Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Antibodies
2.2. Cell Culture
2.3. Preparation of oxLDL
2.4. MTT Assay
2.5. RNA Isolation, Quantitative Real-Time Polymerase Chain Reaction (PCR), and Electrophoresis
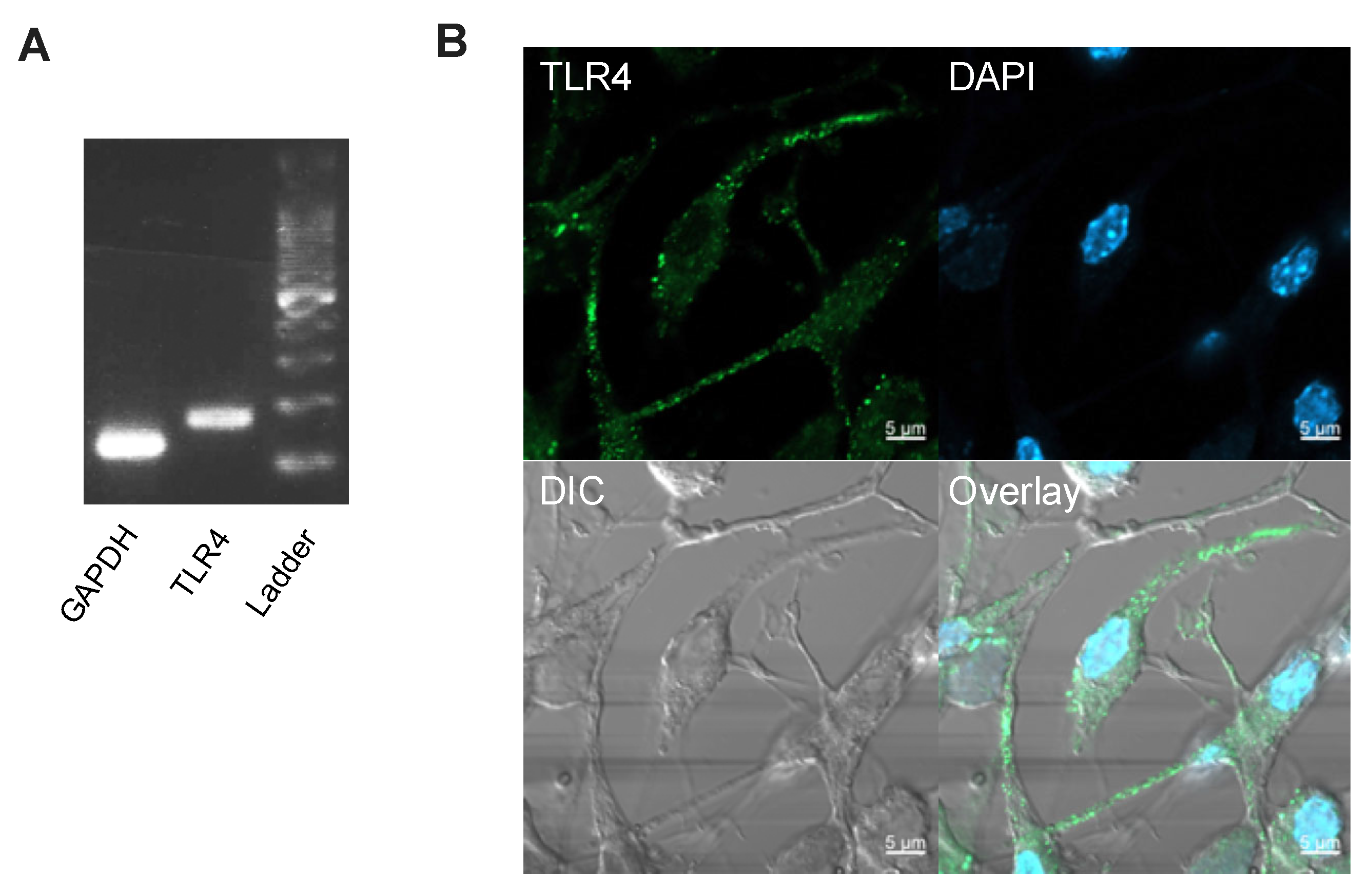
2.6. Immunocytochemistry
2.7. Western Blot
2.8. Caspase-3 Activity Detection
2.9. Statistical Analysis
3. Results
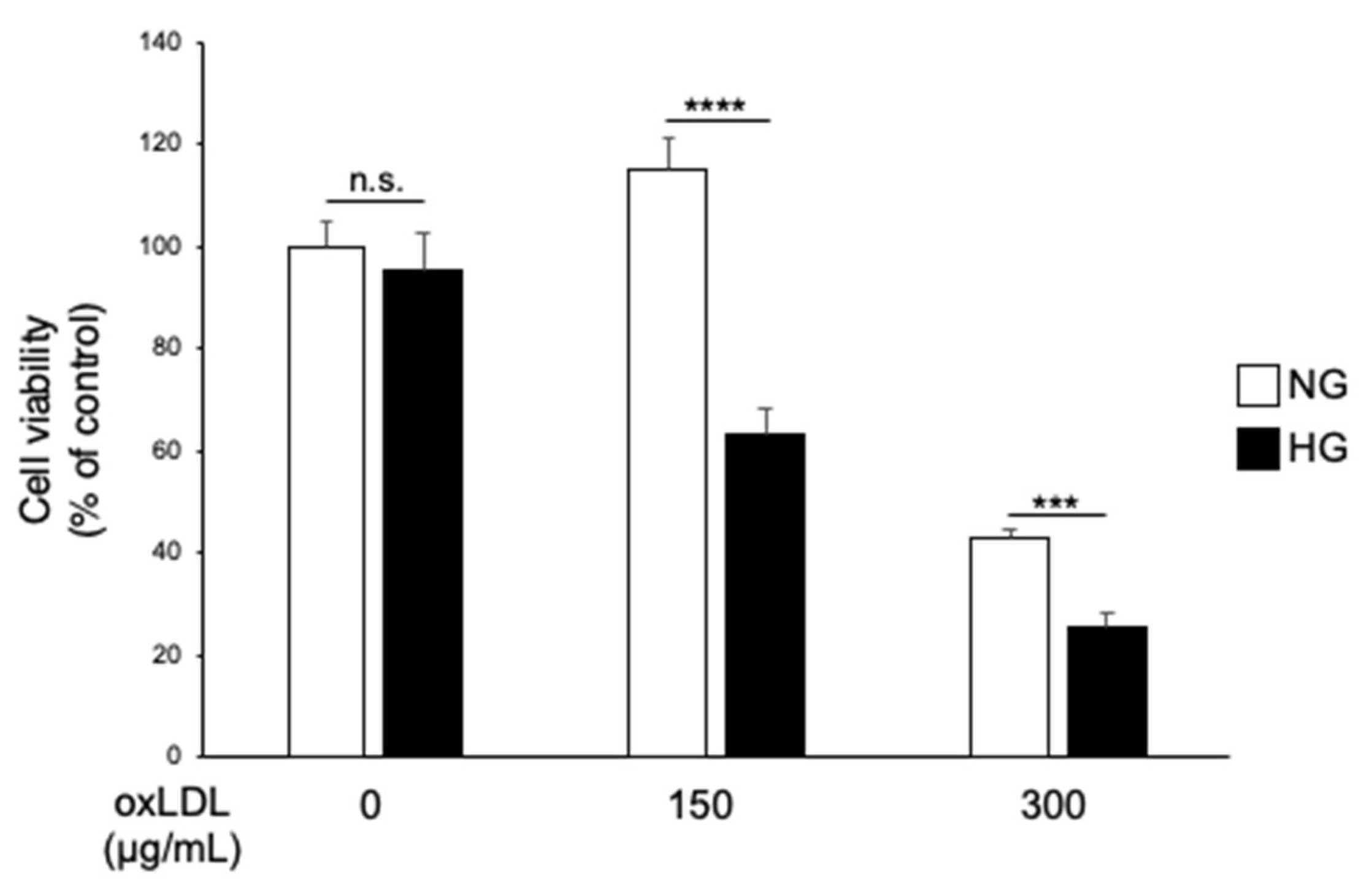
3.1. Hyperglycaemia and oxLDL Treatment Trigger Synergistic Cell Death in IMS32 Cells
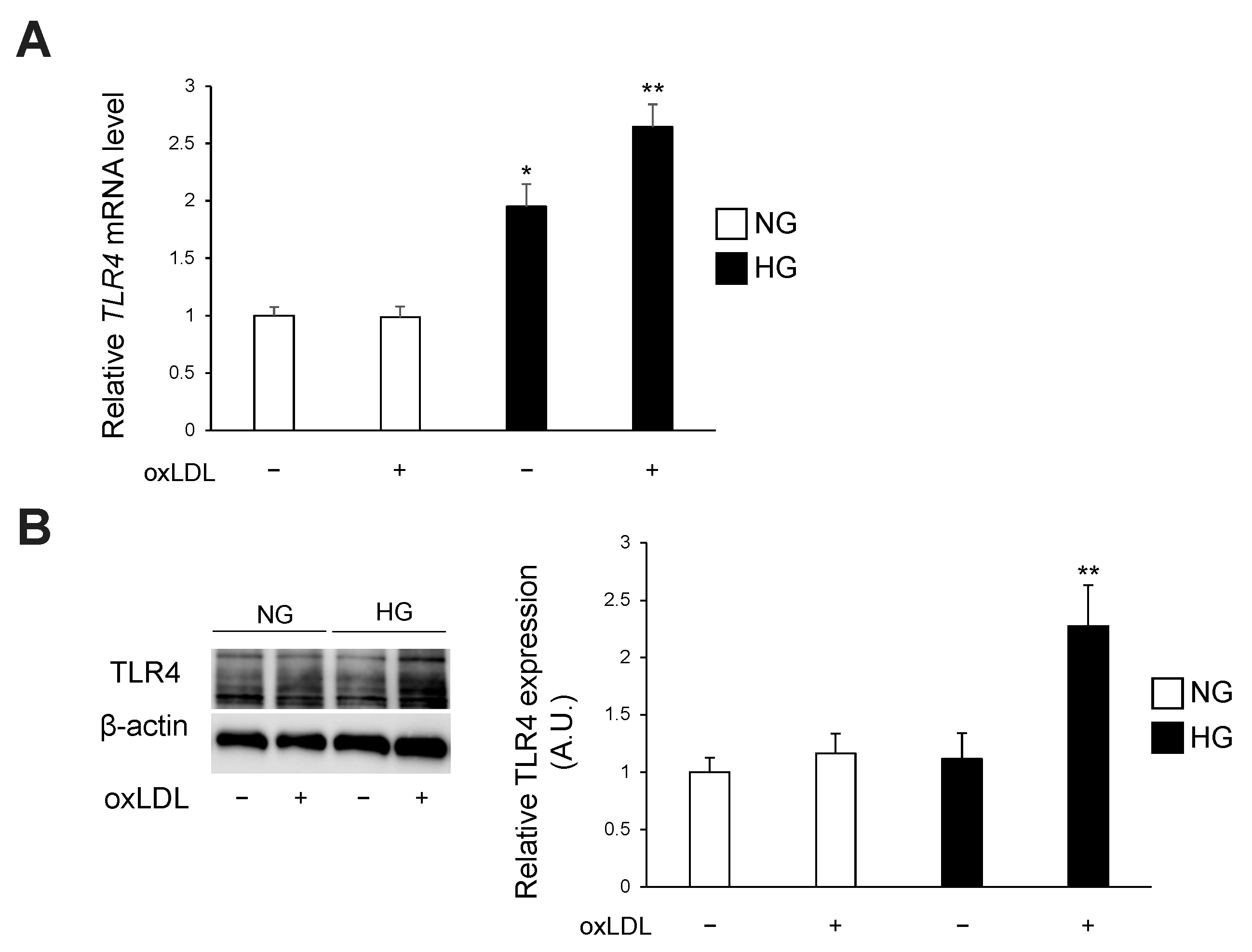
3.2. Hyperglycaemia and oxLDL Treatment Upregulate TLR4 Gene and Protein Expression
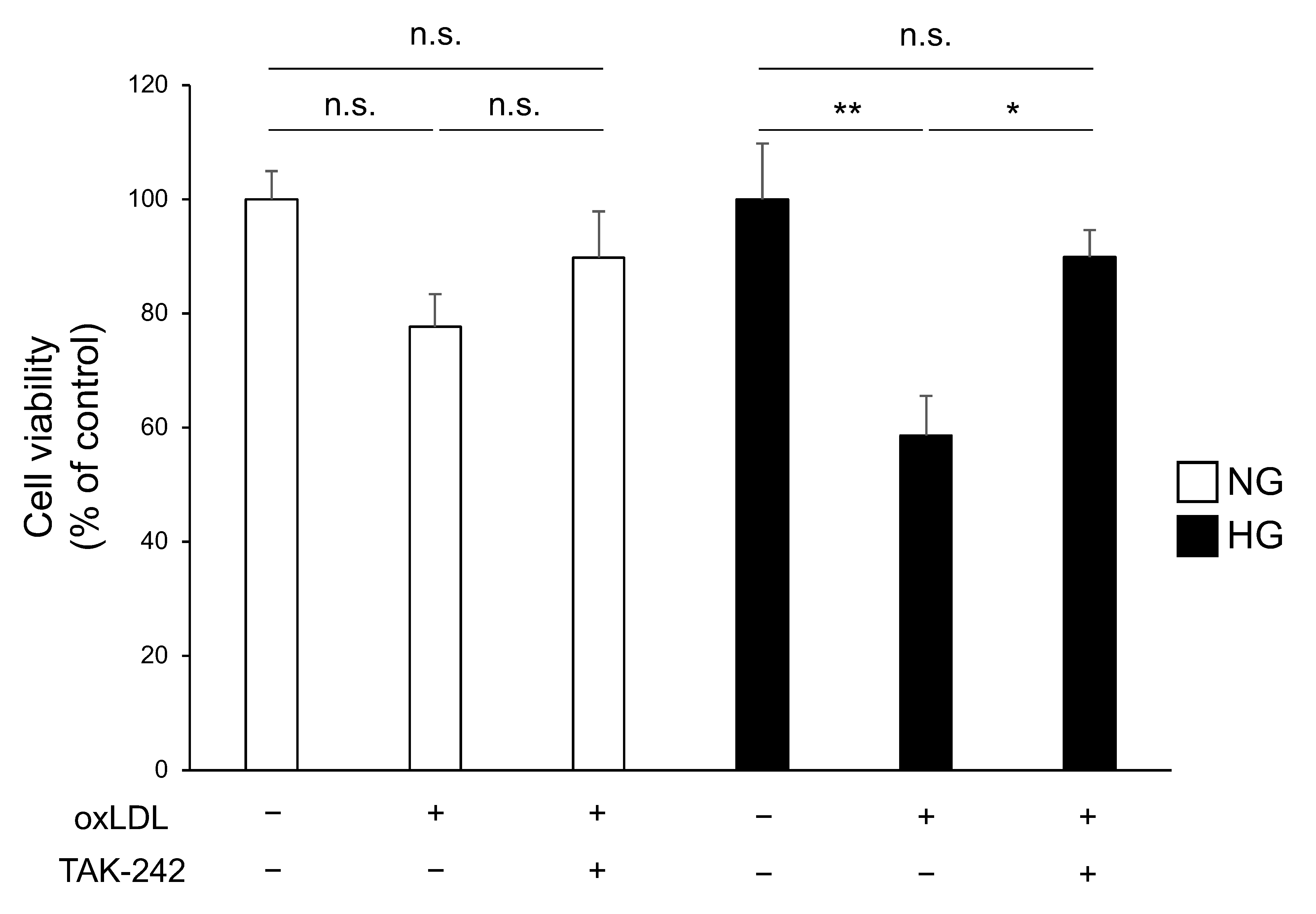
3.3. TLR4 Inhibition Attenuates Cell Death Caused by Hyperglycaemia and oxLDL Treatment
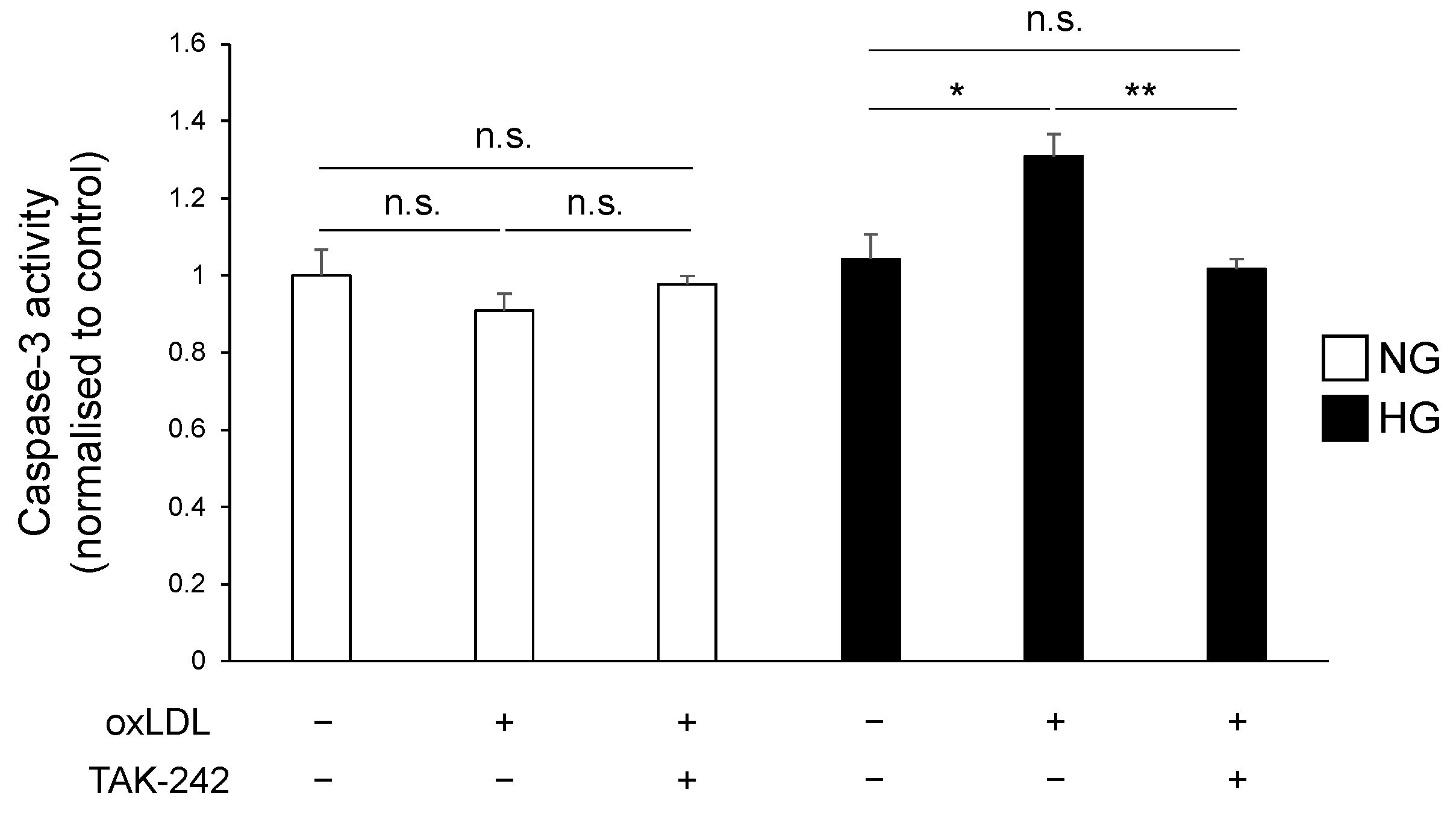
3.4. TLR4 Inhibition Suppressed Hyperglycaemia and oxLDL-Induced Activation of Caspase-3 Pathway
4. Discussion
Author Contributions
Funding
Institutional review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, J.W.; Zilliox, L.A. Diabetic Neuropathies. Continuum 2014, 20, 1226–1240. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [CrossRef]
- Pop-Busui, R.; Boulton, A.J.M.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Nakamura, J.; Kato, K.; Hamada, Y.; Nakayama, M.; Chaya, S.; Nakashima, E.; Naruse, K.; Kasuya, Y.; Mizubayashi, R.; Miwa, K.; et al. A Protein Kinase C-Beta-Selective Inhibitor Ameliorates Neural Dysfunction in Streptozotocin-Induced Diabetic Rats. Diabetes 1999, 48, 2090–2095. [Google Scholar] [CrossRef]
- Koya, D.; King, G.L. Protein Kinase C Activation and the Development of Diabetic Complications. Diabetes 1998, 47, 859–866. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced Glycosylation End Products in Tissue and the Biochemical Basis of Diabetic Complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic Neuropathy: Mechanisms to Management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef]
- Rice, J.B.; Stoll, L.L.; Li, W.-G.; Denning, G.M.; Weydert, J.; Charipar, E.; Richenbacher, W.E.; Miller, F.J., Jr.; Weintraub, N.L. Low-Level Endotoxin Induces Potent Inflammatory Activation of Human Blood Vessels: Inhibition by Statins. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1576–1582. [Google Scholar] [CrossRef]
- Stoll, L.L.; Denning, G.M.; Li, W.-G.; Rice, J.B.; Harrelson, A.L.; Romig, S.A.; Gunnlaugsson, S.T.; Miller, F.J., Jr.; Weintraub, N.L. Regulation of Endotoxin-Induced Proinflammatory Activation in Human Coronary Artery Cells: Expression of Functional Membrane-Bound CD14 by Human Coronary Artery Smooth Muscle Cells. J. Immunol. 2004, 173, 1336–1343. [Google Scholar] [CrossRef]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like Receptor 4 and High-Mobility Group Box-1 Are Involved in Ictogenesis and Can Be Targeted to Reduce Seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, Z.; Zhu, Y.; Shi, L.; Li, Z.; Hou, R.; Zhang, C.; Yao, D. Toll-Like Receptor 4 (TLR4) Expression Affects Schwann Cell Behavior In Vitro. Sci. Rep. 2018, 8, 11179. [Google Scholar] [CrossRef]
- Nowicki, M.; Müller, K.; Serke, H.; Kosacka, J.; Vilser, C.; Ricken, A.; Spanel-Borowski, K. Oxidized Low-Density Lipoprotein (OxLDL)-Induced Cell Death in Dorsal Root Ganglion Cell Cultures Depends Not on the Lectin-like OxLDL Receptor-1 but on the Toll-like Receptor-4. J. Neurosci. Res. 2010, 88, 403–412. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Yang, H.; Lu, Y.; Li, L. Oxidized Low-Density Lipoprotein Induces Apoptosis in Cultured Neonatal Rat Cardiomyocytes by Modulating the TLR4/NF-ΚB Pathway. Sci. Rep. 2016, 6, 27866. [Google Scholar] [CrossRef]
- Xu, S.; Luo, W.; Xu, X.; Qian, Y.; Xu, Z.; Yu, W.; Shan, X.; Guan, X.; Lum, H.; Zhou, H.; et al. MD2 Blockade Prevents OxLDL-Induced Renal Epithelial Cell Injury and Protects against High-Fat-Diet-Induced Kidney Dysfunction. J. Nutr. Biochem. 2019, 70, 47–55. [Google Scholar] [CrossRef]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.M.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H. Vascular Risk Factors and Diabetic Neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef]
- Njajou, O.T.; Kanaya, A.M.; Holvoet, P.; Connelly, S.; Strotmeyer, E.S.; Harris, T.B.; Cummings, S.R.; Hsueh, W.-C. Health ABC Study Association between Oxidized LDL, Obesity and Type 2 Diabetes in a Population-Based Cohort, the Health, Aging and Body Composition Study. Diabetes. Metab. Res. Rev. 2009, 25, 733–739. [Google Scholar] [CrossRef]
- Lin, M.; Yiu, W.H.; Wu, H.J.; Chan, L.Y.Y.; Leung, J.C.K.; Au, W.S.; Chan, K.W.; Lai, K.N.; Tang, S.C.W. Toll-like Receptor 4 Promotes Tubular Inflammation in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 86–102. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased Toll-like Receptor (TLR) Activation and TLR Ligands in Recently Diagnosed Type 2 Diabetic Subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Vincent, A.M.; Hayes, J.M.; McLean, L.L.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Dyslipidemia-Induced Neuropathy in Mice: The Role of OxLDL/LOX-1. Diabetes 2009, 58, 2376–2385. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kakino, A.; Takeshita, H.; Hayashi, N.; Li, L.; Nakano, A.; Hanasaki-Yamamoto, H.; Fujita, Y.; Imaizumi, Y.; Toyama-Yokoyama, S.; et al. Oxidized LDL (OxLDL) Activates the Angiotensin II Type 1 Receptor by Binding to the Lectin-like OxLDL Receptor. FASEB J. 2015, 29, 3342–3356. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Fang, J.; Zhou, H.; Liu, X.; Su, S.B. High Glucose Induces and Activates Toll-like Receptor 4 in Endothelial Cells of Diabetic Retinopathy. Diabetol. Metab. Syndr. 2015, 7, 89. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic Crises in Adult Patients with Diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef]
- Holvoet, P.; Kritchevsky, S.B.; Tracy, R.P.; Mertens, A.; Rubin, S.M.; Butler, J.; Goodpaster, B.; Harris, T.B. The Metabolic Syndrome, Circulating Oxidized LDL, and Risk of Myocardial Infarction in Well-Functioning Elderly People in the Health, Aging, and Body Composition Cohort. Diabetes 2004, 53, 1068–1073. [Google Scholar] [CrossRef]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in Carotid Plaques and Plasma Associates with Plaque Instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Yen, M.H.; Yen, C.H.; Lau, Y.T. Oxidized Low Density Lipoprotein Induces Apoptosis via Generation of Reactive Oxygen Species in Vascular Smooth Muscle Cells. Cardiovasc. Res. 2001, 49, 135–145. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, H.; Wang, X.; Cheng, Z. Upregulation of TLR4 via PKC Activation Contributes to Impaired Wound Healing in High-Glucose-Treated Kidney Proximal Tubular Cells. PLoS ONE 2017, 12, e0178147. [Google Scholar] [CrossRef][Green Version]
- Xu, X.H.; Shah, P.K.; Faure, E.; Equils, O.; Thomas, L.; Fishbein, M.C.; Luthringer, D.; Xu, X.P.; Rajavashisth, T.B.; Yano, J.; et al. Toll-like Receptor-4 Is Expressed by Macrophages in Murine and Human Lipid-Rich Atherosclerotic Plaques and Upregulated by Oxidized LDL. Circulation 2001, 104, 3103–3108. [Google Scholar] [CrossRef]
- Rudofsky, G., Jr.; Reismann, P.; Witte, S.; Humpert, P.M.; Isermann, B.; Chavakis, T.; Tafel, J.; Nosikov, V.V.; Hamann, A.; Nawroth, P.; et al. Asp299Gly and Thr399Ile Genotypes of the TLR4 Gene Are Associated with a Reduced Prevalence of Diabetic Neuropathy in Patients with Type 2 Diabetes. Diabetes Care 2004, 27, 179–183. [Google Scholar] [CrossRef][Green Version]
- Wiggin, T.D.; Sullivan, K.A.; Pop-Busui, R.; Amato, A.; Sima, A.A.F.; Feldman, E.L. Elevated Triglycerides Correlate with Progression of Diabetic Neuropathy. Diabetes 2009, 58, 1634–1640. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated Fatty Acids Activate TLR-Mediated Proinflammatory Signaling Pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A Acts as an Endogenous Ligand of TLR4 to Promote Lipid-Induced Insulin Resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 Ligands Promote Sterile Inflammation through Assembly of a Toll-like Receptor 4 and 6 Heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef]
- Itabe, H.; Obama, T. The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation. Int. J. Mol. Sci. 2023, 24, 5747. [Google Scholar] [CrossRef]
| Genes |
|---|
| ACTB Forward: → 5′-CAT TGC TGA CAG GAT GCA GAA GG-3′ |
| Reverse: → 5′-TGC TGG AAG GTG GAC AGT GAG G-3′ |
| TLR4 Forward: → 5′-TCC CTG CAT AGA GGT AGT TCC-3′ |
| Reverse: → 5′-TCC AGC CAC TGA AGT TCT GA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nihei, W.; Kato, A.; Himeno, T.; Kondo, M.; Nakamura, J.; Kamiya, H.; Sango, K.; Kato, K. Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4. Neurol. Int. 2024, 16, 370-379. https://doi.org/10.3390/neurolint16020027
Nihei W, Kato A, Himeno T, Kondo M, Nakamura J, Kamiya H, Sango K, Kato K. Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4. Neurology International. 2024; 16(2):370-379. https://doi.org/10.3390/neurolint16020027
Chicago/Turabian StyleNihei, Wataru, Ayako Kato, Tatsuhito Himeno, Masaki Kondo, Jiro Nakamura, Hideki Kamiya, Kazunori Sango, and Koichi Kato. 2024. "Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4" Neurology International 16, no. 2: 370-379. https://doi.org/10.3390/neurolint16020027
APA StyleNihei, W., Kato, A., Himeno, T., Kondo, M., Nakamura, J., Kamiya, H., Sango, K., & Kato, K. (2024). Hyperglycaemia Aggravates Oxidised Low-Density Lipoprotein-Induced Schwann Cell Death via Hyperactivation of Toll-like Receptor 4. Neurology International, 16(2), 370-379. https://doi.org/10.3390/neurolint16020027







