Abstract
Cavernous angiomas (CAs) are benign vascular malformations predominantly seen in the brain parenchyma and therefore referred to as intra-axial. Extra-axial dural-based cavernous angiomas, on the other hand, are rare vascular lesions found outside of the brain parenchyma. They occur in the middle fossa and may be easily misdiagnosed as meningiomas due to their extra-axial location. In addition, CAs that are located outside the middle fossa, such as in the convexity, have a better prognosis since they are more surgically accessible. Surgical resection is the main treatment of choice in CAs. However, other options, such as embolization and radiotherapy, may also be considered therapeutic choices or additive treatment options. The pathogenesis of CA and the involvement of other factors (genetics or environmental factors) are still unknown and require further investigation. We are presenting a young man who presented for evaluation of seizure-like events without any family history of neurologic conditions. The physical examination was unremarkable except for a slightly antalgic gait. Imaging studies showed an extra-axial left tentorial mass suggestive of a meningioma, hemangiopericytoma, or other extra-axial lesions. The lesion was resected where its vascular nature was mentioned initially, and the histology proved the diagnosis of cavernous angioma. Here we give an overview of the known pathogenesis, causes, clinical features, and diagnostic and therapeutic options in CA. Better knowledge about CA, its causes, clinical features, and treatment options would help clinicians in early diagnosis and patient management.
1. Introduction
Cavernous angiomas (CAs) are benign vascular malformations that have emerged from enlarged sinusoidal vessels. They appear in clusters and are lined with a thin endothelial wall with no tissue between them. In addition, they do not have elastic lamina or smooth muscles but are occasionally ossified/calcified. Cavernous angiomas are non-neoplastic vascular abnormalities [1,2,3]. Luschka was the first to describe CA, which was incidentally found in a suicidal patient in 1853 [4,5]. The terms “cavernous angioma”, “cavernous hemangioma”, “cavernous malformation”, or “cavernoma” have been used in the literature for these lesions [2,3,6,7]. CAs may occur in the brain parenchyma, spinal cord, and extra-axial regions [1]. Most of the intracranial CAs are intraparenchymal (intra-axial CAs), but extra-axial CAs are rare. In addition, dural-based CAs or CAs in other atypical sites may be misdiagnosed as meningiomas or neoplasms [1,8,9]. We aimed to give an overview of the known pathogenesis, causes, clinical features, and diagnostic and therapeutic options in CA.
2. Case Presentation
A 40-year-old gentleman presented for evaluation of several episodes of brief unresponsiveness, lasting approximately 30 seconds. The patient had a history of four seizure-like episodes after events like having his blood drawn, being in a hot tub, and hitting his knee. No family history of neurologic conditions was noted. The physical examination was unremarkable except for a slightly antalgic gait due to a recent ankle sprain. An unenhanced head CT demonstrated a 1.7 cm hyperdense nodule extending up from the left tentorium. Head MRI with and without contrast identified a 2.2 cm lobulated, mildly T2/Fluid attenuated inversion recovery (FLAIR) hyperintense, T1 isointense, homogeneously enhancing mass along the left tentorium (Figure 1). The lesion also showed small, punctate areas of hypo-intensity on susceptibility-weighted imaging. The differential diagnosis included meningioma and hemangiopericytoma (aka. Solitary fibrous tumor), given the FLAIR hyperintensity and hyperenhancement. The patient underwent a left craniotomy with a resection of the mass. Intraoperatively, the mass arose from the tentorium and extended posteriorly to the wall of the transverse sinus. The lesion was vascular in nature and resembled a cavernous angioma. The lesion was submitted for histopathological examination.
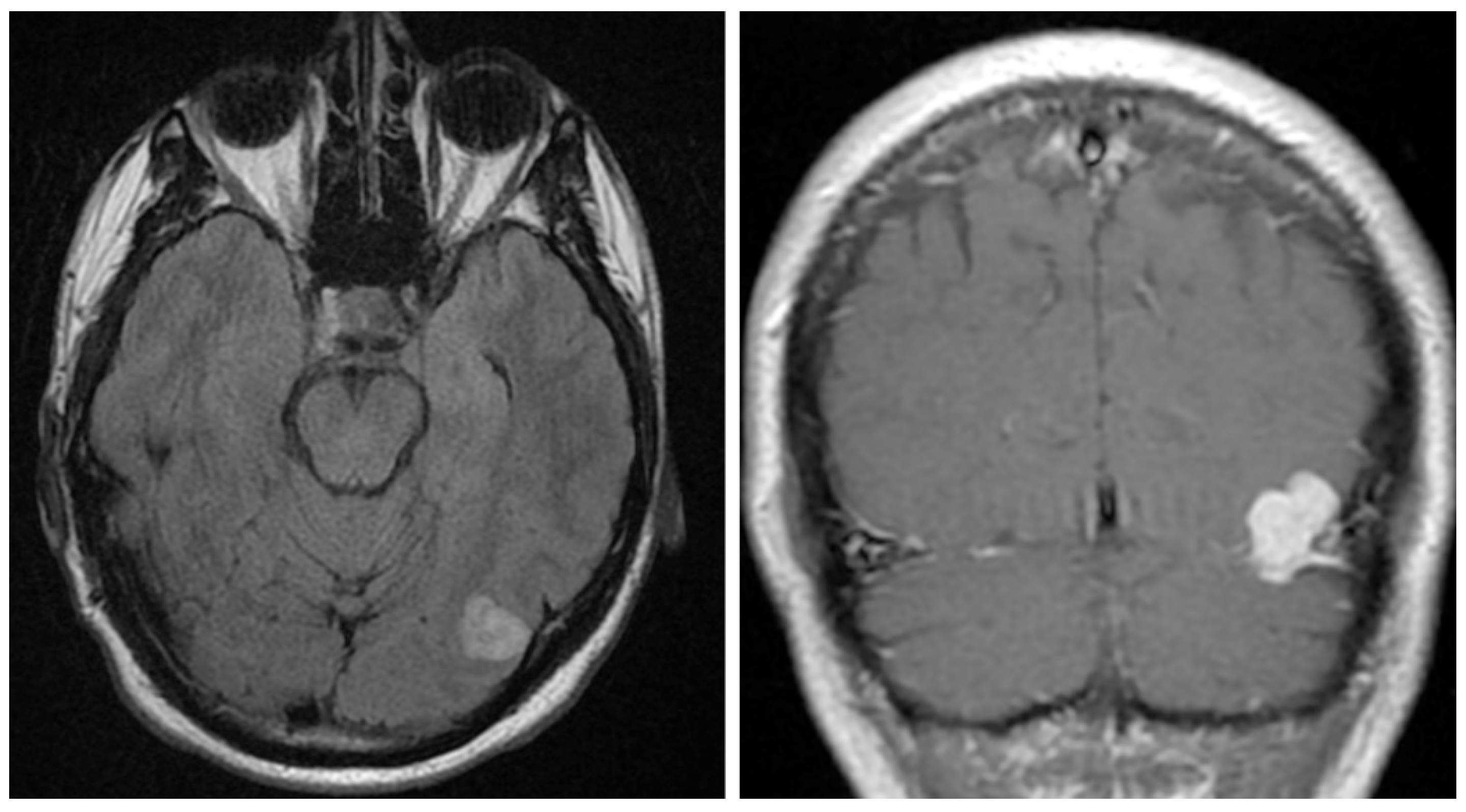
Figure 1.
Imaging studies. Head MRI with and without contrast identified a 2.2 cm lobulated, mildly T2/FLAIR hyperintense (left), T1 isointense, homogeneously enhancing mass along the left tentorium (right).
Standard laboratory procedures were performed to prepare Hematoxylin and Eosin (H&E)-stained glass slides [10], followed by performing standard immunohistochemistry per the manufacturer’s recommendations. A microscopic examination of the lesion revealed clusters of closely juxtaposed vascular channels with thick, hyalinized walls. No intervening neural or glial tissue was identified. The vessels were lined with flattened to cuboidal endothelial cells, and some of the vascular spaces were filled with blood and thrombi. Rare hemosiderin-laden macrophages were noted (Figure 2). Immunohistochemical stain for CD34 (Roche Diagnostics, Rotkreuz, Switzerland—Cat # 790-2927) highlighted the endothelial cells, and trichrome stain highlighted the hyalinized vessel walls. Verhoeff Van Gieson (VVG) (Poly Scientific, Bay Shore, NY, USA—Cat#: k059-16oz) elastic stain was negative for internal elastic lamina, which is a feature of arteries (Figure 3). The absence of an internal elastic lamina ruled out the diagnosis of arteriovenous malformation. To differentiate from meningioma and hemangiopericytoma, histochemical stains for Somatostatin receptor 2 (SSTR2) (performed at Mayo Clinic Laboratory, Rochester, MN, USA), Epithelial membrane antigen (EMA) (Cell MarqueTM Tissue Diagnostics, Rocklin, CA, USA), and signal transducer and activator of transcription 6 (STAT6) (Cell MarqueTM Tissue Diagnostics) were performed, which were all negative. The lesion was also negative for S100 (Roche Diagnostics, Switzerland-Cat# 790-2914) and SOX-10 (Cell MarqueTM Tissue Diagnostics—Cat # 383R-18); which made the diagnosis of neuroma or schwannoma unlikely. In summary, the histological and immunophenotypic findings were compatible with a cavernous angioma (cavernoma). The postoperative course was uneventful, and the patient was discharged on the third postoperative day. At the 1-month follow-up, the patient was doing well. The patient has had no further episodes of unresponsiveness.
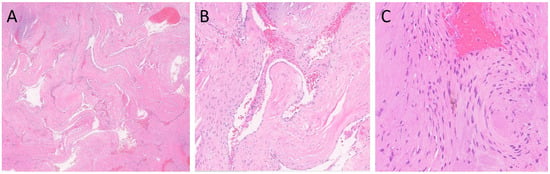
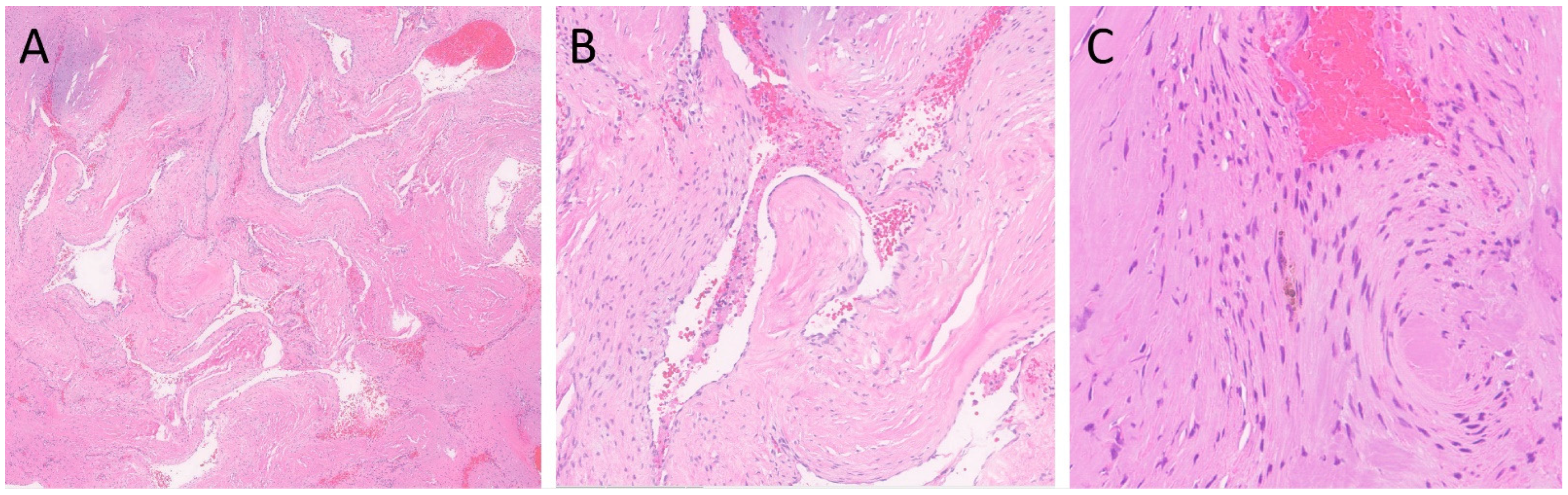
Figure 2.
H&E-stained sections of the lesion. (A) Clusters of closely juxtaposed vascular channels with thick, hyalinized walls (H&E, 20× magnification). (B) The vessels were lined with flattened endothelial cells, and the vascular spaces were filled with blood and thrombi (H&E, 100× magnification). (C) Rare hemosiderin-laden macrophages were present (H&E, 200× magnification).
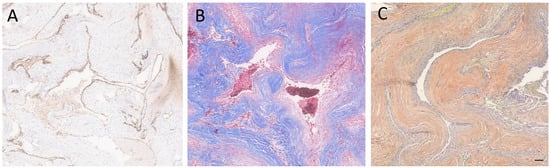
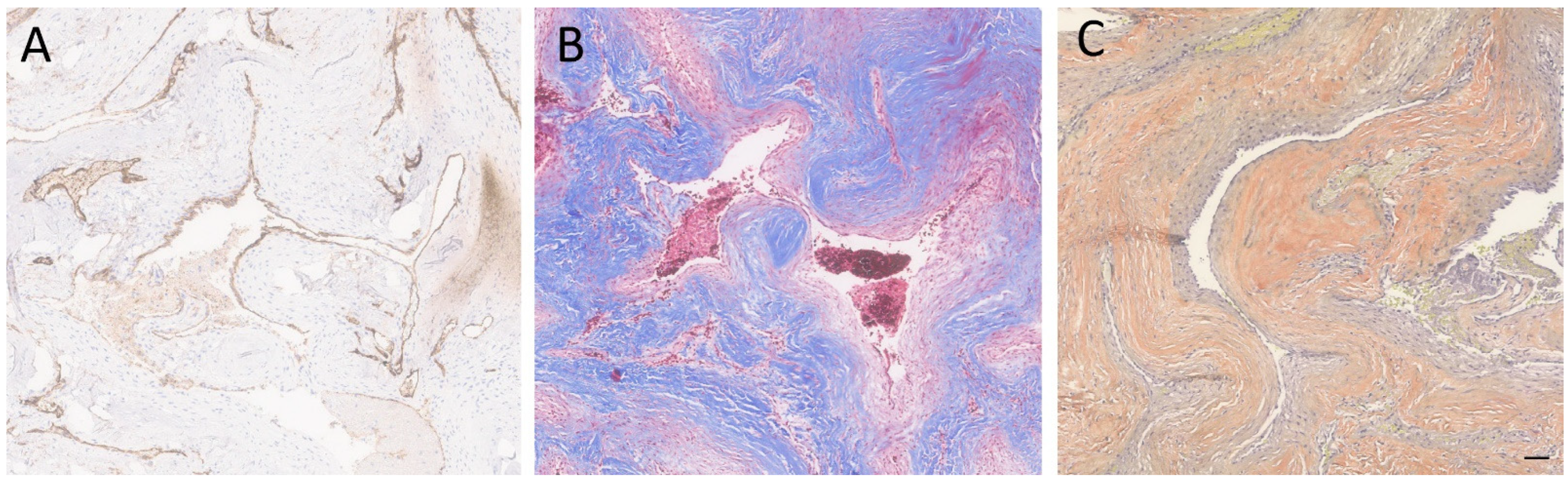
Figure 3.
Special and immunostained sections of the lesion. (A) The immunohistochemical stain for CD34 highlighted the endothelial cells. (B) Immunohistochemical stain for trichrome highlighted the hyalinized vessel walls. (C) A special stain for VVG showed the absence of internal elastic lamina. Scale bar: 100 µm.
We performed a literature search through the National Library of Medicine, the National Center for Biotechnology Information (https://pubmed.ncbi.nlm.nih.gov/, accessed on 1 November 2023), and Google Scholar (https://scholar.google.com/, accessed on 1 November 2023) for similar case reports and included the most relevant cases in this review.
3. Discussion
CAs are benign vascular malformations that account for 3–13% of intracranial vascular malformations and mostly occur in the brain parenchyma (intra-axial CAs). Extra-axial CAs occur in about 0.4% to 2% of intracranial vascular malformations [8]. CAs (or cavernomas) occur in both genders with no specific gender predominance and usually occur in the second to fifth decades of life [1]. However, there have been reports of a female predominance in extra-axial CAs [11]. In addition, CAs in newborns are rare, but there have been some reported cases diagnosed with prenatal ultrasound evaluation [12,13,14]. Gross et al. have reported that intracranial cavernous malformations are located in the supra-tentorial hemisphere (lobar) (66%), brainstem (18%), basal ganglia, thalamus, corpus callosum, or insula (deep supra-tentorial) (9%), and cerebellum (6%) [15]. Lewis et al. (1994) have described two types of extra-axial dural-based CAs [16]. The first type, which consists of the majority of extra-axial CAs, occurs in the dura of the middle cranial fossa. They mostly originate from the sellar and parasellar regions, especially the cavernous sinus [17]. The other type originates from the convexity, cerebral flax, cerebellar flax, tentorium, posterior fossa, anterior fossa (the floor), intrapetrous facial nerve, fifth nerve, eighth nerve, or skull base [2,18,19].
The exact underlying pathogenesis of dural-based cavernomas is still unclear [1]. Since CAs have been reported in the neonate population, it has been suggested that they are probably caused by abnormal vascular development of the embryos. In addition, it has been reported that genetics might have a possible role in the development of CAs [20], although CAs may also develop spontaneously [21,22]. For example, intra-parenchymal CA has been associated with the following cerebral cavernous malformation (CCM) genes: CCM-1, CCM-2, and CCM-3. Most of the patients with CCM and a genetic form have an autosomal dominant pattern and are mostly loss-of-function mutations of the CCM genes. A ‘second hit’ in an existing embryonal nonfunctioning CCM gene causes complete loss of function, leading to the proliferation of the endothelial cells [23,24]. It has been suggested that similar mechanisms may be involved in extra-parenchymal CMs, and consequently, these lesions may be more likely endothelial cell tumors than vascular malformations [24]. CAs may gradually enlarge due to some factors such as thrombosis, engorgement, feeding from adjacent vessels, hemorrhage, hormones, growth, changes in flow, sepsis, trauma, or after surgery [3,25]. It has also been reported that dural-based cavernous malformations (CMs) may change in morphology, increase in size, and develop angiogenesis during pregnancy. These changes have been associated with female hormones (such as estrogen and progesterone) and vascular growth factors (such as vascular endothelial growth factor), which are released during pregnancy [26,27]. For example, in 2021, Ishii et al. reported a case of dural-based CM at the temporal convexity in a pregnant patient that presented with hemorrhage [28]. On the other hand, there have also been reports of dural-based CMs without hemorrhage in pregnancy. Furthermore, the exact association between cavernous hemangioma and meningioma is still unclear. However, there have been suggestions that since both tumors may have a ventricular localization, there may be a collision of the two different tumors due to the migration of tumor cells through the cerebrospinal fluid. In addition, associations between CA and radiation or head traumas have also been suggested [29,30,31].
The clinical presentations of CAs vary and depend on the location and size of the tumor [32]. The majority of CAs are asymptomatic and may be incidentally found on autopsy or imaging [5,33,34]. Dural-based cavernomas have a wide range of non-specific clinical presentations, including seizure (37%), hemorrhage (36%), headache (23%), and neurological deficits (22%). In addition, focal neurological deficits such as sensorimotor deficits, dysphasia, and cranial nerve impairments/palsies are observed in 35–50% of the patients when the motor cortex, speech region, basal ganglia, or brainstem are involved [1,9,17,35]. Dural-based CMs that are located outside of the middle fossa are rarely associated with intracranial hemorrhage [28]. However, there have been reports of subdural hematoma in dural-based CMs located at the dural convexity [36,37].
A definitive diagnosis before surgery is important to plan the surgical technique. However, the radiological and clinical findings in CAs may not be able to distinguish extra-axial CAs from other lesions. For example, there have been reports of misdiagnosing extra-axial CA with meningiomas or neoplasms. Consequently, neurosurgeons may have to alter the excision technique during surgery and change the subsequent treatment plan. Therefore, considering extra-axial CAs as a differential diagnosis during surgery seems necessary [1]. The main differential diagnoses of extra-axial CAs include intra-axial/intraparenchymal cavernomas and meningiomas. Other differential diagnoses include cavernous malformations, metastatic neoplasms, solitary fibrous tumors/hemangiopericytoma, neuromas, high-flow vascular malformations (fistulas and arteriovenous angiomas), schwannomas, lymphomas, and sarcoidosis [1,38,39,40].
Following radiological improvements, the diagnosis of extra-axial CAs is increasing. However, confirmation with pathological evaluation is necessary, as a definite diagnosis of most CAs is only established by histological examination [1,39]. On macroscopic evaluation, CAs appear as purple, mulberry-appearing masses that are multi-loculated. These lesions have multiple irregularly arranged sinusoidal vascular channels that are separated by fibrous strands and fibrous connective tissue stroma. However, there are no neural tissues in between them (2). In addition, they are reported to originate from capillaries. On microscopic evaluation, there is a rim of a single layer of endothelial cells at the vessel wall that do not contain muscle or elastic tissue. In addition, there may be calcifications, ossifications, intravascular thrombosis, and hyalinization [5,39]. However, there are no tight junctions [24]. There have been varying reports on the size and volume range of CAs. For example, a size range of 1 mm to 75 mm, even up to 140 mm (mean size of 14.2 mm), have been reported. In general, sizes of 50–60 mm or more are considered giant CA [25].
Dural-based CAs appear different from a CA that originates from the brain parenchyma on computed tomography (CT) scan and magnetic resonance imaging (MRI) images but resemble a meningioma [41]. Since most dural-based lesions in adults are meningiomas, dural cavernomas with dural attachments may appear as meningiomas [1,42]. Differentiation between meningiomas and dural-based cavernomas with radiological evaluations is usually difficult before surgery. On contrast-enhanced CT scans, meningiomas appear as homogenous or heterogeneous lesions. They usually have a dural tail sign on MRI (with gadolinium). However, angiography of meningiomas is usually negative but may occasionally show a slight vascular redness [18,36]. On CT scans, CAs are well-circumscribed, hyperdense lesions with minimal enhancement after infusion of an iodinated contrast agent, which makes them indistinguishable from a meningioma. They do not appear with adjacent edema or a significant mass effect, and the presence of a dural tail is very rare. Some CAs may contain low-density areas that have been associated with prior thrombosis or cystic degeneration [5,39,43]. Furthermore, dural cavernomas do not cause brain edema, which may be a helpful characteristic of meningioma [24]. On a CT scan, intraparenchymal cavernomas appear as contrast-enhanced and hyperdense lesions [1,44]. Parenchymal cavernomas appear with increased size and recurrent bleeding. The increase in size in these lesions may be due to capillary hyperplasia or thrombosis in the vascular spaces [1,45]. However, bleeding and subarachnoid hemorrhage are very rare in dural-based cavernomas [42,46].
On MRI, intraparenchymal cavernomas appear as iso/hypointense and mixed/hyperintense lesions on T1-weighted and T2-weighted images, respectively [44]. In addition, a peripheral hypointense ring (hemosiderin) surrounding the parenchyma is usually observed on MRI [47]. However, peripheral hemosiderin rings are not commonly seen in dural-based cavernomas [1,44]. Dural-based CAs have a higher intensity and hyperintense signal on T2-weighted imaging compared to meningioma, which is typical for dural CAs [45]. High intensity in T2-weighted imaging has relatively high specificity. MRI and angiography findings can usually establish a preoperative diagnosis [48]. CA in the cavernous sinus of the middle cranial fossa has an intermediate signal intensity in Tl-weighted imaging and has homogeneous hyperintensity in T2-weighted imaging. In addition, following administration of intravenous (IV) gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA), significant and homogeneous enhancement occurs (similar to a meningioma) [41]. Although most dural-based CAs have MRI results similar to those of a meningioma, some may show features resembling those of an intra-axial CA. For example, Vogler et al. reported a case of a dural-based CA in the occiput with MRI presentations similar to an intra-axial CA, including heterogeneous signal intensity in both short and long TR and hemosiderin deposition [5,41].
An angiogram may be used to exclude a tumor [45]. The result of catheter angiography is usually negative in these patients, but a slight vascular blush may be observed in some cases [5]. In addition, the angiogram may show vessel dislocations, widened veins, slight neovascularity, and a flecked tumor blush [33,39]. The sunburst of vessels that radiate outwards from the central vascular pedicle, which is typically seen in meningiomas, has not been reported in CAs [49]. There have been some reports that Thallium201 single-photon emission CT (SPECT) shows low uptake within CA lesions, while in meningioma or malignant tumors it shows high uptake. The high uptake in tumors is because of the increased viability or blood flow of the tumors [49,50]. However, there is controversy over Thallium201 SPECT helping diagnose cavernous sinus hemangioma due to the contradictory results that range from none to mild and significant uptake [34].
The treatment of choice for symptomatic dural-based cavernoma is surgery. Adjuvant therapy would be unnecessary following total surgical removal [1,28]. Uzunoglu et al. recommended considering surgery for extra-axial lesions, especially when the radiological findings are suggestive of a meningioma. Therefore, histopathological confirmation of dural hemangioma would be possible [1]. However, the site of these lesions and MRI results are important in the decision-making process of surgery [1,28]. For example, surgical resection of the CAs involving the cavernous sinus is usually difficult and may be associated with intraoperative blood loss. This may also lead to an incomplete surgical excision. Therefore, embolization, radiotherapy, or radiosurgery may be required in such cases. However, surgical removal of the dural CAs outside the cavernous sinus is usually complete without the need for other therapies [45]. Furthermore, a frozen section may be requested for the diagnosis of CA during surgery [51].
Embolization, radiotherapy, or radiosurgery may be considered in patients with deeper extra-axial lesions [1]. For example, surgical resection could be difficult due to the vascularity of extra-axial CAs, hemorrhage during surgery, cranial nerve involvement, or carotid artery involvement. Therefore, radiotherapy has been suggested as a preoperative or additive treatment option for these patients [39]. A surgical biopsy followed by radiotherapy before surgery has been suggested in CA. However, CA has unpredictable and varying radiosensitivity [52,53]. Furthermore, embolization before surgery could be helpful in cases with high vascularity [3,39]. Among the methods used for sinus CA that decrease the tumor size or vascularity and, in turn, reduce hemorrhage during surgical resection, radiosurgery has been preferred. This is because radiosurgery has shown good clinical outcomes, lower morbidity, and a lower risk of bleeding [54,55].
Furthermore, although CAs are benign lesions, some CAs may grow in size. Different factors have been reported to be involved in the growth of dural CA, including endocrine factors, capillary budding, ectasia, thrombosis of vascular spaces, or hemorrhage, which may be involved in the growth of dural cavernous angioma [37,56]. In addition, dural-based cavernomas located at the cavernous sinus and middle cranial fossa are more vascular and clinically aggressive compared to cavernomas in the convexity or infra-tentorium [41]. Extra-axial intracranial CAs that arise from the cavernous sinus tend to bleed massively during surgery, and, in turn, surgical resection is usually not successful. However, the location of extra-axial intracranial CAs that originate from the convexity is easier for surgical removal, and the bleeding during surgery is much less. Therefore, they have a better prognosis compared to those CAs that originate from the cavernous sinus [43]. Overall, most patients with CA have a good clinical outcome following surgery [25].
4. Conclusions
Extra-axial dural-based CA is a rare intracranial vascular lesion. It usually occurs in the middle fossa. However, those CAs that are located outside the middle fossa, such as in the convexity, have a better prognosis since they are more surgically accessible. Extra-axial dural-based CA may be misdiagnosed as meningioma. Surgical resection remains the main treatment choice in CAs. However, embolization and radiotherapy may also be considered therapeutic choices or additive treatment options. The exact pathogenesis of sporadic CA and the involvement of other factors, such as genetics or environmental factors, are still unknown and require further investigation. Better knowledge about CA, its pathogenesis, causes, clinical features, and treatment options would help clinicians promptly diagnose and manage patients with CA and, in turn, increase their quality of life and clinical outcome.
The following tables summarize the findings of reported cases in the literature based on their locations, as follows. Table 1: Extra-axial cavernous angioma of the cavernous sinus and sellar, parasellar, and intrasellar regions. Table 2. Dural-based cavernous hemangiomas in convexities. Table 3: Cavernous angioma in the falx cerebri. Table 4. Extra-axial cavernoma of the tentorium. Table 5. Extra-axial cavernoma of the cerebellopontine angle (CPA). Table 6. Extra-axial cavernous angioma of the cerebellar falx.

Table 1.
Extra-axial cavernous angioma of the cavernous sinus and sellar, parasellar, and intrasellar regions.

Table 2.
Dural-based cavernous hemangiomas in convexities.

Table 3.
Cavernous angioma in the falx cerebri.

Table 4.
Extra-axial cavernoma of the tentorium.

Table 5.
Extra-axial cavernoma of the cerebellopontine angle (CPA).

Table 6.
Extra-axial cavernous angioma of the cerebellar falx.
Author Contributions
Conceptualization, M.H. and S.H.; methodology, S.H.; patient care and investigative effort, L.G., A.M.A., P.J.C. and N.S.L.; writing—original draft preparation, S.H.; writing—review and editing, S.H. and M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This project involves the pathological features of a rare single case. The data are from a single patient and is not considered generalizable. This proposal is deemed to constitute quality improvement/healthcare oversight. Therefore, there is no requirement for IRB approval.
Informed Consent Statement
No consent is required by the IRB as this is a single case where no identifiable data are presented.
Data Availability Statement
Any data relevant to this manuscript are available by request according to regulations set forth by the KUMC research institution.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uzunoglu, I.; Guvenc, G.; Kizmazoglu, C.; Aydin, H.E.; Kaya, I.; Rezanko, T.A.; Yuceer, N. Cavernous Angioma Mimicking Meningioma. J. Craniofac. Surg. 2019, 30, e218–e220. [Google Scholar] [CrossRef]
- Oommen, A.; Pratap, T.; Chandi, S.; Jalal, M.J.A. Parasellar extra-axial cavernoma mimicking meningioma: A case report. Neuroimmunol. Neuroinflamm. 2017, 4, 16–19. [Google Scholar] [CrossRef]
- Biondi, A.; Clemenceau, S.; Dormont, D.; Deladoeuille, M.; Ricciardi, G.K.; Mokhtari, K.; Sichez, J.P.; Marsault, C. Intracranial extra-axial cavernous (HEM) angiomas: Tumors or vascular malformations? J. Neuroradiol. 2002, 29, 91–104. [Google Scholar]
- Luschka, H. Cavernöse Blutgeschwuelste des Gehirns. Arch. Path. Arch. Path Anat. 1853, 6, 458. [Google Scholar] [CrossRef]
- Vogler, R.; Castillo, M. Dural cavernous angioma: MR features. AJNR Am. J. Neuroradiol. 1995, 16, 773–775. [Google Scholar] [PubMed]
- Simonin, A.; Passaplan, C.; Sancho, S.; Rusconi, A.; Otten, P. Giant Extra-Axial Cavernous Angioma of the Falx: Case Report. Neurosurgery 2019, 84, E211–E214. [Google Scholar] [CrossRef] [PubMed]
- Kashlan, O.N.; Sack, J.A.; Ramnath, S. Cavernous Angioma of the Dural Convexity Mimicking a Meningioma. Austin Neurosurg. Open Access 2014, 1, 1019. [Google Scholar]
- Mori, H.; Koike, T.; Endo, S.; Takii, Y.; Uzuka, T.; Takahashi, H.; Ito, J.; Tanaka, R. Tentorial cavernous angioma with profuse bleeding. Case report. J. Neurosurg. Pediatr. 2009, 3, 37–40. [Google Scholar] [CrossRef]
- Robinson, J.R.; Awad, I.A.; Little, J.R. Natural history of the cavernous angioma. J. Neurosurg. 1991, 75, 709–714. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 3rd ed.; Churchill Livingstone: Edinburgh, Scotland; New York, NY, USA, 2008. [Google Scholar]
- Namba, S. Extracerebral cavernous hemangioma of the middle cranial fossa. Surg. Neurol. 1983, 19, 379–388. [Google Scholar] [CrossRef]
- Saldaña, C.J.; Zimman, H.; Alonso, P.; Mata, P.R. Neonatal cavernous hemangioma of the dura mater: Case report. Neurosurgery 1991, 29, 602–605. [Google Scholar] [CrossRef]
- Canevini, P.; Farneti, A.; Flauto, U. Report of a Case of Cavernous Hemangioma of the Dura Mater in a 2-Day Old Newborn. Folia Hered. Pathol. 1963, 12, 163–166. [Google Scholar]
- Moritake, K.; Handa, H.; Nozaki, K.; Tomiwa, K. Tentorial cavernous angioma with calcification in a neonate. Neurosurgery 1985, 16, 207–211. [Google Scholar] [CrossRef]
- Gross, B.A.; Lin, N.; Du, R.; Day, A.L. The natural history of intracranial cavernous malformations. Neurosurg. Focus 2011, 30, E24. [Google Scholar] [CrossRef]
- Lewis, A.I.; Tew, J.M.; Jr Payner, T.D.; Yeh, H.S. Dural cavernous angiomas outside the middle cranial fossa: A report of two cases. Neurosurgery 1994, 35, 498–504. [Google Scholar] [CrossRef]
- Tarabay, A.; Rocca, A.; Maeder, P.; Simonin, A.; Messerer, M.; Daniel, R.T. Extra-Axial Cavernoma of the Cerebellopontine Angle: A Case Study and Review of Literature. World Neurosurg. 2019, 128, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Yasumoto, Y.; Saeki, H.; Ito, M. Cranial dural cavernous angioma. Clin. Neuroradiol. 2014, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Sasani, M.; Bayhan, M.; Cakiroglu, G.; Ozer, A.F. Extra Axial Cavernous Hemangioma of the Cerebellopontine Angle With Intratumor Hemorrhage: Case Report and Review. Neurosurg. Q. 2010, 20, 23–26. [Google Scholar] [CrossRef]
- Kunishio, K.; Sunami, N.; Yamamoto, Y.; Satoh, T.; Asari, S.; Ohtsuki, Y. A case of convexity cavernous hemangioma associated with meningioma. No Shinkei Geka 1986, 14, 1487–1491. [Google Scholar]
- Quattrocchi, K.B.; Kissel, P.; Ellis, W.G.; Frank, E.H. Cavernous angioma of the tentorium cerebelli. Case Rep. J. Neurosurg. 1989, 71, 935–937. [Google Scholar] [CrossRef]
- Hwang, S.W.; Pfannl, R.M.; Wu, J.K. Convexity dural cavernous malformation with intradural and extradural extension mimicking a meningioma: A case report. Acta Neurochir. 2009, 151, 79–83. [Google Scholar] [CrossRef]
- Riant, F.; Bergametti, F.; Ayrignac, X.; Boulday, G.; Tournier-Lasserve, E. Recent insights into cerebral cavernous malformations: The molecular genetics of CCM. FEBS J. 2010, 277, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; O’Neill, B.R.; Pu, F.; Aziz, K. Giant tentorial cavernous hemangioma: Case report and review of literature. Clin. Neurol. Neurosurg. 2011, 113, 937–942. [Google Scholar] [CrossRef] [PubMed]
- van Lindert, E.J.; Tan, T.C.; Grotenhuis, J.A.; Wesseling, P. Giant cavernous hemangiomas: Report of three cases. Neurosurg. Rev. 2007, 30, 83–92. [Google Scholar] [CrossRef]
- Yamada, S.; Nakase, H.; Nakagawa, I.; Nishimura, F.; Motoyama, Y.; Park, Y.S. Cavernous malformations in pregnancy. Neurol. Med. Chir. 2013, 53, 555–560. [Google Scholar] [CrossRef]
- Haber, J.S.; Kesavabhotla, K.; Ottenhausen, M.; Bodhinayake, I.; Dinkin, M.J.; Segal, A.Z.; Lee, Y.M.; Boockvar, J.A. Conservative management of cavernous sinus cavernous hemangioma in pregnancy. J. Neurosurg. 2014, 120, 1309–1312. [Google Scholar] [CrossRef]
- Ishii, K.; Tanei, T.; Kato, T.; Naito, T.; Tsukamoto, E.; Okada, K.; Hasegawa, T. Dural-based Cavernous Malformation at the Temporal Convexity Presenting with Hemorrhage in a Pregnant Woman: Case Report. NMC Case Rep. J. 2021, 8, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Weigel, J.; Neher, M.; Schrey, M.; Wünsch, P.H.; Steiner, H.H. Collision Tumor Composed of Meningioma and Cavernoma. J. Korean Neurosurg. Soc. 2017, 60, 102–107. [Google Scholar] [CrossRef]
- Kilani, M.; Darmoul, M.; Hammedi, F.; Ben Nsir, A.; Hattab, M.N. Cavernous hemangioma of the skull and meningioma: Association or coincidence? Case Rep. Neurol. Med. 2015, 2015, 716837. [Google Scholar] [CrossRef]
- Dubovoy, A.V.; Jafarov, V.M.; Voronina, E.I. Supratentorial dural-based collision of cavernoma and meningioma: A case report. Chin. Neurosurg. J. 2018, 4, 17. [Google Scholar] [CrossRef]
- Bteich, F.; Kassab, C.; El Hage, G.; Moussa, R.; Abadjian, G.A.; Bou-Nassif, R. Atypical Presentation of Parietal Convexity Dural-Based Cavernous Hemangioma: A Case Report and Review of Literature. World Neurosurg. 2019, 128, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Garcia-Bengochea, F.; Ballinger, W.E., Jr.; Mickle, J.P.; Quisling, R.G. Cavernous angioma: A review of 126 collected and 12 new clinical cases. Neurosurgery 1986, 18, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Dörner, L.; Buhl, R.; Hugo, H.H.; Jansen, O.; Barth, H.; Mehdorn, H.M. Unusual locations for cavernous hemangiomas: Report of two cases and review of the literature. Acta Neurochir. 2005, 147, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Del Curling, O., Jr.; Kelly, D.L., Jr.; Elster, A.D.; Craven, T.E. An analysis of the natural history of cavernous angiomas. J. Neurosurg. 1991, 75, 702–708. [Google Scholar] [CrossRef]
- Li, G.; Zhai, X.; Zhang, Y.; Liang, P.; Wu, X.; Hou, K. Dural-Based Cavernous Malformation at the Cerebral Convexity: Report of Two Pediatric Patients. World Neurosurg. 2018, 112, 81–85. [Google Scholar] [CrossRef]
- Suzuki, K.; Kamezaki, T.; Tsuboi, K.; Kobayashi, E. Dural cavernous angioma causing acute subdural hemorrhage--case report. Neurol. Med. Chir. 1996, 36, 580–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jadik, S.; Stan, A.C.; Dietrich, U.; Pietilä, T.A.; Elsharkawy, A.E. Intraparenchymal meningioma mimicking cavernous malformation: A case report and review of the literature. J. Med. Case Rep. 2014, 8, 467. [Google Scholar] [CrossRef]
- Puca, A.; Colosimo, C.; Tirpakova, B.; Lauriola, L.; Di Rocco, F. Cavernous hemangioma extending to extracranial, intracranial, and orbital regions. Case report. J. Neurosurg. 2004, 101, 1057–1060. [Google Scholar] [CrossRef]
- Ghanta, R.K.; Tangella, P.; Koti, K.; Dandamudi, S. A rare case of an extra-axial cavernous angioma in the cerebellopontine angle. J. Neurosci. Rural. Pract. 2013, 4, 210–212. [Google Scholar]
- Shen, W.C.; Chenn, C.A.; Hsue, C.T.; Lin, T.Y. Dural cavernous angioma mimicking a meningioma and causing facial pain. J. Neuroimaging 2000, 10, 183–185. [Google Scholar] [CrossRef]
- Melone, A.G.; Delfinis, C.P.; Passacantilli, E.; Lenzi, J.; Santoro, A. Intracranial extra-axial cavernous angioma of the cerebellar falx. World Neurosurg. 2010, 74, 501–504. [Google Scholar] [CrossRef]
- Revuelta, R.; Teixeira, F.; Rojas, R.; Juambelz, P.; Romero, V.; Valdes, J. Cavernous hemangiomas of the dura mater at the convexity. Report of a case and therapeutical considerations. Neurosurg. Rev. 1994, 17, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Zabramski, J.M.; Wascher, T.M.; Spetzler, R.F.; Johnson, B.; Golfinos, J.; Drayer, B.P.; Brown, B.; Rigamonti, D.; Brown, G. The natural history of familial cavernous malformations: Results of an ongoing study. J. Neurosurg. 1994, 80, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.P.; You, C.; Mao, Q. Convexity dural cavernous haemangioma mimicking meningioma: A case report. Br. J. Neurosurg. 2016, 30, 345–347. [Google Scholar] [CrossRef]
- Eisenberg, M.B.; Al-Mefty, O.; DeMonte, F.; Burson, G.T. Benign nonmeningeal tumors of the cavernous sinus. Neurosurgery 1999, 44, 949–954. [Google Scholar] [CrossRef]
- Zhu, W.-Z.; Qi, J.-P.; Zhan, C.-J.; Shu, H.-G.; Zhang, L.; Wang, C.-Y.; Xia, L.-M.; Hu, J.-W.; Feng, D.-Y. Magnetic resonance susceptibility weighted imaging in detecting intracranial calcification and hemorrhage. Chin. Med. J. 2008, 121, 2021–2025. [Google Scholar] [CrossRef]
- Yonezawa, U.; Ikawa, F.; Hamasaki, O.; Hidaka, T.; Kurokawa, Y.; Onuma, H. A case of cavernous angioma at the convexity in the dura mater: Characteristics of images in the literature. No. Shinkei. Geka 2014, 42, 731–735. [Google Scholar]
- Kim, J.-S.; Yang, S.-H.; Kim, M.-K.; Hong, Y.-K. Cavernous Angioma in the Falx Cerebri: A Case Report. JKMS 2006, 21, 950–953. [Google Scholar] [CrossRef]
- Seo, Y.; Fukuoka, S.; Sasaki, T.; Takanashi, M.; Hojo, A.; Nakamura, H. Cavernous sinus hemangioma treated with gamma knife radiosurgery: Usefulness of SPECT for diagnosis—Case report. Neurol. Med. Chir. 2000, 40, 575–580. [Google Scholar] [CrossRef]
- Turan, A.; Unlu, H.A.; Yigit, H.; Yilmaz, O.; Yakut, Z.I. Intrasellar Cavernous Hemangioma: MRI Findings of a Very Rare Lesion. J. Med. Cases 2013, 4, 719–721. [Google Scholar] [CrossRef][Green Version]
- Rigamonti, D.; Pappas, C.T.; Spetzler, R.F.; Johnson, P.C. Extracerebral cavernous angiomas of the middle fossa. Neurosurgery 1990, 27, 306–310. [Google Scholar] [CrossRef]
- Shi, J.; Hang, C.; Pan, Y.; Liu, C.; Zhang, Z. Cavernous hemangiomas in the cavernous sinus. Neurosurgery. 1999, 45, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Zhang, X.J.; Xu, Y.Y.; Wang, W. Gamma Knife Surgery for Cavernous Sinus Hemanginoma: A Report of 32 Cases. World Neurosurg. 2016, 94, 18–25. [Google Scholar] [CrossRef]
- Chuang, C.C.; Jung, S.M.; Yang, J.T.; Chang, C.N.; Pai, P.C. Intrasellar cavernous hemangioma. J. Clin. Neurosci. 2006, 13, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.B.; Lombardi, D.; Scheithauer, B.; Nichols, D.A. Extra-axial cavernous hemangiomas involving the dural sinuses. J. Neurosurg. 1990, 73, 187–192. [Google Scholar] [CrossRef]
- Kamrin, R.B.; Buchsbaum, H.W. Large vascular malformations of the brain not visualized by serial angiography. Arch. Neurol. 1965, 13, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.E.; Liwnicz, B.H.; Mandybur, T.I. Giant pituitary cavernous hemangioma: Case report. J. Neurosurg. 1980, 53, 124–126. [Google Scholar] [CrossRef]
- Buonaguidi, R.; Canapicci, R.; Mimassi, N.; Ferdeghini, M. Intrasellar cavernous hemangioma. Neurosurgery 1984, 14, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, Y.; de Tribolet, N. Cavernous hemangioma in the cavernous sinus: Case report. Neurosurgery 1990, 26, 126–128. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Hashimoto, R.; Nagahama, S.; Nagata, Y. Intrasellar cavernous angioma in neurofibromatosis. Hum. Pathol. 1991, 22, 623–624. [Google Scholar] [CrossRef]
- Chhang, W.H.; Khosla, V.K.; Radotra, B.D.; Kak, V.K. Large cavernous haemangioma of the pituitary fossa: A case report. Br. J. Neurosurg. 1991, 5, 627–629. [Google Scholar] [CrossRef]
- Lombardi, D.; Giovanelli, M.; de Tribolet, N. Sellar and parasellar extra-axial cavernous hemangiomas. Acta Neurochir. 1994, 130, 47–54. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Wilson, C.B. Intrasellar cavernous hemangioma. Case report. J. Neurosurg. 2001, 94, 520–522. [Google Scholar] [CrossRef]
- Jeon, S.C.; Yi, J.S.; Yang, J.H.; Lee, I.W. Intrasellar Cavernous Hemangioma. J. Korean Neurosurg. Soc. 2004, 36, 163–165. [Google Scholar]
- Ma, L.C.; Li, W.Y.; Chen, W.Q.; Wu, Y.K. Intrasellar cavernous hemangioma. Neurol. India 2014, 62, 95–96. [Google Scholar] [CrossRef]
- Wu, X.; Yu, H.; Zhao, G.; Wang, L.; Liu, Y.; Li, Y. Intrasellar Cavernous Hemangioma: A Case Report and Literature Review. Transl. Neurosci. Clin. 2017, 3, 111–115. [Google Scholar] [CrossRef]
- Das, S.; Ang, L.C.; Ramsay, D. Intrasellar cavernous hemangioma presenting as pituitary adenoma: A report of two cases and review of the literature. Clin. Neuropathol. 2018, 37, 64–67. [Google Scholar] [CrossRef]
- Al-Sharydah, A.; Al-Suhibani, S.; Al-Jubran, S.; Al-Abdulwahhab, A.; Al-Bar, M.; Al-Jehani, H.; Al-Issawi, W. Endoscopic management of Atypical sellar cavernous hemangioma: A case report and review of the literature. Int. J. Surg. Case Rep. 2018, 42, 161–164. [Google Scholar] [CrossRef]
- Chibbaro, S.; Cebula, H.; Ganau, M.; Gubian, A.; Todeschi, J.; Lhermitte, B.; Proust, F.; Noel, G. Multidisciplinary management of an intra-sellar cavernous hemangioma: Case report and review of the literature. J. Clin. Neurosci. 2018, 52, 135–138. [Google Scholar] [CrossRef]
- Pan, X.; Shen, J.; Ma, Y.; Lou, H.; Weng, Y.; Zhan, R. Imaging characteristics of Intrasellar cavernous hemangioma: A case report. Medicine 2020, 99, e23405. [Google Scholar] [CrossRef]
- Al-Saiari, S.; Al-Orabi, K.; Farag, A.; Brinji, Z.; Azzouz, A.; Mohammed, T.; Mushtaq, D.; Hamouda, W. Intrasellar cavernous hemangiomas: A case report with a comprehensive review of the literature. Surg. Neurol. Int. 2021, 12, 58. [Google Scholar] [CrossRef]
- Okada, J.; Hara, M.; Takeuchi, K. Dural haemangioma with extracranial component. Acta Neurochir. 1977, 36, 111–115. [Google Scholar] [CrossRef]
- Ito, J.; Konno, K.; Sato, I.; Kameyama, S.; Takeda, S. Convexity cavernous hemangioma, its angiographic and CT findings. Report of a case (author’s transl). No Shinkei 1978, 30, 737–747. [Google Scholar]
- Perry, J.R.; Tucker, W.S.; Chui, M.; Bilbao, J.M. Dural cavernous hemangioma: An under-recognized lesion mimicking meningioma. Can. J. Neurol. Sci. 1993, 20, 230–233. [Google Scholar]
- McKechnie, S.; Harper, C.; Besser, M. Durally-based occipital cavernous haemangioma indistinguishable from meningioma. J. Clin. Neurosci. 1998, 5, 105–108. [Google Scholar] [CrossRef]
- Hyodo, A.; Yanaka, K.; Higuchi, O.; Tomono, Y.; Nose, T. Giant interdural cavernous hemangioma at the convexity: Case. illustration. J. Neurosurg. 2000, 92, 503. [Google Scholar] [CrossRef]
- Joshi, V.; Muzumdar, D.; Dange, N.; Goel, A. Supratentorial convexity dural-based cavernous hemangioma mimicking a meningioma in a child. Pediatr. Neurosurg. 2009, 45, 141–145. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Matsumori, T.; Taguchi, Y.; Koizumi, H. Supratentorial high convexity intradural extramedullary cavernous angioma: Case report. Neurol. Med. Chir. 2010, 50, 328–329. [Google Scholar] [CrossRef][Green Version]
- Di Vitantonio, H.; De Paulis, D.; Ricci, A.; Marzi, S.; Dehcordi, S.R.; Galzio, R.J. Cavernous hemangioma of the dura mater mimicking meningioma. Surg. Neurol. Int. 2015, 6 (Suppl. 13), S375–S378. [Google Scholar]
- Pelluru, P.K.; Rajesh, A.; Uppin, M.S. Dural-based giant cavernous hemangioma mimicking a meningioma: Lessons learnt. Neurol. India 2017, 65, 1173–1176. [Google Scholar]
- Bhide, A.; Velho, V.; Pujari, M.; Jain, N. Dural-Based Cavernous Hemangioma Mimicking Convexity Meningioma—Case Report. Indian J. Neurosurg. 2018, 7, 280–283. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Ma, L.; Wu, C.; Zhao, Y.; Jiang, Z. Multiple nodular dural cavernous angiomas occluding superior sagittal sinus and destructing calvarium: Case report and literature review. J. Clin. Neurosci. 2018, 58, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, L. Considerazioni su un caso di angioma della falce. Riv. Patol. Nerv. Ment. 1947, 68, 214–226. [Google Scholar]
- Kaga, A.; Isono, M.; Mori, T.; Kusakabe, T.; Okada, H.; Hori, S. Cavernous angioma of falx cerebri; case report. No Shinkei Geka 1991, 19, 1079–1083. [Google Scholar] [PubMed]
- McCormick, W.F.; Boulter, T.R. Vascular malformations (“angiomas”) of the dura mater. J. Neurosurg. 1966, 25, 309–311. [Google Scholar] [CrossRef]
- Huber, P. Vascular abnormalities and vascular tumors of the A. carotis externa and of the dura. Fortschr. Geb. Rontgenstr. Nuklearmed. 1968, 109, 325–335. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kikuchi, H.; Nagata, I.; Yamagata, S. A case of tentorial cavernous angioma. No Shinkei Geka 1988, 16, 403–407. [Google Scholar] [PubMed]
- Lee, A.G.; Parrish, R.G.; Goodman, J.C. Homonymous hemianopsia due to a dural cavernous hemangioma. J. Neuroophthalmol. 1998, 18, 250–254. [Google Scholar] [CrossRef]
- Yoshimura, J.; Tsukamoto, Y.; Sano, M.; Hasegawa, H.; Nishino, K.; Saito, A.; Fukuda, M.; Okamoto, K.; Fujii, Y. Successful removal of a huge hypervascular tentorial cavernous angioma after preoperative endovascular embolization. J. Neurosurg. Pediatr. 2014, 14, 43–47. [Google Scholar] [CrossRef]
- Iplikçioğlu, A.C.; Benli, K.; Bertan, V.; Ruacan, S. Cystic cavernous hemangioma of the cerebellopontine angle: Case report. Neurosurgery 1986, 19, 641–642. [Google Scholar] [CrossRef]
- Goel, A.; Achwal, S.; Nagpal, R.D. Dural cavernous haemangioma of posterior cranial fossa. J. Postgrad. Med. 1993, 39, 222–223. [Google Scholar] [PubMed]
- Brunori, A.; Chiappetta, F. Cystic extra-axial cavernoma of the cerebellopontine angle. Surg. Neurol. 1996, 46, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rowed, D.W.; Cheung, G.; Ang, L.C. Cavernous malformation presenting as an extra-axial cerebellopontine angle mass: Case report. Neurosurgery 1997, 40, 187–190. [Google Scholar] [PubMed]
- Ferrante, L.; Acqui, M.; Trillò, G.; Antonio, M.; Nardacci, B.; Celli, P. Cavernous angioma of the VIIIth cranial nerve. A case report. Neurosurg. Rev. 1998, 21, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Vajramani, G.V.; Devi, B.I.; Hegde, T.; Srikanth, S.G.; Shankar, S.K. Cystic cavernous malformation of the cerebellopontine angle. Clin. Neurol. Neurosurg. 1998, 100, 133–137. [Google Scholar] [CrossRef]
- Beskonakli, E.; Kaptanoglu, E.; Okutan, O.; Solaroglu, I.; Taskin, Y. Extra-axial cavernomas of the cerebellopontine angle involving the seventh-eighth nerve complex. Neurosurg. Rev. 2002, 25, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.R.; Albuquerque, F.C.; Zabramski, J.M.; Spetzler, R.F. Surgical management of cavernous malformations involving the cranial nerves. Neurosurgery 2003, 53, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.B.; Johnson, M.D.; Thompson, R.C. Cystic cavernous malformation of the cerebellopontine angle. Case illustration. J. Neurosurg. 2005, 103, 931. [Google Scholar] [CrossRef]
- Albanese, A.; Sturiale, C.L.; D’Alessandris, Q.G.; Capone, G.; Maira, G. Calcified extra-axial cavernoma involving lower cranial nerves: Technical case report. Neurosurgery 2009, 64 (Suppl. 3), onsE135–onsE136. [Google Scholar] [CrossRef]
- Engh, J.A.; Kostov, D.; Martin MB, S.; Yeaney, G.; Rothfus, W.; Hirsch, B.; Kassam, A.B. Cavernous malformation tumors: A case study and review of the literature. Otol. Neurotol. 2010, 31, 294–298. [Google Scholar] [CrossRef]
- Huang, H.; Xu, K.; Qu, L.; Li, Y.; Yu, J. Cystic cavernous malformation of the cerebellopontine angle: Case report and literature review. World J. Surg. Oncol. 2011, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Wada, K.; Sakakibara, F.; Takeuchi, S.; Nagatani, K. A cystic haemorrhagic lesion located in the cerebellopontine angle cistern. J. Clin. Neurosci. 2012, 19, 1608. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, W.; Zhao, Y. Coexistence of extra-axial cavernous malformation and cerebellar developmental venous anomaly in the cerebellopontine angle. World Neurosurg. 2012, 78, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Kamiyama, H.; Nakamura, T.; Nakajima, H.; Tokugawa, J. Dural cavernous hemangioma of the cerebellar falx. Neurol. Med. Chir. 2009, 49, 410–412. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).