Gut Microbiota, Mild Cognitive Impairment and Dementia: A Systematic Review
Abstract
1. Introduction
1.1. Definition and Prevalence of Dementia
1.2. Importance of Non-Neurological Factors in Neurodegenerative Diseases
1.3. Emerging Role of the Gut–Brain Axis in Cognitive Health
1.4. Rationale and Aim of the Study
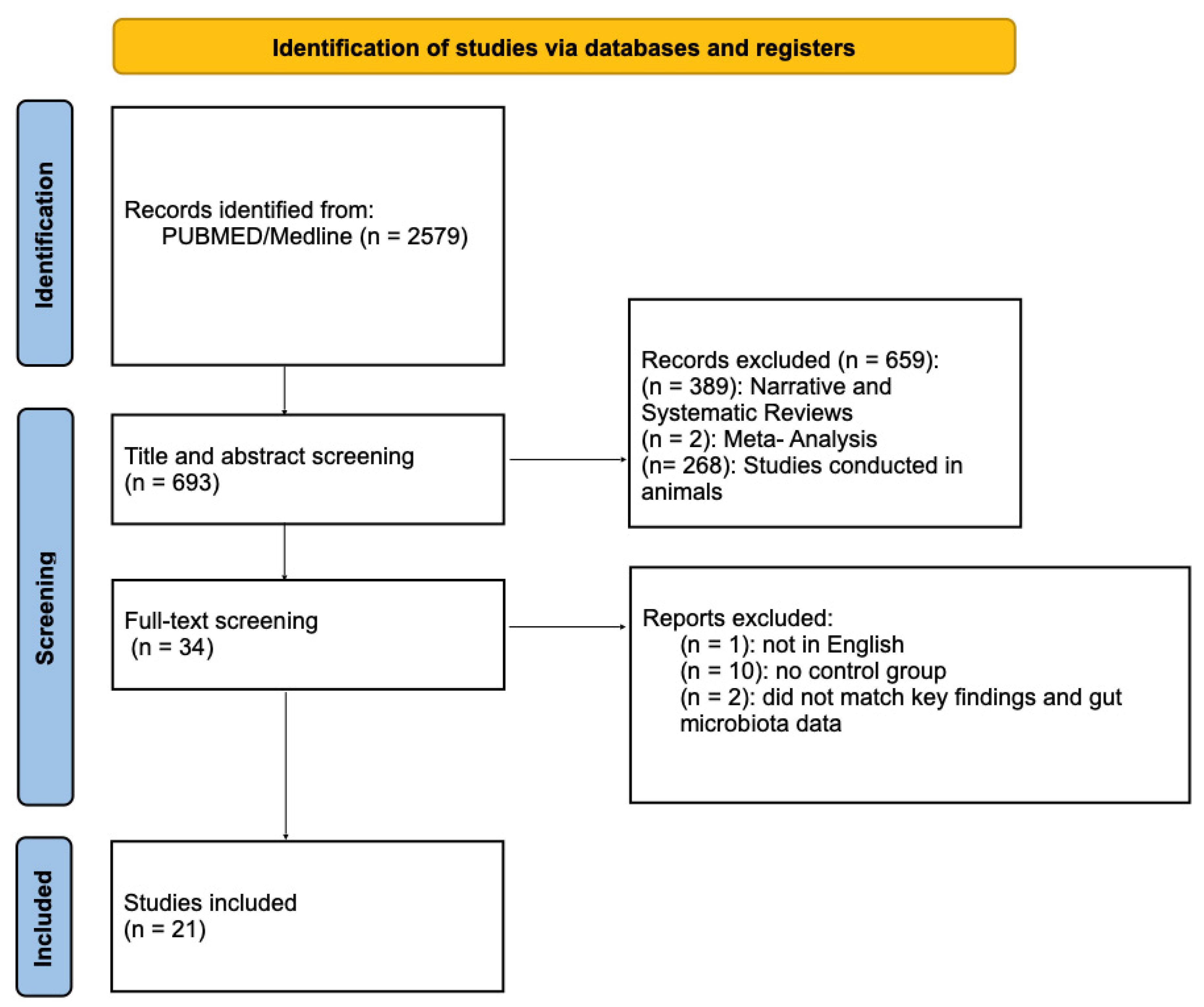
2. Material and Methods
- -
- Population (P): Adults (≥55 years, with a mean age between 65 and 75 years) including MCI and AD;
- -
- Intervention (I): Evaluation of gut microbiota composition;
- -
- Comparator (C): Normal controls (NC) or healthy control participants;
- -
- Outcome (O): Measures of cognitive performance (e.g., MMSE, MoCA, ADAS-Cog), neurodegenerative biomarkers (e.g., amyloid-β, phosphorylated tau), microbial diversity indices, and taxonomic changes.
2.1. Review Aim and Scope
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection and Data Extraction
2.5. Data Synthesis
3. Results
4. Discussion
Limitation and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Byeon, G.; Kang, D.W.; Kim, Y.; Kim, G.H.; Kim, K.W.; Kim, H.J.; Na, S.; Park, K.H.; Park, Y.H.; Suh, J.; et al. Clinical Practice Guidelines for Dementia: Recommendations for the Pharmacological Treatment of Behavioral and Psychological Symptoms. Dement. Neurocogn. Disord. 2025, 24, 24–43. [Google Scholar] [CrossRef]
- World Alzheimer Reprr 2015. Available online: https://www.alzint.org/u/WorldAlzheimerReport2015.pdf (accessed on 1 April 2025).
- Global Dementia Observatory. Available online: https://www.who.int/data/gho/data/themes/global-dementia-observatory-gdo (accessed on 1 April 2025).
- Fołta, J.; Rzepka, Z.; Wrześniok, D. The Role of Inflammation in Neurodegenerative Diseases: Parkinson’s Disease, Alzheimer’s Disease, and Multiple Sclerosis. Int. J. Mol. Sci. 2025, 26, 5177. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, Y.; Tomar, S.; Das, A.; Prajapati, J.L.; Singh, A.P.; Bodake, S.H.; Namdeo, K.P. Chronic inflammation in obesity and neurodegenerative diseases: Exploring the link in disease onset and progression. Mol. Biol. Rep. 2025, 52, 424. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef]
- Meng, X.; D’Arcy, C. Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS ONE 2012, 7, e38268. [Google Scholar] [CrossRef]
- Cardona, M.; Andrés, P. Are social isolation and loneliness associated with cognitive decline in ageing? Front. Aging Neurosci. 2023, 15, 1075563. [Google Scholar] [CrossRef]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut microbiota, cognitive frailty and dementia in older individuals: A systematic review. Clin. Interv. Aging 2018, 13, 1497–1511. [Google Scholar] [CrossRef]
- Nouvenne, A.; Ticinesi, A.; Tana, C.; Prati, B.; Catania, P.; Miraglia, C.; De’ Angelis, G.L.; Di Mario, F.; Meschi, T. Digestive disorders and Intestinal microbiota. Acta Biomed. 2018, 89, 47–51. [Google Scholar]
- Ticinesi, A.; Nouvenne, A.; Tana, C.; Prati, B.; Cerundolo, N.; Miraglia, C.; De’ Angelis, G.L.; Di Mario, F.; Meschi, T. The impact of intestinal microbiota on bio-medical research: Definitions, techniques and physiology of a “new frontier”. Acta Biomed. 2018, 89, 52–59. [Google Scholar]
- Aljumaah, M.R.; Bhatia, U.; Roach, J.; Gunstad, J.; Azcarate Peril, M.A. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin. Nutr. 2022, 41, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.C.; Lin, C.C.; Chiu, Y.L.; Koh, S.H.; Liu, Y.C.; Chuang, Y.F. Compositional and functional gut microbiota alterations in mild cognitive impairment: Links to Alzheimer’s disease pathology. Alzheimers Res. Ther. 2025, 17, 122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wanapaisan, P.; Chuansangeam, M.; Nopnipa, S.; Mathuranyanon, R.; Nonthabenjawan, N.; Ngamsombat, C.; Thientunyakit, T.; Muangpaisan, W. Association between Gut Microbiota with Mild Cognitive Impairment and Alzheimer’s Disease in a Thai Population. Neurodegener. Dis. 2022, 22, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, G.; Lai, H.; Li, Z.; Shen, M.; Li, C.; Kwan, P.; O’Brien, T.J.; Wu, T.; Yang, S.; et al. Characterizing Gut Microbiota in Older Chinese Adults with Cognitive Impairment: A Cross-Sectional Study. J. Alzheimers Dis. 2024, 101, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Takabayashi, K.; Kamagata, K.; Nishimoto, Y.; Togashi, Y.; Yamauchi, Y.; Ogaki, K.; Li, Y.; Hatano, T.; Motoi, Y.; et al. Free water in gray matter linked to gut microbiota changes with decreased butyrate producers in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Dis. 2024, 193, 106464. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Penalver Bernabe, B.; Xia, Y.; Sanchez-Flack, J.; Lamar, M.; Schiffer, L.; Castellanos, K.; Fantuzzi, G.; Maki, P.; Fitzgibbon, M.; et al. Comparing the gut microbiome of obese, African American, older adults with and without mild cognitive impairment. PLoS ONE 2023, 18, e0280211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saji, N.; Murotani, K.; Hisada, T.; Tsuduki, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: A cross-sectional study conducted in Japan. Sci. Rep. 2019, 9, 19227. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, X.; Wu, J.; Xiao, Z.; Wu, W.; Ding, S.; Zheng, L.; Liang, X.; Luo, J.; Ding, D.; et al. Altered Gut Microbiota and Its Clinical Relevance in Mild Cognitive Impairment and Alzheimer’s Disease: Shanghai Aging Study and Shanghai Memory Study. Nutrients 2022, 14, 3959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, E.J.; Kim, J.S.; Park, S.E.; Seo, S.H.; Cho, K.M.; Kwon, S.J.; Lee, M.H.; Kim, J.H.; Son, H.S. Association between Mild Cognitive Impairment and Gut Microbiota in Elderly Korean Patients. J. Microbiol. Biotechnol. 2023, 33, 1376–1383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am. J. Clin. Nutr. 2021, 114, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Yang, K.; He, B.; Du, W.; Cai, Y.; Han, Y. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: A cross-sectional analysis from the SILCODE study. Alzheimers Res. Ther. 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, C.; Lin, L.; Lin, H.; Wang, X.; Han, Y.; Liu, S.L. Altered Gut Microbiota in Adults with Subjective Cognitive Decline: The SILCODE Study. J. Alzheimers Dis. 2021, 82, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Lwere, K.; Muwonge, H.; Sendagire, H.; Sajatovic, M.; Williams, S.M.; Gumukiriza-Onoria, J.L.; Buwembo, D.; Buwembo, W.; Nassanga, R.; Nakimbugwe, R.; et al. of the gut microbiome in Alzheimer disease and mild cognitive impairment among older adults in Uganda: A case-control study. Medicine 2025, 104, e42100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yıldırım, S.; Nalbantoğlu, Ö.U.; Bayraktar, A.; Ercan, F.B.; Gündoğdu, A.; Velioğlu, H.A.; Göl, M.F.; Soylu, A.E.; Koç, F.; Gülpınar, E.A.; et al. Stratification of the Gut Microbiota Composition Landscape across the Alzheimer’s Disease Continuum in a Turkish Cohort. mSystems 2022, 7, e0000422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, J.; Tan, H.; Cheng, Y.; Ma, X.; Jiang, S.; Hou, X.; Li, S.; Shi, L.; Li, P.; Xu, H.; et al. Air particulate pollution exposure associated with impaired cognition via microbiota gut-brain axis: An evidence from rural elderly female in northwest China. Environ. Sci. Pollut. Res. Int. 2024, 31, 6398–6410. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Tsuduki, T.; Murotani, K.; Hisada, T.; Sugimoto, T.; Kimura, A.; Niida, S.; Toba, K.; Sakurai, T. Relationship between the Japanese-style diet, gut microbiota, and dementia: A cross-sectional study. Nutrition 2022, 94, 111524. [Google Scholar] [CrossRef] [PubMed]
- Teigen, L.M.; McCarter, S.J.; Ziegert, Z.; Staley, C.; Grant, K.M.; Gupta, V.K.; Zhao, X.; St Louis, E.K.; Kantarci, K.; Lowe, V.J.; et al. Taxonomic intestinal microbiota differences in Lewy body spectrum disease and cohabitant controls. Park. Relat. Disord. 2024, 129, 107176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, M.; Peng, J.; Huang, X.; Xiao, L.; Huang, F.; Zuo, Z. Gut Microbiome Features of Chinese Patients Newly Diagnosed with Alzheimer’s Disease or Mild Cognitive Impairment. J. Alzheimers Dis. 2021, 80, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, Y.Q.; Guo, K.; Xue, M.; Gan, Y.; Wang, K.; Xu, D.B.; Tu, Q.Y. Elderly Patients with Mild Cognitive Impairment Exhibit Altered Gut Microbiota Profiles. J. Immunol. Res. 2021, 2021, 5578958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ticinesi, A.; Tana, C.; Nouvenne, A. The intestinal microbiome and its relevance for functionality in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 4–12. [Google Scholar] [CrossRef]
- Rozera, T.; Pasolli, E.; Segata, N.; Ianiro, G. Machine Learning and Artificial Intelligence in the Multi-Omics Approach to Gut Microbiota. Gastroenterology 2025, 169, 487–501. [Google Scholar] [CrossRef]
- Gemikonakli, G.; Mach, J.; Zhang, F.; Bullock, M.; Tran, T.; El-Omar, E.; Hilmer, S.N. Polypharmacy With High Drug Burden Index (DBI) Alters the Gut Microbiome Overriding Aging Effects and Is Reversible With Deprescribing. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Song, J.; Jiang, H.; Wei, B.; Wang, H. Association between the dietary index for gut microbiota and cardiometabolic multimorbidity: Systemic immune-inflammation index and systemic inflammatory response index. Front. Nutr. 2025, 12, 1591799. [Google Scholar] [CrossRef]
- Sadagopan, A.; Mahmoud, A.; Begg, M.; Tarhuni, M.; Fotso, M.; Gonzalez, N.A.; Sanivarapu, R.R.; Osman, U.; Latha Kumar, A.; Mohammed, L. Understanding the Role of the Gut Microbiome in Diabetes and Therapeutics Targeting Leaky Gut: A Systematic Review. Cureus 2023, 15, e41559. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The gut microbiome and hypertension. Nat. Rev. Nephrol. 2023, 19, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Pedroza Matute, S.; Iyavoo, S. Exploring the gut microbiota: Lifestyle choices, disease associations, and personal genomics. Front. Nutr. 2023, 10, 1225120. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Siebieszuk, A.; Witkowska, A.M. The Role of Gut Microbiome in Sleep Quality and Health: Dietary Strategies for Microbiota Support. Nutrients 2024, 16, 2259. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Prokopidis, K.; Chambers, E.; Ni Lochlainn, M.; Witard, O.C. Mechanisms Linking the Gut-Muscle Axis With Muscle Protein Metabolism and Anabolic Resistance: Implications for Older Adults at Risk of Sarcopenia. Front. Physiol. 2021, 12, 770455. [Google Scholar] [CrossRef] [PubMed]
- Casati, M.; Ferri, E.; Azzolino, D.; Cesari, M.; Arosio, B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp. Gerontol. 2019, 124, 110639. [Google Scholar] [CrossRef]
- Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Morella, I.; Negro, M.; Dossena, M.; Brambilla, R.; D’Antona, G. Gut-muscle-brain axis: Molecular mechanisms in neurodegenerative disorders and potential therapeutic efficacy of probiotic supplementation coupled with exercise. Neuropharmacology 2023, 240, 109718. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
| Study | Type of Study | No. of Patients Included | Gender | Analysis | Cognitive Alteration | Cognitive Test | Altered Taxa | Dietary Issues | Treatment | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Aljumaah et al., 2022 [13] | Randomized, double-blind, placebo-controlled trial | 169 middle-aged (52–59 years) and older adults (60–75 years) | Placebo group: 28 M 55 F Probiotic group: 38 M 48 F | 16S rRNA sequencing | MCI | MMSE | ↑ Prevotella ruminicola, ↑ Bacteroides thetaiotaomicron, ↑ Dehalobacterium | Not reported | Daily supplementation with LGG | p = 0.0017 |
| Li B. et al., 2019 [14] | Cross-sectional observational study | 30 MCI 30 AD 30 NC | MCI 12 M AD 15 M NC 13 M | 16S rRNA (fecal + blood) | MCI/AD | MMSE | ↑ Escherichia spp. in both MCI/AD, reduced microbial diversity | Not reported | No intervention applied | p = 0.001 |
| Fan et al., 2025 [15] | Observational cross-sectional study | 439 individuals: 119 MCI 320 NC | MCI 51 M NC 111 M | Shotgun metagenomic sequencing (MetaPhlAn4, Illumina NovaSeq) of fecal samples | MCI | MMSE, CTT1, CTT2, Digit Span, DSST, Logical Memory (LMI/LMII), Semantic Verbal Fluency, Stroop, Boston Naming Test | ↓ Akkermansia muciniphila; mixed Bacteroides, Ruminococcus species | Vegeterian → 19 Normal, 4 MCI Tea consuptionFrequently → 73 Normal, 24 MCI Coffee consumption → 122 Normal, 30 MCI | No intervention applied | p = 0.004 |
| Wanapaisan et al., 2022 [16] | Cross-sectional observational | 52 total: 20 NC 12 MCI 20 AD | NC 8 M MCI 6 M AD 10 M | 16S rRNA sequencing of stool samples | MCI/AD | Diagnosis based on clinical, cognitive assessments (CDR, MMSE), plus brain imaging (MRI and amyloid PET) | ↑ Escherichia–Shigella, Bacteroides, Holdemanella, Romboutsia, Megamonas; ↓ Faecalibacterium, Agathobacter, Clostridiales | AD group consumed significantly fewer vegetables than controls Rice was the most consumed carbohydrate across all groups | No intervention applied | p < 0.0001 |
| Wang et al., 2024 [17] | Cross-sectional observational study | 229 older adults: 74 MCI 131 NC | MCI 50 M NC 68 M | 16S rRNA sequencing of fecal samples | MCI | MMSE classification | a-diversity lower in MCI vc NCI, ↑ Megamonas, Blautia, Pseudomonas, Stenotrophomonas, Veillonella | Not reported | No intervention applied | p < 0.001 |
| Yamashiro et al., 2024 [18] | Cross-sectional observational | 56 participants: 19 NC 19 MCI 18 AD | NC 7 M MCI 6 M AD 8 M | 16S rRNA sequencing of fecal samples | MCI/AD | MMSE, MoCA, ADAS-Cog, CDR + free water MRI (FW) | ↓ Anaerostipes, Lachnospiraceae UCG-004, [Ruminococcus] gnavus group | Not reported | No intervention applied | p = 0.003 |
| McLeod et al., 2023 [19] | Case–control observational | 60 obese, African American adults (55–76 yrs): 30 MCI 30 NC | NC 21 M MCI 21 M | 16S rRNA sequencing of fecal samples | MCI | MoCA scores | ↓ Parabacteroides distasonis; ↑ Dialister invisus, Streptococcus, Methanobrevibacter | Mediterranean diet: non-refined grains, potatoes, fruit, vegetables, legumes and nuts, fish, olive oil, alcohol, red meat and processed meat, poultry, and full-fat dairy products | No intervention applied | p = 0.04 |
| Saji et al., 2019 [20] | Cross-sectional observational | 82 Japanese older adults: 61 MCI 21 NC | MCI 28 M NC 11 M | 16S rRNA sequencing of fecal samples | MCI | MMSE, CDR, ADAS-Cog, RCPM, FAB, LM-WMSR I, LM-WMSR II | ↑ Bacteroides | Not reported | No intervention applied | p = 0.009 |
| Zhu Z. et al., 2022 [21] | Cross-sectional observational | 302 older adults: 94 NC 125 MCI 83 AD | NC 36 M MCI 49 M AD 30 M | 16S rRNA sequencing of fecal samples | MCI/AD | CDR | ↑ Erysipelatoclostridiaceae, Patescibacteria, Saccharimonadales; ↓ Alistipes, Faecalibacterium | Not reported | No intervention applied | p < 0.05 |
| Kim E-J et al., 2023 [22] | Cross-sectional observational | 80 elderly Koreans: 40 MCI 40 NC | MCI 6 M NC 2 M | 16S rRNA sequencing of fecal samples | MCI | MoCA scores | ↑ Bacteroides, Eubacterium nodatum group, Oribacterium, Rikenellaceae RC9; ↓ Prevotella, Coprococcus, Akkermansia | Not reported | No intervention applied | p < 0.05 |
| Zhang X. et al., 2021 [23] | Case–control observational | 127 participants: 75 MCI, 52 NC (Chinese) | MCI 36 M NC 24 M | 16S rRNA sequencing of fecal samples | MCI | MoCA scores, MMSE | ↑ Proteobacteria, Gammaproteobacteria; ↓ Faecalibacterium, Alistipes, Ruminococcaceae | Chinese Dietary Guidelines Index 2018: carbohydrates, grains and mixed beans, fruit, vegetables, soybean and nuts, meat and poultry, eggs, aquatic products, oil, salt, and alcohol | Diet quality | p = 0.008 |
| Sheng C. et al., 2022 [24] | Cross-sectional observational | 88 community-dwelling older adults: 34 CN– (Aβ−) 32 CN+ (Aβ+ preclinical AD) 22 CI (11 MCI, 11 AD) | CN- 8 M CN+ 10 M CI (MCI 8 M, AD 5 M) | 16S rRNA sequencing of fecal samples | MCI/AD | AVLT, STT-A, STT-B, AFT, BNT, MoCA-B, FAQ, HAMD, HAMA | ↑ Bacteroidetes; ↓ Firmicutes, Deltaproteobacteria, Desulfovibrionaceae, Faecalibacterium, Bilophila | Not reported | No intervention applied | p = 0.003 |
| Sheng C. et al., 2021 [25] | Cross-sectional observational study | 105 participants: 38 NC 53 SCD 14 CI (including MCI/AD) | NC 15 M SCD 10 M CI 4 M | 16S rRNA sequencing of fecal samples | MCI/AD | SCD-Q9, AVLT-H, STT-A, STT-B, AFT, BNT, MoCA-B, FAQ, HAMD, HAMA | ↓ Faecalibacterium, Clostridium | Not reported | No intervention applied | p = 0.021 |
| Lwere K. et al., 2025 [26] | Cross-sectional observational | 104 older adults in Uganda: 77 AD 14 MCI 13 NC (≥60 year) | AD 14 M MCI 3 M NC 4 M | 16S rRNA sequencing of fecal samples | MCI/AD | MoCA, DSM--V/ICD--11 criteria | ↑ Hafnia-Obesumbacterium, Dickeya; ↓ Novosphingobium, Staphylococcus | Not reported | No intervention applied | p < 0.05 |
| Yıldırım S. et al., 2022 [27] | Cross-sectional observational | 125 participants: 51 NC 27 MCI 47 AD Turkish cohort | NC 28 M MCI 16 M AD 24 M | 16S rRNA amplicon sequencing (fecal) | AD | MMSE, CDR | ↑ Bacteroides, Escherichia/Shigella, Subdoligranulum, Bilophila, Alistipes; ↓ Faecalibacterium, Lachnospiraceae, Ruminococcaceae | Not reported | No intervention applied | p = 0.04 |
| Yuan et al., 2024 [28] | Cross-sectional observational | 120 rural Chinese elderly: 81 AD 39 NC | AD 40 M NC 15 M | 16S rRNA sequencing of fecal samples | AD | AD8 questionnaire | ↑ Escherichia–Shigella, Akkermansia, Monoglobus; ↓ Faecalibacterium | Not reported | No intervention applied | p = 0.0235 |
| Saji N. et al., 2022 [29] | Cross-sectional study | 85 participants: 23 AD 42 MCI 20 NC | 33 M | 16S rRNA sequencing of fecal samples | AD | MMSE, CDR | ↑ Faecalibacterium, Ruminococcus, Gemmiger; ↓ Bifidobacterium, Actinomyces, Parabacteroides | Japanese Diet: rice, miso, fish and shellfish, green and yellow vegetables, seaweed, pickles, fruit, soybeans and soybeanderived foods, mushrooms, beef and pork, chicken, green tea, and coffee | Adherence to Japanese diet | p = 0.006 |
| Teigen L.M. et al., 2024 [30] | Cross-sectional observational | 27 MCI. 11 iRBD, 39 cohabitant controls, 19 not cohabitants unrelated healthy controls | iRBD 9 M Cohabitant Controls 5 M | 16S rRNA sequencing of fecal samples | MCI | MoCA | ↑ Blautia, Collinsella; ↓ Bacteroides, Alistipes, Faecalibacterium, Lachnospiraceae | Not reported | No intervention applied | p < 0.0083 |
| Guo M. et al., 2021 [31] | Cross-sectional observational | 56 Chinese participants: 18 AD 20 MCI 18 NC | AD 2 M MCI 4 M NC 4 M | 16S rRNA sequencing of fecal samples | MCI/AD | MMSE, MoCA | ↓ Bacteroides, Lachnospira, Ruminiclostridium_9; ↑ Prevotella | Not reported | No intervention applied | p < 0.001 |
| Liu P. et al., 2019 [32] | Cross-sectional prospective | 97 Chinese participants: 32 NC 32 MCI 33 AD | NC 16 M MCI 14 M AD 19 M | 16S rRNA sequencing of fecal samples | MCI/AD | MMSE, MoCA, CDR scores | ↓ Firmicutes, Clostridiaceae, Ruminococcus, Lachnospira; ↑ Proteobacteria, Enterobacteriaceae, Escherichia/Shigella | The majority of healthy controls were patient’s spouses with the same diet | No intervention applied | p = 0.008 |
| Pan et al., 2021 [33] | Case–control observational | 22 MCI 26 NC | MCI 8 M NC 7 M | 16S rRNA sequencing of fecal samples | MCI | MMSE. IADL | ↑ Staphylococcus intermedius, S. lentus; ↓ Bacteroides salyersiae, B. gallinarum | Not reported | No intervention applied | p = 0.048 |
| Study | Microbiota Intervention | Microbiota Changes | Marker | ||
|---|---|---|---|---|---|
| Pre | Post | p Value | |||
| Aljumaah et al., 2022 [13] | Daily supplementation with probiotic Lactobacillus rhamnosus GG (LGG) vs. placebo for 3 months | Prevotella ruminicola, Bacteroides thetaiotaomicron and Bacteroides xylanisolvens were increased in MCI | In the MCI group receiving LGG, there was a decrease in the abundance of Prevotella and Dehalobacterium | p = 0.0017 | Microbiota biomarkers: Prevotella and Dehalobacterium |
| Zhang X. et al., 2021 [23] | Diet quality (CDG-2018): higher diet quality Lower diet quality | ↓ Faecalibacterium, Roseburia | ↑ Faecalibacterium, Roseburia ↑ Escherichia/Shigella | p < 0.005 | microRNAs, dietary indices |
| Saji N. et al., 2022 [29] | Adherence to Japanese diet (rJDI12) Beneficial components: white rice, miso soup, fish and shellfish, green and yellow vegetables, seaweed, pickles, green tea, soybeans, fruit, mushrooms, coffee Less beneficial components: beef and pork | ↓ SCFA-producers | ↑Bifidobacterium, Clostridium butyricum and better cognition ↓ SCFA-producers | Participants without dementia consumed more fish and shellfish (p = 0.048), mushrooms (p = 0.015), soybeans and soybean-derived foods (p = 0.013), coffee (p = 0.024). | MMSE, dietary adherence scores |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tana, C.; Moffa, S.; Tana, M.; Ucciferri, C.; Moffa, L. Gut Microbiota, Mild Cognitive Impairment and Dementia: A Systematic Review. Neurol. Int. 2025, 17, 155. https://doi.org/10.3390/neurolint17100155
Tana C, Moffa S, Tana M, Ucciferri C, Moffa L. Gut Microbiota, Mild Cognitive Impairment and Dementia: A Systematic Review. Neurology International. 2025; 17(10):155. https://doi.org/10.3390/neurolint17100155
Chicago/Turabian StyleTana, Claudio, Samanta Moffa, Marco Tana, Claudio Ucciferri, and Livia Moffa. 2025. "Gut Microbiota, Mild Cognitive Impairment and Dementia: A Systematic Review" Neurology International 17, no. 10: 155. https://doi.org/10.3390/neurolint17100155
APA StyleTana, C., Moffa, S., Tana, M., Ucciferri, C., & Moffa, L. (2025). Gut Microbiota, Mild Cognitive Impairment and Dementia: A Systematic Review. Neurology International, 17(10), 155. https://doi.org/10.3390/neurolint17100155









