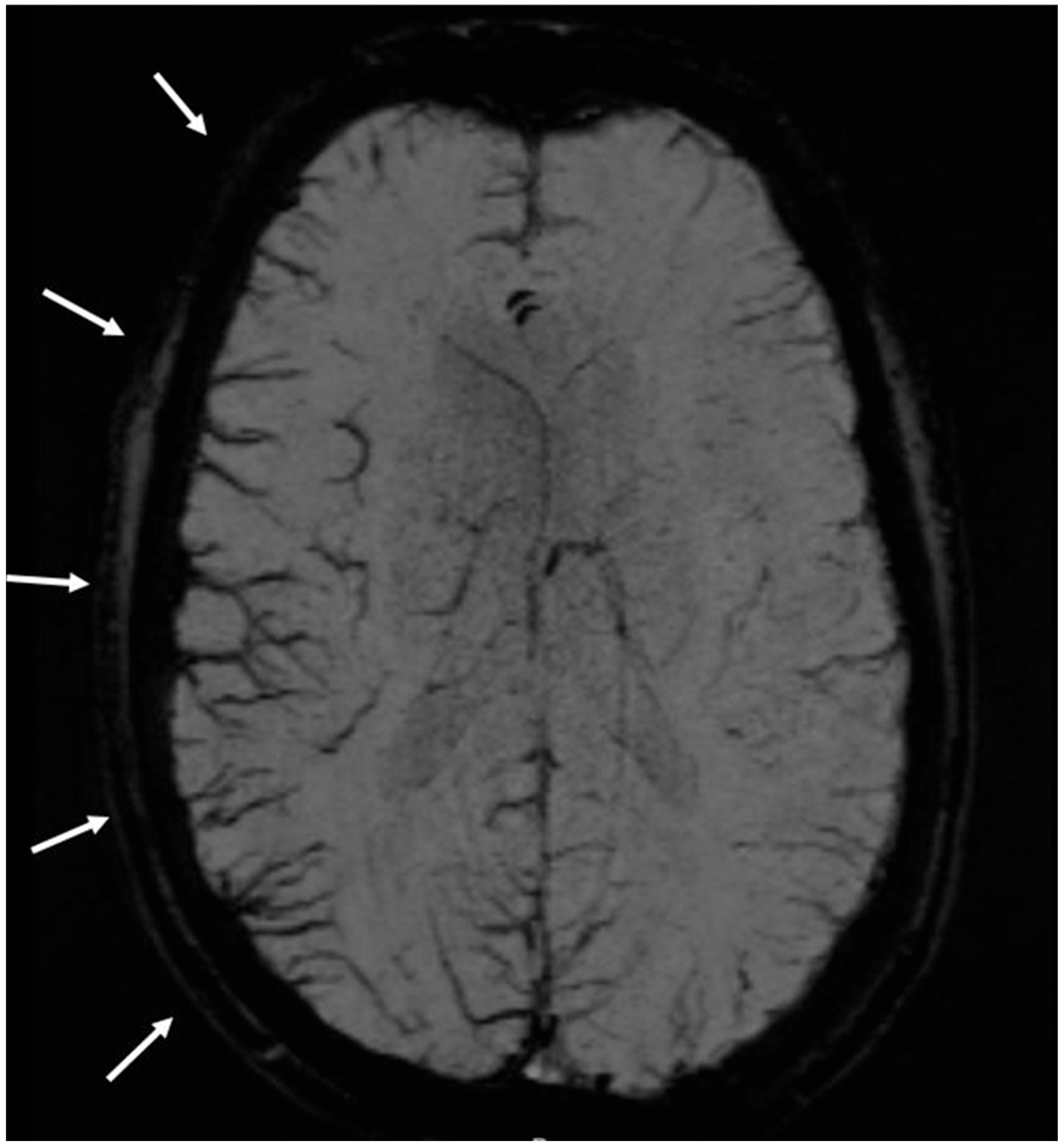
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Goadsby, P.J. Migraine, aura, and cortical spreading depression: Why are we still talking about it? Ann. Neurol. 2001, 49, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Bolay, H.; Vuralli, D.; Goadsby, P.J. Aura and Head pain: Relationship and gaps in the translational models. J. Headache Pain 2019, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Burstein, R.; Ashina, M.; Tfelt-Hansen, P. Origin of pain in migraine: Evidence for peripheral sensitisation. Lancet Neurol. 2009, 8, 679–690. [Google Scholar] [CrossRef]
- Shatillo, A.; Koroleva, K.; Giniatullina, R.; Naumenko, N.; Slastnikova, A.; Aliev, R.; Bart, G.; Atalay, M.; Gu, C.; Khazipov, R.; et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience 2013, 253, 341–349. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of meningeal nociceptors by cortical spreading depression: Implications for migraine with aura. J. Neurosci. 2010, 30, 8807–8814, Erratum in: J. Neurosci. 2010, 30, 10259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Levy, D.; Kainz, V.; Noseda, R.; Jakubowski, M.; Burstein, R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011, 69, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Harriott, A.M.; Chung, D.Y.; Uner, A.; Bozdayi, R.O.; Morais, A.; Takizawa, T.; Qin, T.; Ayata, C. Optogenetic Spreading Depression Elicits Trigeminal Pain and Anxiety Behavior. Ann. Neurol. 2021, 89, 99–110. [Google Scholar] [CrossRef]
- Bogdanov, V.B.; Bogdanova, O.V.; Lombard, A.; Chauvel, V.; Multon, S.; Kot, L.I.; Makarchuk, M.Y.; Schoenen, J. Cortical spreading depression decreases Fos expression in rat periaqueductal gray matter. Neurosci. Lett. 2015, 585, 138–143. [Google Scholar] [CrossRef]
- Zhao, J.; Levy, D. Modulation of intracranial meningeal nociceptor activity by cortical spreading depression: A reassessment. J. Neurophysiol. 2015, 113, 2778–2785. [Google Scholar] [CrossRef]
- Ebersberger, A.; Schaible, H.G.; Averbeck, B.; Richter, F. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache? Ann. Neurol. 2001, 49, 7–13. [Google Scholar] [CrossRef]
- Fioravanti, B.; Kasasbeh, A.; Edelmayer, R.; Skinner, D.P., Jr.; Hartings, J.A.; Burklund, R.D.; De Felice, M.; French, E.D.; O Dussor, G.; Dodick, D.W.; et al. Evaluation of cutaneous allodynia following induction of cortical spreading depression in freely moving rats. Cephalalgia 2011, 31, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Wolthausen, J.; Sternberg, S.; Gerloff, C.; May, A. Are cortical spreading depression and headache in migraine causally linked? Cephalalgia 2009, 29, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, V.B.; Multon, S.; Chauvel, V.; Bogdanova, O.V.; Prodanov, D.; Makarchuk, M.Y.; Schoenen, J. Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol. Dis. 2011, 41, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, M.; Akman, Ö.; Selekler, H.; Komsuoğlu, S.; Ateş, N. Does metoprolol inhibit the cortical spreading depression? Acute effects of systematic metropol on CSD in rats. Cephalalgia 2007, 27, 1010–1013. [Google Scholar] [CrossRef]
- Silberstein, S.; Schoenen, J.; Göbel, H.; Diener, H.; Elkind, A.; Klapper, J.; Howard, R. Tonabersat, a gap-junction modulator: Efficacy and safety in two randomized, placebo-controlled, dose-ranging studies of acute migraine. Cephalalgia 2009, 29, 17–27. [Google Scholar] [CrossRef]
- Haacke, E.M.; Xu, Y.; Cheng, Y.C.; Reichenbach, J.R. Susceptibility weighted imaging (SWI). Magn. Reson. Med. 2004, 52, 612–618. [Google Scholar] [CrossRef]
- Leao, A.A.P.; Morison, R.S. Propagation of spreading cortical depression. J. Neurophysiol. 1945, 8, 33–45. [Google Scholar] [CrossRef]
- Kellner-Weldon, F.; Lehmann, V.F.; Breiding, P.S.; Grunder, L.; Muri, R.; Pastore-Wapp, M.; Bigi, S.; Wiest, R.; El-Koussy, M.; Slavova, N. Findings in susceptibility weighted imaging in pediatric patients with migraine with aura. Eur. J. Paediatr. Neurol. 2020, 28, 221–227. [Google Scholar] [CrossRef]
- Scutelnic, A.; Petroulia, V.; Schraml, L.; Jung, S.; Branca, M.; Beyeler, M.; Fischer, U.; Wiest, R.; Slavova, N.; Schankin, C.J. The “Index Vein” as a sign for migraine aura in the emergency setting. Cephalalgia 2023, 43, 3331024221132010. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Slavova, N.; Denier, N.; El-Koussy, M.; Wiest, R.; Kellner-Weldon, F.; Fischer, U.; Schankin, C.J. The index vein pointing to the origin of the migraine aura symptom: A case series. Neurology 2020, 94, e2577–e2580. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Haan, J.; Blokland, J.K.; Arndt, J.W.; Minnee, P.; Zwinderman, A.H.; Pauwels, E.K.; Saxena, P.R. Cerebral blood flow during migraine attacks without aura and effect of sumatriptan. Arch. Neurol. 1995, 52, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Sanchez del Rio, M.; Bakker, D.; Wu, O.; Agosti, R.; Mitsikostas, D.D.; Østergaard, L.; Wells, W.A.; Rosen, B.R.; Sorensen, G.; Moskowitz, M.A.; et al. Perfusion weighted imaging during migraine: Spontaneous visual aura and headache. Cephalagia 1999, 19, 701–707. [Google Scholar] [CrossRef]
- Woods, R.P.; Iacoboni, M.; Mazziotta, J.C. Brief report: Bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N. Engl. J. Med. 1994, 331, 1689–1692. [Google Scholar] [CrossRef]
- Denuelle, M.; Fabre, N.; Payoux, P.; Chollet, F.; Geraud, G. Posterior cerebral hypoperfusion in migraine without aura. Cephalalgia 2008, 28, 856–862. [Google Scholar] [CrossRef]
- Bednarczyk, E.M.; Remler, B.; Weikart, C.; Nelson, A.D.; Reed, R.C. Global cerebral blood flow, blood volume, and oxygen metabolism in patients with migraine headache. Neurology 1998, 50, 1736–1740. [Google Scholar] [CrossRef]
- Lauritzen, M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994, 117, 199–210. [Google Scholar] [CrossRef]
- Larner, A.J. Transient global amnesia: Model, mechanism, hypothesis. Cortex 2022, 149, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hong, S.M.; Park, I.S.; Choi, H.G. Association Between Migraine and Benign Paroxysmal Positional Vertigo Among Adults in South Korea. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 307–312. [Google Scholar] [CrossRef]
- Schulte, L.H.; Allers, A.; May, A. Hypothalamus as a mediator of chronic migraine: Evidence from high-resolution fMRI. Neurology 2017, 88, 2011–2016. [Google Scholar] [CrossRef]
- Maniyar, F.H.; Sprenger, T.; Monteith, T.; Schankin, C.; Goadsby, P.J. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014, 137, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.; Fischer-Schulte, L.; May, A. Aura phenomena do not initiate migraine attacks-Findings from neuroimaging. Headache 2013, 63, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
| Co-Morbidities and Vascular Risk Factors | Migraine with Aura | Migraine Without Aura | Controls | p Value (Bonferroni) | Post-Hoc Test | ||
|---|---|---|---|---|---|---|---|
| p Value MA vs. MO † | p Value MA vs. C † | p Value MO vs. C † | |||||
| age | 39+/−15 | 36+/−11 | 61+/−13 | < 0.001 | 0.1 | <0.001 | <0.001 |
| female sex | 68 | 86 | 55 | < 0.001 | 0.09 | 0.059 | <0.001 |
| arterial hypertension | 15 | 6 | 33 | < 0.001 | 0.03 | 0.003 | <0.001 |
| obesity * | 1 | 3 | 3 | 1 | 0.62 | 0.62 | 1 |
| atrial fibrillation | 2 | 0 | 5 | 0.18 | 0.49 | 0.24 | 0.059 |
| diabetes mellitus | 6 | 4 | 8 | 1 | 0.52 | 0.57 | 0.23 |
| cigarette smoking | 17 | 9 | 14 | 0.72 | 0.93 | 0.55 | 0.39 |
| family history of stroke/myocardial infarction | 0 | 1 | 2 | 1 | 1 | 0.49 | 1 |
| chronic alcohol abuse †† | 4 | 1 | 4 | 1 | 0.36 | 1 | 0.36 |
| Illicit drugs | 2 | 1 | 2 | 1 | 1 | 1 | 1 |
| depression | 2 | 11 | 8 | 0.12 | 0.01 | 0.052 | 0.46 |
| chronic kidney failure | 0 | 1 | 3 | 0.51 | 1 | 0.24 | 0.62 |
| sleep apnea | 2 | 0 | 4 | 1 | 0.49 | 0.4 | 0.12 |
| dyslipidemia | 9 | 3 | 20 | <0.001 | 0.07 | 0.02 | <0.001 |
| No | Sex | Age | Diagnosis at Discharge | Co-Morbidities | Vascular Risk Factors |
|---|---|---|---|---|---|
| 1 | male | 49 | benign positional vertigo | history of migraine without aura, fungal sinusitis ethmoidalis | arterial hypertension |
| 2 | female | 71 | transient global amnesia | restless-legs syndrome | none |
| 3 | male | 59 | transient global amnesia | none | arterial hypertension |
| 4 | female | 56 | benign positional vertigo | hypothyreosis, osteoarthritis | arterial hypertension, smoking, dyslipidemia |
| 5 | female | 35 | idiopathic facial nerve palsy | none | arterial hypertension |
| 6 | female | 74 | transient global amnesia | IgG (1&3) antibody deficiency syndrome | none |
| 7 | male | 65 | transient global amnesia | none | none |
| 8 | female | 42 | migraine without aura | none | depression |
| 9 | male | 44 | migraine without aura | none | none |
| 10 | male | 52 | migraine without aura | none | arterial hypertension |
| Symptoms | SWI Changes Present (N = 26) | SWI Changes Absent (N = 74) | p Value |
|---|---|---|---|
| Visual symptoms n (%) | 16 (62) | 49 (66) | 0.66 |
| Sensory symptoms n (%) | 16 (62) | 37 (50) | 0.31 |
| Motor symptoms n (%) | 4 (15) | 14 (19) | 0.68 |
| Speech disturbance n (%) | 17 (65) | 24 (32) | 0.003 |
| Coordination problems n (%) | 3 (12) | 1 (1) | 0.053 |
| Vertigo n (%) | 3 (12) | 11 (15) | 0.67 |
| Confusion n (%) | 0 | 1 (1) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutelnic, A.; Klail, T.; Moor, D.; Slavova, N.; Petroulia, V.; Jung, S.; Branca, M.; Fischer, U.; Riederer, F.; Wiest, R.; et al. Susceptibility Weighted Imaging in Migraine with and Without Aura: A Case–Control Study. Neurol. Int. 2025, 17, 104. https://doi.org/10.3390/neurolint17070104
Scutelnic A, Klail T, Moor D, Slavova N, Petroulia V, Jung S, Branca M, Fischer U, Riederer F, Wiest R, et al. Susceptibility Weighted Imaging in Migraine with and Without Aura: A Case–Control Study. Neurology International. 2025; 17(7):104. https://doi.org/10.3390/neurolint17070104
Chicago/Turabian StyleScutelnic, Adrian, Tomas Klail, Diego Moor, Nedelina Slavova, Valentina Petroulia, Simon Jung, Mattia Branca, Urs Fischer, Franz Riederer, Roland Wiest, and et al. 2025. "Susceptibility Weighted Imaging in Migraine with and Without Aura: A Case–Control Study" Neurology International 17, no. 7: 104. https://doi.org/10.3390/neurolint17070104
APA StyleScutelnic, A., Klail, T., Moor, D., Slavova, N., Petroulia, V., Jung, S., Branca, M., Fischer, U., Riederer, F., Wiest, R., & Schankin, C. J. (2025). Susceptibility Weighted Imaging in Migraine with and Without Aura: A Case–Control Study. Neurology International, 17(7), 104. https://doi.org/10.3390/neurolint17070104






