Perioperative Predictors of Early Spinal Cord Stimulator Removal: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
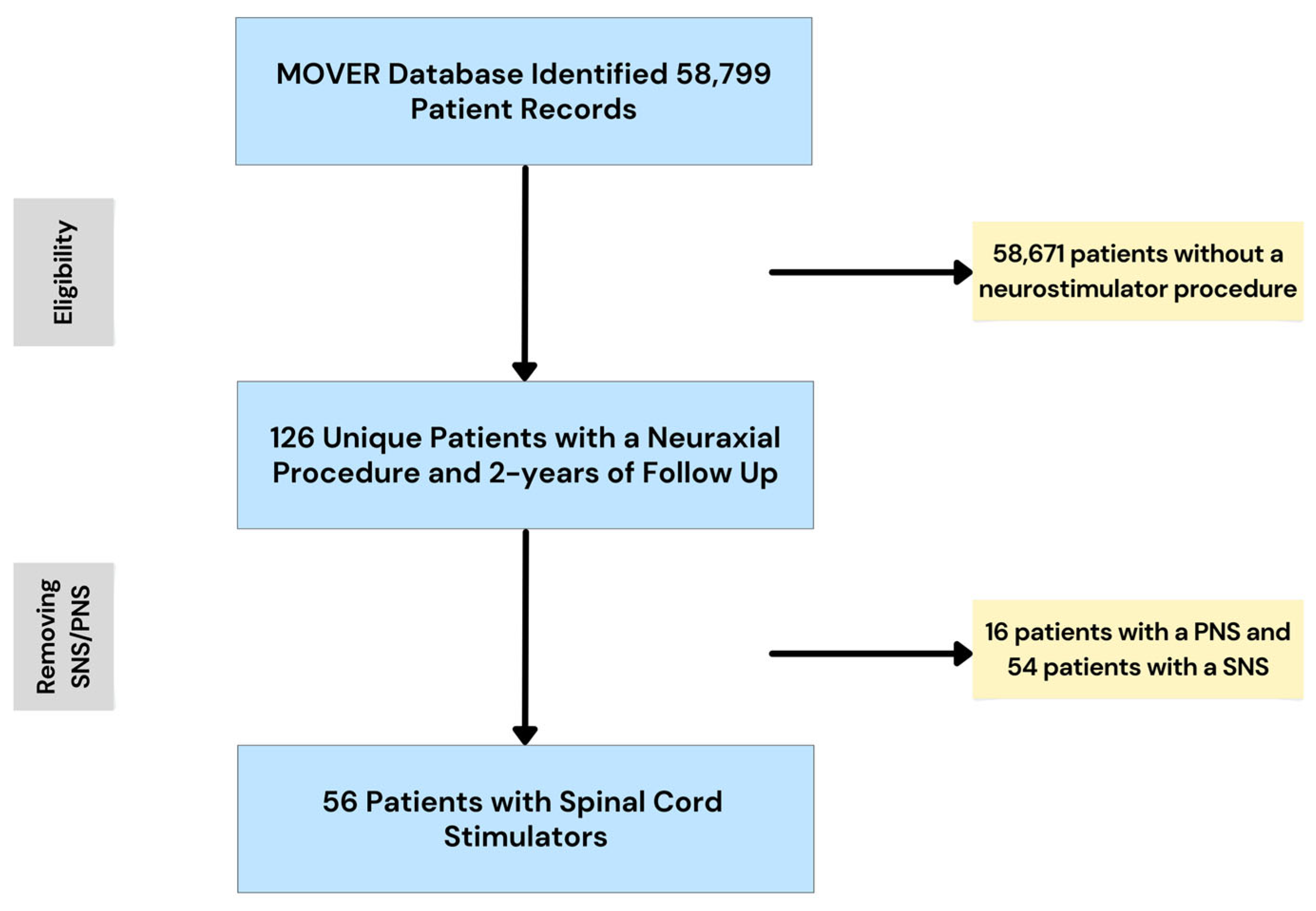
2.2. Participants
2.3. Data Preparation
2.4. Predictors
2.5. Sample Size
2.6. Missing Data
2.7. Analytical Methods
2.8. Class Imbalance
2.9. Fairness
2.10. Model Output
3. Results
3.1. Cohort Demographics
3.2. Multivariable Logistic Regression Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| AUC–ROC | Area Under the Curve—Receiver Operating Characteristic |
| CI | Confidence Interval |
| CPT | Current Procedural Terminology |
| EHR | Electronic Health Record |
| FDR | False Discovery Rate |
| HIPAA | Health Insurance Portability and Accountability Act |
| ICD | International Classification of Diseases |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LOS | Length of Stay |
| MAC | Monitored Anesthesia Care |
| MOVER | Medical Informatics Operating room Vitals and Events Repository |
| OR | Odds Ratio |
| PHI | Protected Health Information |
| PNS | Peripheral Nerve Stimulator |
| RFECV | Recursive Feature Elimination with Cross-Validation |
| SCS | Spinal Cord Stimulator/Spinal Cord Stimulation |
| SD | Standard Deviation |
| SMOTE | Synthetic Minority Oversampling Technique |
| SNS | Sacral Nerve Stimulator |
References
- Thomson, S.; Huygen, F.; Prangnell, S.; De Andres, J.; Baranidharan, G.; Belaid, H.; Berry, N.; Billet, B.; Cooil, J.; De Carolis, G.; et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e-health tool. Eur. J. Pain 2020, 24, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, A.Z.; Chang, H.H.; DiSilvestro, K.; Veeramani, A.; McDonald, C.; Zhang, A.S.; Daniels, A. Spinal Cord Stimulation via Percutaneous and Open Implantation: Systematic Review and Meta-Analysis Examining Complication Rates. World Neurosurg. 2021, 154, 132–143.e131. [Google Scholar] [CrossRef] [PubMed]
- Gatzinsky, K.; Brink, B.; Eygloardottir, K.L.; Hallen, T. Long-term explantation risk in patients with chronic pain treated with spinal cord or dorsal root ganglion stimulation. Reg. Anesth. Pain Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Boulos, R.; Malik, T.M.; Abd-Elsayed, A.; Essandoh, M.K.; Khan, S.; Nguyen, A.; Weaver, T.E. Identifying Predictors for Early Percutaneous Spinal Cord Stimulator Explant at One and Two Years: A Retrospective Database Analysis. Neuromodulation 2023, 26, 124–130. [Google Scholar] [CrossRef]
- Rauck, R.L.; Loudermilk, E.; Thomson, S.J.; Paz-Solis, J.F.; Bojrab, L.; Noles, J.; Vesper, J.; Atallah, J.; Roth, D.; Hegarty, J.; et al. Long-term safety of spinal cord stimulation systems in a prospective, global registry of patients with chronic pain. Pain Manag. 2023, 13, 115–127. [Google Scholar] [CrossRef]
- Bir, S.C.; Konar, S.; Maiti, T.; Nanda, A.; Guthikonda, B. Neuromodulation in intractable pain management: Outcomes and predictors of revisions of spinal cord stimulators. Neurosurg. Focus 2016, 40, E4. [Google Scholar] [CrossRef]
- Dougherty, M.C.; Woodroffe, R.W.; Wilson, S.; Gillies, G.T.; Howard, M.A., 3rd; Carnahan, R.M. Risk Factors and Survival Analysis of Spinal Cord Stimulator Explantation. Neuromodulation 2021, 24, 61–67. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Royds, J.; Al-Kaisy, O.; Palmisani, S.; Pang, D.; Smith, T.; Padfield, N.; Harris, S.; Wesley, S.; Yearwood, T.L.; et al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg. Anesth. Pain Med. 2020, 45, 883–890. [Google Scholar] [CrossRef]
- Kirketeig, T.; Soreskog, E.; Jacobson, T.; Karlsten, R.; Zethraeus, N.; Borgstrom, F. Real-world outcomes in spinal cord stimulation: Predictors of reported effect and explantation using a comprehensive registry-based approach. Pain Rep. 2023, 8, e1107. [Google Scholar] [CrossRef]
- Samad, M.; Angel, M.; Rinehart, J.; Kanomata, Y.; Baldi, P.; Cannesson, M. Medical Informatics Operating Room Vitals and Events Repository (MOVER): A public-access operating room database. JAMIA Open 2023, 6, ooad084. [Google Scholar] [CrossRef]
- Mekhail, N.; Azer, G.; Saweris, Y.; Mehanny, D.S.; Costandi, S.; Mao, G. The Impact of Tobacco Cigarette Smoking on Spinal Cord Stimulation Effectiveness in Chronic Spine-Related Pain Patients. Reg. Anesth. Pain Med. 2018, 43, 768–775. [Google Scholar] [CrossRef]
- Patel, S.K.; Gozal, Y.M.; Saleh, M.S.; Gibson, J.L.; Karsy, M.; Mandybur, G.T. Spinal cord stimulation failure: Evaluation of factors underlying hardware explantation. J. Neurosurg. Spine 2020, 32, 133–138. [Google Scholar] [CrossRef]
- Murin, P.J.; Murin, P.J.; Lima de Mendonca, Y.; Martins, Y.C. Identification of Perioperative Risk Factors for Early Sacral Nerve Stimulator Explantation: A Single-Center Retrospective Cohort Study. J. Clin. Med. 2025, 14, 2363. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Guillaume Lemaıtre, F.N.; Christos, K. Aridas. Imbalanced-learn: A Python Toolbox to Tackle the Curse of Imbalanced Datasets in Machine Learning. J. Mach. Learn. Res. 2017, 18, 1–5. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Jain, S.V.; Panjeton, G.D.; Martins, Y.C. Relationship Between Sleep Disturbances and Chronic Pain: A Narrative Review. Clin Pract. 2024, 14, 2650–2660. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Ng, S.-M.; Yin, M.X.; Chan, J.S.; Chan, C.H.; Fong, T.C.; Li, A.; So, K.-F.; Yuen, L.-P.; Chen, J.-P.; Chung, K.-F. Impact of mind–body intervention on proinflammatory cytokines interleukin 6 and 1β: A three-arm randomized controlled trial for persons with sleep disturbance and depression. Brain Behav. Immun. 2022, 99, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Bretherton, B.; de Ridder, D.; Crowther, T.; Black, S.; Whelan, A.; Baranidharan, G. Men and Women Respond Equally Well to Spinal Cord and Dorsal Root Ganglion Stimulation. Neuromodulation 2022, 25, 1015–1023. [Google Scholar] [CrossRef]
- Grabnar, M.; Wilson, R. Sex Differences in Rates of Spinal Cord Stimulation Therapy and Spinal Cord Stimulator Explants: A Propensity-Score Matched Analysis. Neuromodulation, 2025; in press. [Google Scholar] [CrossRef]
- Conic, R.R.; Caylor, J.; Cui, C.L.; Reyes, Z.; Nelson, E.; Yin, S.; Lerman, I. Sex-specific differences in the efficacy of traditional low frequency versus high frequency spinal cord stimulation for chronic pain. Bioelectron. Med. 2022, 8, 8. [Google Scholar] [CrossRef]
- Mekhail, N.; Costandi, S.; Saweris, Y.; Armanyous, S.; Chauhan, G. Impact of biological sex on the outcomes of spinal cord stimulation in patients with chronic pain. Pain Pract. 2022, 22, 432–439. [Google Scholar] [CrossRef]
- Mogil, J.S.; Parisien, M.; Esfahani, S.J.; Diatchenko, L. Sex differences in mechanisms of pain hypersensitivity. Neurosci. Biobehav. Rev. 2024, 163, 105749. [Google Scholar] [CrossRef] [PubMed]
- Beletsky, A.; Liu, C.; Alexander, E.; Hassanin, S.W.; Vickery, K.; Loomba, M.; Winston, N.; Chen, J.; Gabriel, R.A. The Association of Psychiatric Comorbidities With Short-Term and Long-Term Outcomes Following Spinal Cord Stimulator Placement. Neuromodulation 2023, 26, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Weaver, T. Response to the Letter to the Editor Regarding: “Identifying Predictors for Early Percutaneous Spinal Cord Stimulator Explant at One and Two Years: A Retrospective Database Analysis”. Neuromodulation 2023, 26, 710. [Google Scholar] [CrossRef]
- Goudman, L.; Moens, M.; Kelly, S.; Young, C.; Pilitsis, J.G. Incidence of Infections, Explantations, and Displacements/Mechanical Complications of Spinal Cord Stimulation During the Past Eight Years. Neuromodulation 2024, 27, 1082–1089. [Google Scholar] [CrossRef]
- Kang, K.; Glicksman, M.; Ho, J.; Hoang, K.; Phung, A.; Madabhushi, S.; Hasoon, J.; Yazdi, C.; Fonseca, A.C.; Kaye, A.D.; et al. Single Institutional Cross-Sectional Phone Survey Study: Evaluation of Causes for Loss to Follow-up After Spinal Cord Stimulator Implantation. Pain Physician 2024, 27, 441–446. [Google Scholar] [CrossRef]
- Huygen, F.; Soulanis, K.; Rtveladze, K.; Kamra, S.; Schlueter, M. Spinal Cord Stimulation vs. Medical Management for Chronic Back and Leg Pain: A Systematic Review and Network Meta-Analysis. JAMA Netw. Open 2024, 7, e2444608. [Google Scholar] [CrossRef]


| Variable | Overall | Explantation | No Explantation | p-Value |
|---|---|---|---|---|
| Number of Patients | 56 | 14 | 42 | |
| Sex | ||||
| Male n (%) | 25 (44.6%) | 7 (50.0%) | 18 (42.9%) | 0.7593 |
| Female n (%) | 31 (55.4%) | 7 (50.0%) | 24 (57.1%) | 0.7593 |
| Age (years ± SD) | 60.0 ± 14.2 | 62.6 ± 13.7 | 59.1 ± 14.5 | 0.2735 |
| ASA Score (IQR) | 3.0 (2–3) | 3.0 (2–3) | 3.0 (2–3) | 0.3613 |
| Anesthesia Type | ||||
| Monitored Anesthesia Care n (%) | 12 (21.4%) | 3 (21.4%) | 9 (21.4%) | >0.9999 |
| General Anesthesia n (%) | 44 (78.6%) | 11 (78.6%) | 38 (78.6%) | >0.9999 |
| SCS Lead Type | ||||
| Paddle Lead n (%) | 31 (55.4%) | 4 (28.6%) | 19 (45.2%) | >0.9999 |
| Percutaneous Lead n (%) | 13 (23.2%) | 2 (14.3%) | 8 (19.0%) | >0.9999 |
| Unspecified n (%) | 12 (21.4%) | 8 (57.1%) | 15 (35.7%) | N/A |
| Length of Stay (days ± SD) | 0.7 ± 0.3 | 2.4 ± 5.5 | 0.6 ± 0.9 | 0.1632 |
| ICU Admission n (%) | 4 (7.1%) | 1 (7.1%) | 3 (7.1%) | >0.9999 |
| Possible Indications for SCS * | ||||
| Failed Back Surgery n (%) | 9 (16.1%) | 3 (21.4%) | 6 (14.3%) | 0.6759 |
| Peripheral Neuropathy n (%) | 7 (12.5%) | 1 (7.1%) | 6 (14.3%) | 0.6662 |
| Low Back Pain n (%) | 39 (69.6%) | 8 (57.1%) | 31 (73.8%) | 0.3171 |
| Cervical Pain n (%) | 8 (14.3%) | 2 (14.3%) | 6 (14.3%) | >0.9999 |
| Urinary Dysfunction n (%) | 8 (14.3%) | 3 (21.4%) | 5 (11.9%) | 0.3981 |
| Past Medical History | ||||
| Cerebrovascular Disease | 2 (3.6%) | 1 (7.1%) | 1 (2.4%) | 0.4409 |
| Obstructive Sleep Apnea n (%) | 8 (14.3%) | 3 (21.4%) | 5 (11.9%) | 0.3981 |
| Sleep Disorder n (%) | 10 (17.9%) | 4 (28.6%) | 6 (14.3%) | 0.2472 |
| Hypertension n (%) | 15 (26.8%) | 3 (21.4%) | 12 (28.6%) | 0.7364 |
| Hyperlipidemia n (%) | 14 (25.0%) | 5 (35.7%) | 9 (21.4%) | 0.3045 |
| Atrial Fibrillation n (%) | 5 (8.9%) | 0 (0.0%) | 5 (11.9%) | 0.3163 |
| Diabetes Mellitus n (%) | 6 (10.7%) | 0 (0.0%) | 6 (14.3%) | 0.3195 |
| Chronic Kidney Disease n (%) | 3 (5.4%) | 2 (14.3%) | 1 (2.4%) | 0.1510 |
| Anxiety n (%) | 7 (12.5%) | 1 (7.1%) | 6 (14.3%) | 0.6662 |
| Depression n (%) | 10 (17.9%) | 1 (7.1%) | 9 (21.4%) | 0.4226 |
| Fibromyalgia n (%) | 3 (5.4%) | 2 (14.3%) | 1 (2.4%) | 0.1510 |
| Irritable Bowel Syndrome n (%) | 3 (5.4%) | 2 (14.3%) | 1 (2.4%) | 0.1510 |
| Obesity n (%) | 8 (14.3%) | 4 (28.6%) | 4 (9.5%) | 0.0970 |
| Migraine n (%) | 2 (3.6%) | 1 (7.1%) | 1 (2.4%) | 0.4409 |
| Musculoskeletal Pain n (%) | 10 (17.9%) | 3 (21.4%) | 7 (16.7%) | 0.6984 |
| Arthritis n (%) | 10 (17.9%) | 5 (35.7%) | 5 (11.9%) | 0.0998 |
| Malignancy n (%) | 5 (8.9%) | 2 (14.3%) | 3 (7.1%) | 0.5898 |
| Social History | ||||
| Opioid Use n (%) | 7 (12.5%) | 2 (14.3%) | 5 (11.9%) | >0.9999 |
| Illicit Substance Use n (%) | 1 (1.8%) | 0 (0.0%) | 1 (2.4%) | >0.9999 |
| Tobacco Products n (%) | 3 (5.4%) | 0 (0.0%) | 3 (7.1%) | 0.5652 |
| Variable | Odds Ratio | Lower CI | Upper CI | p-Value | FDR-Adjusted p-Value |
|---|---|---|---|---|---|
| Cardiovascular Disease | 2.0385 | 0.9047 | 4.5932 | 0.0857 | 0.1286 |
| Sleep Disorders | 3.8792 | 1.3636 | 11.0355 | 0.0110 | 0.0497 |
| Hypertension | 0.4709 | 0.1795 | 1.2357 | 0.1260 | 0.1620 |
| Atrial Fibrillation | 0.3138 | 0.0659 | 1.4939 | 0.1455 | 0.1636 |
| Diabetes Mellitus | 0.1368 | 0.0248 | 0.7559 | 0.0226 | 0.0507 |
| Urinary Dysfunction | 2.6935 | 1.1718 | 6.1916 | 0.01963 | 0.0507 |
| ICU Admission | 0.6039 | 0.2953 | 1.2349 | 0.1670 | 0.1670 |
| Female Sex | 0.2388 | 0.1025 | 0.5562 | 0.0009 | 0.0081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murin, P.J.; Murin, P.J.; Jain, S.V.; Martins, Y.C. Perioperative Predictors of Early Spinal Cord Stimulator Removal: A Retrospective Cohort Study. Neurol. Int. 2025, 17, 100. https://doi.org/10.3390/neurolint17070100
Murin PJ, Murin PJ, Jain SV, Martins YC. Perioperative Predictors of Early Spinal Cord Stimulator Removal: A Retrospective Cohort Study. Neurology International. 2025; 17(7):100. https://doi.org/10.3390/neurolint17070100
Chicago/Turabian StyleMurin, Peyton J., Patrick J. Murin, Sejal V. Jain, and Yuri Chaves Martins. 2025. "Perioperative Predictors of Early Spinal Cord Stimulator Removal: A Retrospective Cohort Study" Neurology International 17, no. 7: 100. https://doi.org/10.3390/neurolint17070100
APA StyleMurin, P. J., Murin, P. J., Jain, S. V., & Martins, Y. C. (2025). Perioperative Predictors of Early Spinal Cord Stimulator Removal: A Retrospective Cohort Study. Neurology International, 17(7), 100. https://doi.org/10.3390/neurolint17070100







