The Neuroanatomical Correlates of Bladder Filling: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction and Statistical Analysis
3. Results
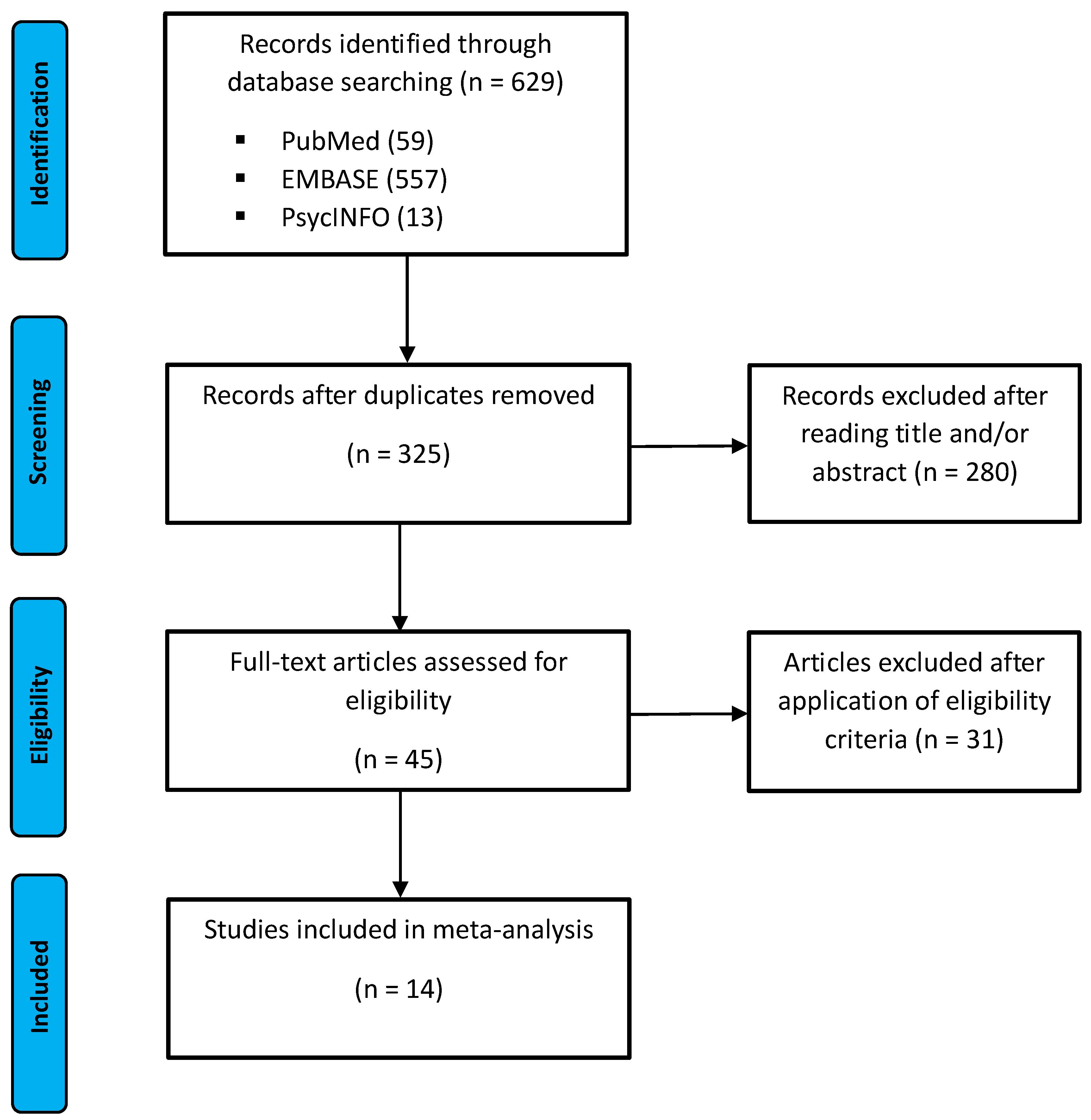
3.1. Study Selection
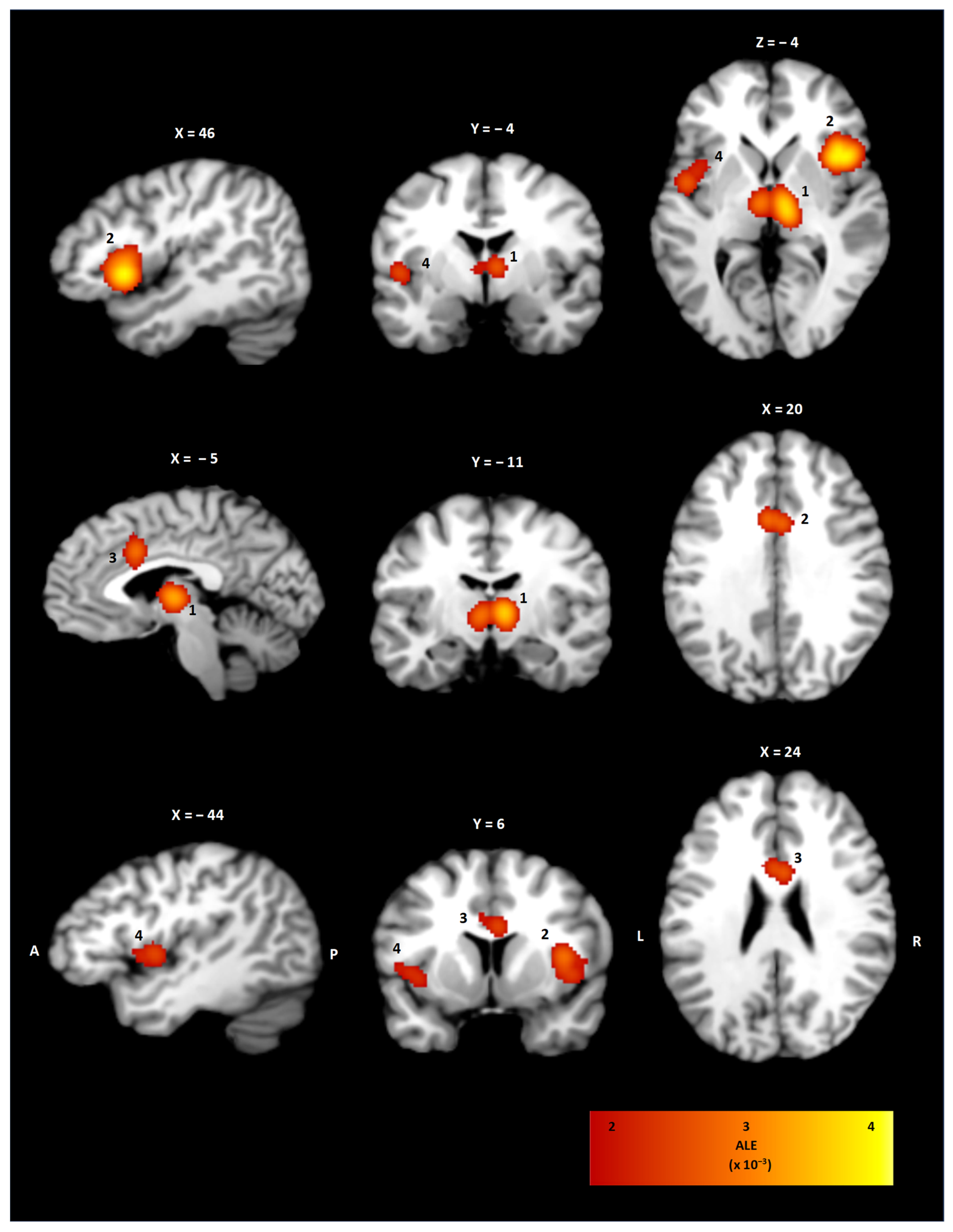
3.2. ALE Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| ALE | Activation likelihood estimation |
| BA | Brodmann area |
| DRG | Dorsal root ganglia |
| fMRI | Functional magnetic resonance imaging |
| FWHM | Full width half maximum |
| GABA | Gamma aminobutyric acid |
| MeSH | Medical Subject Heading |
| MNI | Montreal Neurological Imaging |
| n.a. | Not available |
| PCC | Posterior cingulate cortex |
| PMC | Pontine micturition center |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSC | Pontine storage center |
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| T | Tesla |
| VAS | Visual Analogue Scale |
| VMA | Visceromotor areas |
References
- de Groat, W.C. Central neural control of the lower urinary tract. In Ciba Foundation Symposium 151-Neurobiology of Incontinence: Neurobiology of Incontinence: Ciba Foundation Symposium 151 Sep 28; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 27–56. [Google Scholar]
- Anger, J.T.; Saigal, C.S.; Stothers, L.; Thom, D.H.; Rodríguez, L.V.; Litwin, M.S.; Urologic Diseases of America Project. The prevalence of urinary incontinence among community dwelling men: Results from the National Health and Nutrition Examination survey. J. Urol. 2006, 176, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Gray, M. Urinary incontinence in men: Current status and future directions. Nurs. Res. 2004, 53, S36–S41. [Google Scholar] [PubMed]
- Thomas, T.M.; Plymat, K.R.; Blannin, J.; Meade, T.W. Prevalence of urinary incontinence. Br. Med. J. 1980, 281, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Milsom, I.; Gyhagen, M. The prevalence of urinary incontinence. Climacteric 2019, 22, 217–222. [Google Scholar]
- Mahony, D.T.; Laferte, R.O.; Blais, D.J. Integral storage and voiding reflexes. Neurophysiologic concept of continence and micturition. Urology 1977, 9, 95–106. [Google Scholar] [PubMed]
- Perez, N.E.; Godbole, N.P.; Amin, K.; Syan, R.; Gater, D.R., Jr. Neurogenic bladder physiology, pathogenesis, and management after spinal cord injury. J. Pers. Med. 2022, 12, 968. [Google Scholar] [PubMed]
- Abrams, P.; Blaivas, J.G.; Stanton, S.L.; Andersen, J.T. Standardisation of terminology of lower urinary tract function. Neurourol. Urodyn. 1988, 7, 403–427. [Google Scholar] [CrossRef]
- Dorsher, P.T.; McIntosh, P.M. Neurogenic bladder. Adv. Urol. 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brittain, K.R.; Peet, S.M.; Castleden, C.M. Stroke and incontinence. Stroke 1998, 29, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Buchman, N.M.; Leurgans, S.E.; Shah, R.J.; VanderHorst, V.; Wilson, R.S.; Bachner, Y.G.; Tanne, D.; Schneider, J.A.; Bennett, D.A.; Buchman, A.S. Urinary incontinence, incident parkinsonism, and Parkinson’s disease pathology in older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 1295–1301. [Google Scholar]
- Murphy, A.M.; Bethoux, F.; Stough, D.; Goldman, H.B. Prevalence of stress urinary incontinence in women with multiple sclerosis. Int. Neurourol. J. 2012, 16, 86. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The emerging science of interoception: Sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Robinson, D.R.; Gebhart, G.F. Inside information-The unique features of visceral sensation. Mol. Interv. 2008, 8, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002, 25, 433–469. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. NeuroImage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Griffiths, D.; Derbyshire, S.; Stenger, A.; Resnick, N. Brain control of normal and overactive bladder. J. Urol. 2005, 174, 1862–1867. [Google Scholar] [CrossRef]
- Zhang, H.; Reitz, A.; Kollias, S.; Summers, P.; Curt, A.; Schurch, B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage 2005, 24, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, U.; Boy, S.; Svensson, J.; Michels, L.; Reitz, A.; Candia, V.; Kleiser, R.; Kollias, S.; Schurch, B. Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve—An fMRI study in healthy women. Neuroimage 2008, 41, 682–689. [Google Scholar] [PubMed]
- Griffiths, D.J.; Tadicy, S.D.; Schaefer, W.; Resnick, N.M. Cerebral control of the lower urinary tract: How age-related changes might predispose to urge incontinence. Neuroimage 2009, 47, 981–986. [Google Scholar]
- Nardos, R.; Gregory, W.T.; Krisky, C.; Newell, A.; Nardos, B.; Schlaggar, B.; Fair, D.A. Examining mechanisms of brain control of bladder function with resting state functional connectivity MRI. Neurourol. Urodyn. 2014, 33, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Krhut, J.; Holy, P.; Tintera, J.; Zachoval, R.; Zvara, P. Brain activity during bladder filling and pelvic floor muscle contractions: A study using functional magnetic resonance imaging and synchronous urodynamics. Int. J. Urol. 2014, 21, 169–174. [Google Scholar] [PubMed]
- Gao, Y.; Liao, L.; Blok, B.F. A resting-state functional MRI study on central control of storage: Brain response provoked by strong desire to void. Int. Urol. Nephrol. 2015, 47, 927–935. [Google Scholar] [PubMed]
- Leitner, L.; Walter, M.; Jarrahi, B.; Wanek, J.; Diefenbacher, J.; Michels, L.; Liechti, M.D.; Kollias, S.S.; Kessler, T.M.; Mehnert, U. A novel infusion-drainage device to assess lower urinary tract function in neuro-imaging. BJU Int. 2017, 119, 305–316. [Google Scholar] [PubMed]
- Walter, M.; Leitner, L.; Michels, L.; Liechti, M.D.; Freund, P.; Kessler, T.M.; Kollias, S.; Mehnert, U. Reliability of supraspinal correlates to lower urinary tract stimulation in healthy participants–a fMRI study. Neuroimage 2019, 191, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.; Gao, Y.; Liao, L. Responses of functional brain networks to bladder control in healthy adults: A study using regional homogeneity combined with independent component analysis methods. Int. Urol. Nephrol. 2021, 53, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.F.; Sturms, L.M.; Holstege, G. Brain activation during micturition in women. Brain A J. Neurol. 1998, 121, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Critchley, H.D.; Dolan, R.J.; Fowler, C.J. Changes in brain activity following sacral neuromodulation for urinary retention. J. Urol. 2005, 174, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, U.; Michels, L.; Zempleni, M.Z.; Schurch, B.; Kollias, S. The supraspinal neural correlate of bladder cold sensation—An fMRI study. Hum. Brain Mapp. 2011, 32, 835–845. [Google Scholar] [PubMed]
- Yin, Y.; Shuke, N.; Okizaki, A.; Sato, J.; Aburano, T.; Li, Y.; Kaneko, S.; Mizunaga, M.; Yachiku, S. Cerebral activation during withholding urine with full bladder in healthy men using 99mTc-HMPAO SPECT. J. Nucl. Med. 2006, 47, 1093–1098. [Google Scholar]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef]
- Andersson, K.E.; Gratzke, C. Pharmacology of the lower urinary tract. Textb. Neurogenic Bladder 2008, 24, 95–114. [Google Scholar]
- Morrison, J.F.B. The activation of bladder wall afferent nerves. Exp. Physiol. 1999, 84, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, K.; Nishijima, S.; Miyazato, M.; Ogawa, Y. Central nervous control of micturition and urine storage. J. Smooth Muscle Res. 2005, 41, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Griffiths, D.; De Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Schellino, R.; Boido, M.; Vercelli, A. The dual nature of Onuf’s nucleus: Neuroanatomical features and peculiarities, in health and disease. Front. Neuroanat. 2020, 14, 572013. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.J. The pontine micturition centres. Scand. J. Urol. Nephrol. 2002, 36, 21–26. [Google Scholar] [CrossRef]
- Rahman, M.; Siddick, A. Neuroanatomy, Pontine Micturition Center. In StatPearls [Internet]; Updated 9 September 2021; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Andersson, K.E.; Arner, A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef] [PubMed]
- Kendroud, S.; Fitzgerald, L.A.; Murray, I.V.; Hanna, A. Physiology, Nociceptive Pathways. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470255/ (accessed on 10 April 2025).
- Grodd, W.; Kumar, V.J.; Schüz, A.; Lindig, T.; Scheffler, K. The anterior and medial thalamic nuclei and the human limbic system: Tracing the structural connectivity using diffusion-weighted imaging. Sci. Rep. 2020, 10, 10957. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Ceunen, E.; Vlaeyen, J.W.S.; Van Diest, I. On the origin of interoception. Front. Psychol. 2016, 7, 743. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef]
- Damasio, A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 1413–1420. [Google Scholar]
- Stephani, C.; Fernandez-Baca, V.; Maciunas, R.; Koubeissi, M.; Lüders, H.O. Functional neuroanatomy of the insular lobe. Brain Struct. Funct. 2011, 216, 137–149. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Ungerleider, L.G.; Haxby, J.V. ‘What’ and ‘where’ in the human brain. Curr. Opin. Neurobiol. 1994, 4, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129 Pt 3, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Voogd, J.; van Huijzen, C. Telencephalon: Hippocampus and related structures. In The Human Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2008; pp. 361–400. [Google Scholar]
- Critchley, H.D.; Harrison, N.A. Visceral influences on brain and behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef]
- Friston, K.J.; Daunizeau, J.; Kilner, J.; Kiebel, S.J. Action and behavior: A free-energy formulation. Biol. Cybern. 2010, 102, 227–260. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Pezzulo, G.; Rigoli, F.; Friston, K.J. Active inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 2015, 134, 17–35. [Google Scholar]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of anterior cingulate cortex to behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.; Tadic, S.D.; Schaefer, W.; Resnick, N.M. Cerebral control of the bladder in normal and urge-incontinent women. NeuroImage 2007, 37, 1–7. [Google Scholar] [CrossRef]
- Griffiths, D.; Tadic, S.D. Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 2008, 27, 466–474. [Google Scholar]
- Griffiths, D.; Clarkson, B.; Tadic, S.D.; Resnick, N.M. Brain mechanisms underlying urge incontinence and its response to pelvic floor muscle training. J. Urol. 2015, 194, 708–715. [Google Scholar] [CrossRef]
- Griffiths, D. Clinical studies of cerebral and urinary tract function in elderly people with urinary incontinence. Behav. Brain Res. 1998, 92, 151–155. [Google Scholar] [CrossRef]
- Maurice-Williams, R.S. Micturition symptoms in frontal tumours. J. Neurol. Neurosurg. Psychiatry 1974, 37, 431–436. [Google Scholar] [CrossRef]
- Kuroiwa, Y.; Tohgi, H.; Ono, S.; Itoh, M. Frequency and urgency of micturition in hemiplegic patients: Relationship to hemisphere laterality of lesions. J. Neurol. 1987, 234, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Vargas, E.; Sörös, P.; Shoemaker, J.K.; Hachinski, V. Human cerebral circuitry related to cardiac control: A neuroimaging meta-analysis. Ann. Neurol. 2016, 79, 709–716. [Google Scholar] [PubMed]
- Abrams, P.; Andersson, K.E.; Birder, L.; Brubaker, L.; Cardozo, L.; Chapple, C.; Cottenden, A.; Davila, W.; De Ridder, D.; Dmochowski, R.; et al. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol. Urodyn. 2010, 29, 213–240. [Google Scholar] [PubMed]
- Chou, Y.C.; Jiang, Y.H.; Harnod, T.; Lee, H.T.; Kuo, H.C. Stroke and lower urinary tract symptoms: A neurosurgical view. Urol. Sci. 2019, 30, 8–13. [Google Scholar]
- Tuong, N.E.; Klausner, A.P.; Hampton, L.J. A review of post-stroke urinary incontinence. Can. J. Urol. 2016, 23, 8265–8270. [Google Scholar]
- Weissbart, S.J.; Bhavsar, R.; Rao, H.; Wein, A.J.; Detre, J.A.; Arya, L.A.; Smith, L.A. Specific Changes in Brain Activity during Urgency in Women with Overactive Bladder after Successful Sacral Neuromodulation: A Functional Magnetic Resonance Imaging Study. J. Urol. 2018, 200, 382–388. [Google Scholar] [CrossRef]
- Tadic, S.D.; Zdaniuk, B.; Griffiths, D.; Rosenberg, L.; Schäfer, W.; Resnick, N.M. Effect of biofeedback on psychological burden and symptoms in older women with urge urinary incontinence. J. Am. Geriatr. Soc. 2007, 55, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Versace, V.; Sebastianelli, L.; Brigo, F.; Golaszewski, S.; Christova, M.; Saltuari, L.; Trinka, E. Transcranial magnetic stimulation and bladder function: A systematic review. Clin. Neurophysiol. 2019, 130, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.S.; Kim, S.G. Spatial and temporal limits in cognitive neuroimaging with fMRI. Trends Cogn. Sci. 1999, 3, 207–216. [Google Scholar] [CrossRef]
- Camargo, E.E. Brain SPECT in neurology and psychiatry. J. Nucl. Med. 2001, 42, 611–623. [Google Scholar] [PubMed]

| Author (Year) | Study Design | Imaging Modality | Sample Size (% Female) | Mean Age ± SD (Years) | Overview on Conditions | Foci |
|---|---|---|---|---|---|---|
| Blok et al. (1998) [30] | Prospective experimental study | PET | 18 (100%) | 27.0 ± n.a. | 15 min prior to micturition while withholding urine (scan 1), during micturition (scan 2), 15 min after micturition (scan 3), 30 min after micturition, contrasts between each condition | 2 |
| Dasgupta et al. (2005) [31] | Prospective experimental study | PET | 8 (100%) | n.a. | Healthy participants and patients with urinary retention due to sphincter overactivity had 6 scans with empty bladder and full bladder; conditions with a sacral neurostimulator in patients with either empty or full bladder | 3 |
| Griffiths et al. (2005) [20] | Prospective experimental study | 3 T fMRI | 12 (92%) | n.a. | Trials with empty bladder and after infusion with normal saline at a rate of 60 mL/min via urine catheter; intravesical pressure was monitored | 10 |
| Zhang et al. (2005) [21] | Prospective experimental study | 3 T fMRI | 12 (0%) | 23.8 ± 0.65 | Conditions of empty and full bladder with either relaxed or contracted pelvic floor muscles; participants were instructed to drink until a feeling of bladder fullness was perceived, instruction to voluntarily contract the pelvic floor under both empty and full bladder conditions | 10 |
| Yin et al. (2006) [33] | Prospective experimental study | SPECT | 15 (0%) | 32.7 ± 7.3 | Each participant was scanned with an empty bladder or during withholding urine with a full bladder | 4 |
| Mehnert et al. (2008) [22] | Prospective experimental study | 3 T fMRI | 8 (100%) | 24.3 ± n.a. | Conditions of either empty or filled bladder by saline infusion via urethral catheter and an alternating resting period repeated five times, intermittent genital nerve stimulation | 17 |
| Griffiths et al. (2009) [23] | Prospective experimental study | 3 T fMRI | 10 (100%) | n.a. | CConditions of bladder filling via urethral catheter by infusion and partial withdrawal of bladder volume with up to 6 cycles | 6 |
| Study ID | Population | Intervention | ||||
|---|---|---|---|---|---|---|
| Author (Year) | Study Design | Imaging Modality | Sample Size (% Female) | Mean Age ± SD (Years) | Overview on Conditions | Foci |
| Mehnert et al. (2011) [32] | Prospective experimental study | PET | 14 (100%) | 24.8 ± n.a. | Infusion of maximally 100 mL cold normal saline via urethral catheter, conditions of empty bladder, sensation of filled bladder, two conditions of continuous draining for 10 cycles | 6 |
| Nardos et al. (2014) [24] | Prospective experimental study | 3 T fMRI | 20 (100%) | 56.2 ± n.a. | Infusion of normal saline via transurethral catheter at an infusion rate of 50 mL/min until urge to void, phase of withdrawal, trials with empty and filled bladder | 3 |
| Kruht et al. (2014) [25] | Prospective experimental study | 3 T fMRI | 23 (100%) | n.a. | Infusion of normal saline into the bladder at a rate of 50 mL/min, conditions of empty bladder, first sensation and strong desire to void, first filling with 100 mL normal saline and then rapid filling with additional 25 mL and withdrawal, two trials of filling and emptying | 8 |
| Gao et al. (2015) [26] | Prospective experimental study | 3 T fMRI | 30 (73%) | 29.8 ± 5.9 | Conditions of empty bladder after voiding and strong desire to void followed by phase of drinking water, evaluation of the desire to void on a VAS | 11 |
| Leitner et al. (2017) [27] | prospective experimental study | 3 T fMRI | 33 (48%) | 35.3 ± 11.0 | 8 blocks consisting of infusion of 100 mL normal saline, plateau of perceived filling, rating of desire to void, short rest period, withdrawal of 100 mL normal saline, plateau phase after 100 mL normal saline have been withdrawn, rating of desire to void and pain level | 27 |
| Walter et al. (2019) [28] | Prospective experimental study | 3 T fMRI | 20 (50%) | 39.2 ± 11.6 | 8 blocks of normal saline infusion via transurethral catheter, plateau phase of filled bladder, rating of desire to void, rest condition, withdrawal of 100 mL normal saline, plateau phase of filled bladder, rating of desire to void, resting condition, the first measurement was followed by a second scan after 5–8 weeks and follow-up interview | 23 |
| Pang et al. (2021) [29] | Prospective experimental study | 3 T fMRI | 20 (50%) | 51.5 ± 5.6 | 2 resting state trials with an empty bladder and a full bladder; between both resting state scans, the bladder was filled with 200 mL of normal saline via urethral catheter, repeated infusion and withdrawal | 8 |
| Cluster | Brain Region | BA | x | y | z | Volume (mm3) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| 1 | Right/Left Thalamus | - | 6 | −14 | 4 | 9352 | 3.5 |
| 2 | Right Insula | 44 | 41 | 15 | 4 | 10,928 | 3.7 |
| 3 | Right/Left Cingulate Cortex | 24 | −1 | 12 | 29 | 3392 | 2.7 |
| 4 | Left Insula | 13 | −44 | 3 | 3 | 2912 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, C.; Kaufmann, A. The Neuroanatomical Correlates of Bladder Filling: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies. Neurol. Int. 2025, 17, 156. https://doi.org/10.3390/neurolint17100156
Müller C, Kaufmann A. The Neuroanatomical Correlates of Bladder Filling: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies. Neurology International. 2025; 17(10):156. https://doi.org/10.3390/neurolint17100156
Chicago/Turabian StyleMüller, Christoph, and Albert Kaufmann. 2025. "The Neuroanatomical Correlates of Bladder Filling: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies" Neurology International 17, no. 10: 156. https://doi.org/10.3390/neurolint17100156
APA StyleMüller, C., & Kaufmann, A. (2025). The Neuroanatomical Correlates of Bladder Filling: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies. Neurology International, 17(10), 156. https://doi.org/10.3390/neurolint17100156





