Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential
Abstract
1. Anthocyanins: Chemistry and Occurrence
1.1. Biosynthesis of Anthocyanins
1.2. Chemistry of Anthocyanins
1.3. Stability of Anthocyanins
1.3.1. The Effect of Temperature on Anthocyanin Stability
1.3.2. The Effect of Light on Anthocyanin Stability
1.3.3. The Effect of Storage Time on Anthocyanin Stability
1.3.4. The Effect of the Other Prominent Factors on Anthocyanin Stability
2. Materials
3. Metabolic Digestion, Bioavailability, and Therapeutic Effects under Nutraceutical and Pharmaceutical Perspectives
3.1. Metabolic Digestion and Bioavailability of Anthocyanins
| Cell Models (In Vitro) | ||||||
|---|---|---|---|---|---|---|
| Cell Model | Anthocyanin Source | Anthocyanin Dose | Duration (h) | AUC(3) | Transport Efficiency | References |
| MKN-28 (gastric cell) | Red wine extract | 200 μM | 3 | 4–9% | [63] | |
| Caco-2 (intestinal cell) | Red wine extract | 200 μM | 3 | 3–5% | [63] | |
| MKN-28 (gastric cell) | Grape skin extract Mv3glc, vitisin A, oxovitisin, methylpyrano-Mv3glc | 100 μM | 3 | 5–7% | [68] | |
| MKN-28 (gastric cell) | Red wine extract | 50 μg/mL | 3 | 4–8% | [69] | |
| MKN-28 (gastric cell) | Commercial standard | 500 μM | 3 | 6.38–10.44% | [59] | |
| Dp 3-O-glucoside | ||||||
| Cy 3-O-glucoside | ||||||
| Mv 3-O-glucoside | ||||||
| Caco-2 (intestinal cell) | Grape | 1766.1 μg/mL | 4 | 0.35% (Mv3glc) | [70] | |
| Caco-2 (intestinal cell) | Grape/blueberry extract | 2613 μM/L | 1.5 | 0.005–0.06% | [71] | |
| Human studies | ||||||
| Anthocyanin Source (Intake) | Anthocyanin Dose (Total Intake) | Cmax (1) | Tmax(h) (2) | AUC (3) | UrinaryExcretion | References |
| Purple wheat bars (160 g) | 6.7 mg | 6.1 μM | 0–2 | 3.8 nmol × h/L | 0.19% | [64] |
| Purple wheat crackers (120 g) | 6.7 mg | 4.5 μM | 0–2 | 3.7 nmol × h/L | 0.19% | [64] |
| Blackcurrant extract | Dp 3-O-rutinoside: 290 µMol | 8.6 nmol/L | 1.5 | 30.5 nmol × h/L | [72] | |
| Cy 3-O-rutinoside: 273 µMol | 9.8 nmol/L | 1.4 | 30.8 nmol × h/L | |||
| Table red wine (250 mL) | 221.86 mg | 32.29 mg/mL | 2.0 | [73] | ||
| Young port wine (150 mL) | 48.94 mg | 5.90 mg/mL | 1.5 | [73] | ||
| Aronia berry extract (500 mg) | Cy 3-O-galactoside: 32.52 mg | 0.004 mg/mg | 4.67 | 0.016 mg × h/mg | [74] | |
| Cy 3-O-glucoside | 0.010 mg/mg | 6.00 | 0.118 mg × h/mg | |||
| Cy 3-O-arabinoside: 11.72 mg | 0.020 mg/mg | 4.00 | 0.088 mg × h/mg | |||
| Dealcoholized red wine (100 mL) | 22.1 mg | 7.01 nmol | 0.5 | [75] | ||
| Strawberry juice (34.7 mg) | Cy 3-O-glucoside: 7.8 µMol | 0.6 nmol/L | 2.1 | 1.7 nmol × h/L(10 h) | [76] | |
| Pg glucuronide | 38.0 nmol/L | 1.7 | 123.8 nmol × h/L(10 h) | |||
| Pg-3-O-glucoside: 58.8 µMol | 5.2 nmol/L | 1.3 | 15.0 nmol × h/L(10 h) | |||
| Pg 3-O-rutinoside: 9.7 µMol | 0.4 nmol/L | 1.9 | 1.4 nmol × h/L(10 h) | |||
| Σ = 76.6 µMol | ||||||
| Tart cherry juice (60 mL) | 62.47 mg/L | 2.75 µg × h/mL | 1 | 106.4 µg × h/mL | [77] | |
| Grape/blueberry juice (330 mL) | 3,4-dihydroxybenzoic acid | 7.6 nmol/L | 1 | 568 nmol × min/L | [71] | |
| Cy 3-O-glucoside | 0.10 nmol/L | 1 | 6 nmol × min/L | |||
| Dp 3-O-glucoside | 0.18 nmol/L | 1.1 | 10 nmol × min/L | |||
| Mv 3-O-glucoside | 1.5 nmol/L | 1.1 | 103 nmol × min/L | |||
| Mv 3-O-glucuronide | 1.1 nmol/L | 2 | 114 nmol × min/L | |||
| Pn 3-O-glucuronide | 1.1 nmol/L | 1.8 | 114 nmol × min/L | |||
| Pn 3-O-glucoside | 1.7 nmol/L | 1 | 52 nmol × min/L | |||
| Pt 3-O-glucoside | 0.8 nmol/L | 1 | 12 nmol × min/L | |||
| Σ = 841 mg/L | 1.21 nmol/L | |||||
| Red raspberries (300 g) | 292 µMol | 0.1–180 nmol/L | 1–1.5 | 0.007% (1–1.5 h) | [65] |
3.2. Therapeutic Effects of Anthocyanins
| Disease | Pathophysiology of Disease | Effect of Anthocyanins | References | |||
|---|---|---|---|---|---|---|
| ACNs Source | Dose | Animal Model/Cell Line | Effect | |||
| Neurodegenerative: Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) | Neuron loss is generally associated with oxidative stress, neuroinflammation, and excitotoxicity that triggered the macromolecule oxidations, mitochondrial dysfunctions, deposition of protein aggregates (amyloid-β, α-synuclein, and DNA-binding protein-43, etc.), and calcium overloads by the over stimulation of glutamate receptors. | Black mulberry extract | 500 µg/mL | Drosophila model of AD | Reduced the amyloid-β formation and enhanced motor dysfunctions by inhibiting BACE (beta-secretase)-1 activity. | [101] |
| Grape skin extracts rich in Del 3-glu and Mal 3,5-di glu | 50 mg/kg | Senescence-accelerated prone mice 8 (SAMP8) model of AD | Improved spatial learning and memory. | [102] | ||
| Blueberry extract | 50–100 mg/kg | MPDP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced PD mice | Enhanced motor coordination and increased brain contents of dopamine, tyrosine hydroxylase, SOD, and GPx. | [103] | ||
| Protocatechuic acid as a derivative of cyn 3-glu | 100 mg/kg | hSOD1 G93A mouse model of ALS | Extend the survival, improved the balance and motor function, and reduced the biomarkers of oxidative stress and neuroinflammation. | [104] | ||
| Cardiovascular: Coronary artery disease (CAD), cerebrovascular disease (CVD), peripheral artery disease (PAD), and aortic atherosclerosis. | Lessened or lacking blood flow throughout the blood vessels prompted by atherosclerotic plaques, arterial stiffness, and endothelial dysfunction. | Key mechanisms of anthocyanins include: (1) Lipid metabolism, for example: lower serum triglycerides, total- and LDL-cholesterols, increase HDL-cholesterol; (2) Improve endothelial function; (3) Decreased oxidative stress, lipid peroxidation, and inflammatory gene expression. | [89,104,105] | |||
| Purple sweet potato anthocyanins | 100–200 mg/kg/day | Mice model with doxorubicin-induced cardiotoxicity | Reduced inflammatory factors (TNF-α and nitric oxide), level of myocardial enzymes (lactic dehydrogenase and creatine kinase), trimethylamine-N-oxide as a risk factor of cardiovascular damage in serum and heart tissue. | [91] | ||
| Diabetes | Long-term metabolic disorder characterized by high blood glucose, insulin hormone level, and insulin resistance in the body. Type 2/insulin resistant diabetes are common. | Therapeutic potential of anthocyanins related to lower hyperglycemia and glycosylated hemoglobin (HbA1c) levels; regulate digestive enzymes (α-amylase and α-glucosidase) via binding their catalytic cites; protection of pancreatic β cells due to anti-inflammatory and antioxidant properties, etc. | [106] | |||
| Pg 3-glu from wild raspberry | 150 mg/kg | db/db diabetic mice model | Show hyperglycemia-lowering effect by modifying the gut microbiota composition and support the intestinal barrier function. Increased the short-chain fatty acids (especially acetic, propionic, butyric and valeric acids) as a part of their protective action. | [107] | ||
| Obesity | Energy imbalance is a primary cause triggered by high energy unbalanced diet, and sedentary life.Epigenetic susceptibility, and oxidative stress, and inflammation in adipose tissue are other major factors. Excessive adiposity advances to comorbidities, including type 2 diabetes, hypertension, cardiovascular disorders, inflammatory bowel disease, AD, PD, cancer, etc. (Sivamaruthi et al. 2020). | Sweet cherry anthocyanins | 40–200 mg/kg | High-fat dieted C57BL/6 mice | Decreased body weight, adipocytes size, serum parameters (glucose, triglyceride, total cholesterol, LDL-cholesterol), liver triglycerides, and in the hepatic lipids, expression of cytokines (IL-6 and TNF-α) reduced, and antioxidant enzyme activities (SOD and GPx) increased. | [94] |
| Purple and black wheat anthocyanins | 45 and 1575 µg/day | High-fat diet (HFD) induced obese mice | Lower weight gain and fat pad weight; enhanced lipid homeostasis with lower serum lipid parameters (triglyceride, total cholesterol, LDL-cholesterol); higher glucose tolerance and insulin resistance in adipose tissues; upregulated expression of β-oxidation marker genes coding anti-oxidative enzymes. | [108] | ||
| Cancer | A genetic disease consists of many types (carcinomas, leukemia, lymphoma, sarcoma, melanoma, etc.) mainly characterized by abnormal cell proliferation that can damage normal body tissues. Genetic changes mainly initiated by oxidative stress alter cancer-causing genes (oncogenes), tumor suppressor genes (anti-oncogenes), and DNA repair genes that controlling cell growth, division, and mutations that lead to cancer pathogenesis. | Pure Cyn 3-glu | 0.4 mg/mL | Drosophila model with a malignant tumor | Suppressed the tumor growth and metastasis of tumor cells. | [109] |
| Anthocyanins from the fruits of Vitis coignetiae Pulliat (AIMs) | 100 µg/mL | Hep3B human hepatocellular carcinoma cells | Inhibited the cell proliferation and invasion. | [93] | ||
| 50 µg/g | Athymic nude mouse model with Hep3B xenograft tumor | Reduce tumor growth and inhibited the activation of NF-κB pathway and expression of their proteins (cyclin D1, COX-2, MMP (mitochondrial membrane potential)-2, MMP-9, and Bc1-xL) that involve in proliferation, metastasis, and anti-apoptosis of tumor cells. | ||||
| Retinal degeneration | Age-related macular degeneration triggered by photooxidations of retinal cells results in blurred vision and advanced vision loss. | Bilberry anthocyanin extract | 500 mg/kg | Light induced retinal damaged rabbit model | Show protective effect on retina via down-regulate the photooxidation-induced expression of inflammatory cytokine (IL-6) and inflammatory response of NF-κB pathway; up-regulate the heme oxygenase-1 expression. | [98] |
4. Industrial/Technological Applications of Anthocyanins
4.1. Anthocyanins As Natural Dyes in the Food Industry
4.2. Applications of Anthocyanins as Prebiotics Ingredients
4.3. Innovation Potential of Anthocyanins in the Industrial Fields
5. Sustainable Sources of Anthocyanins
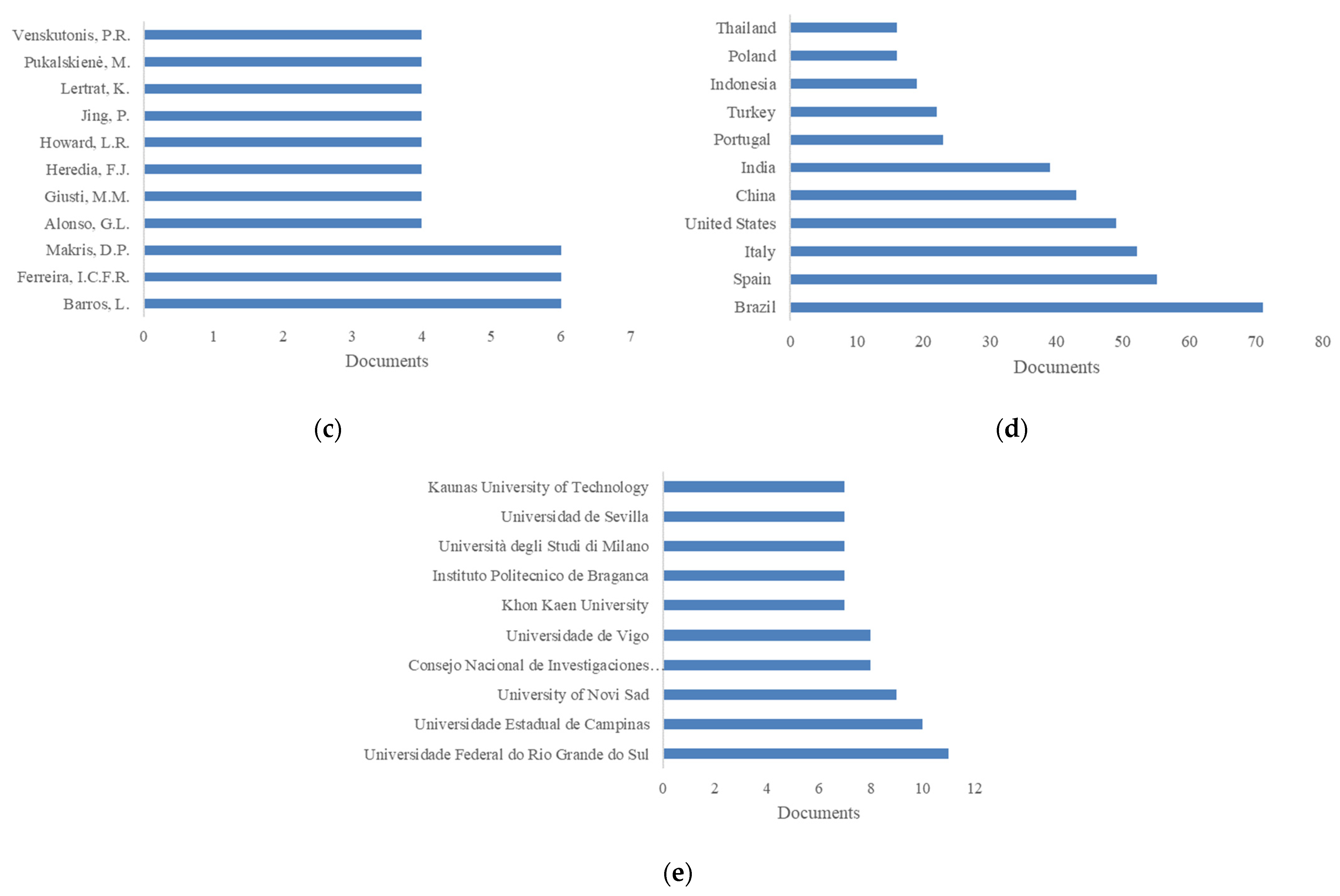
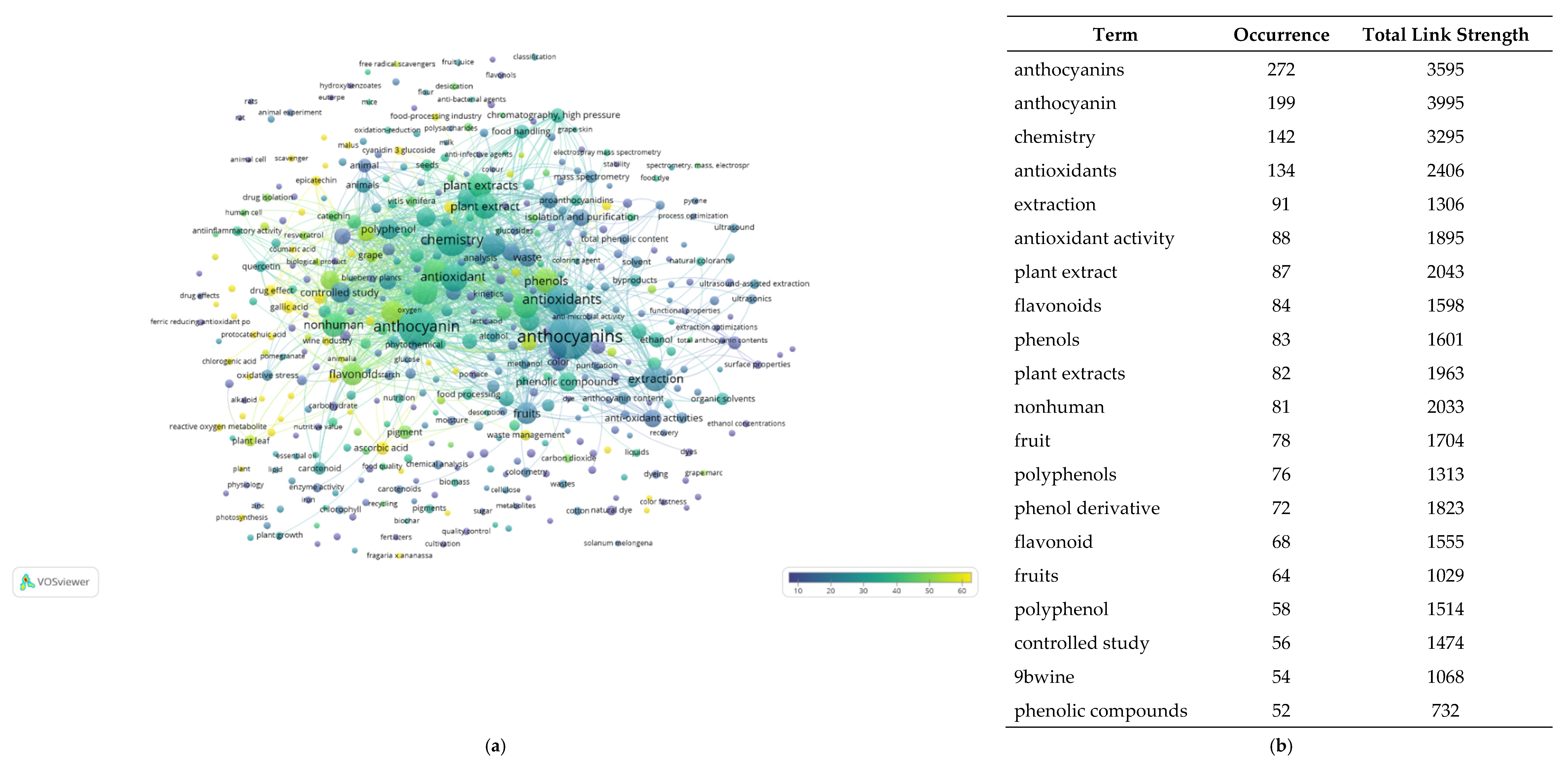
5.1. Sustainable Sources of Anthocyanins: Quantitative Research Literature Analysis
5.2. Anthocyanins and Databases: A Picture of the State of the Art
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chaves-Silva, S.; Santos, A.L.d.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.P.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants—Tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef]
- Filip, M.; Vlassa, M.; Copaciu, F.; Coman, V. Identification of anthocyanins and anthocyanidins from berry fruits by chromatographic and spectroscopic techniques to establish the juice authenticity from market. JPC-J. Planar Chromatogr.-Mod. TLC 2012, 25, 534–541. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally Occurring Anthocyanin, Structure, Functions and BiosyntheticPathway in Fruit Plants. J. Plant Biochem. Physiol. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Z.; Guan, L.; Zheng, T.; Jiu, S.; Zhu, X.; Jia, H.; Fang, J. Changes of Anthocyanin Component Biosynthesis in ‘Summer Black’ Grape Berries after the Red Flesh Mutation Occurred. J. Agric. Food Chem. 2018, 66, 9209–9218. [Google Scholar] [CrossRef]
- Manela, N.; Oliva, M.; Ovadia, R.; Sikron-Persi, N.; Ayenew, B.; Fait, A.; Galili, G.; Perl, A.; Weiss, D.; Oren-Shamir, M. Phenylalanine and tyrosine levels are rate-limiting factors in production of health promoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front Plant Sci. 2015, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. MATE Transporters Facilitate Vacuolar Uptake of Epicatechin 3′-O-Glucoside for Proanthocyanidin Biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef]
- Kallam, K.; Appelhagen, I.; Luo, J.; Albert, N.; Zhang, H.; Deroles, S.; Hill, L.; Findlay, K.; Andersen, Ø.M.; Davies, K.; et al. Aromatic Decoration Determines the Formation of Anthocyanic Vacuolar Inclusions. Curr. Biol. 2017, 27, 945–957. [Google Scholar] [CrossRef]
- Chanoca, A.; Kovinich, N.; Burkel, B.; Stecha, S.; Bohorquez-Restrepo, A.; Ueda, T.; Eliceiri, K.W.; Grotewold, E.; Otegui, M.S. Anthocyanin Vacuolar Inclusions Form by a Microautophagy Mechanism. Plant Cell 2015, 27, 2545–2559. [Google Scholar] [CrossRef]
- Casañal, A.; Zander, U.; Muñoz, C.; Dupeux, F.; Luque, I.; Botella, M.A.; Schwab, W.; Valpuesta, V.; Marquez, J.A. The strawberry pathogenesis-related 10 (PR-10) Fra a proteins control flavonoid biosynthesis by binding to metabolic intermediates. J. Biol. Chem. 2013, 288, 35322–35332. [Google Scholar] [CrossRef]
- Jia, D.; Li, Z.; Dang, Q.; Shang, L.; Shen, J.; Leng, X.; Wang, Y.; Yuan, Y. Anthocyanin Biosynthesis and Methylation of the MdMYB10 Promoter Are Associated with the Red Blushed-Skin Mutant in the Red Striped-Skin “Changfu 2” Apple. J. Agric. Food Chem. 2020, 68, 4292–4304. [Google Scholar] [CrossRef]
- Khusnutdinov, E.; Sukhareva, A.; Panfilova, M.; Mikhaylova, E. Anthocyanin Biosynthesis Genes as Model Genes for Genome Editing in Plants. Int. J. Mol. Sci. 2021, 22, 8752. [Google Scholar] [CrossRef]
- Enaru, B.; Socaci, S.; Farcas, A.; Socaciu, C.; Danciu, C.; Stanila, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Amp Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Fu, M.; Tang, W.; Liu, J.J.; Gong, X.Q.; Kong, L.; Yao, X.M.; Jing, M.; Cai, F.Y.; Li, X.T.; Ju, R.J. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Daravingas, G.; Cain, R.F. Changes in the Anthocyanin Pigments of Raspberries During Processing and Storage. J. Food Sci. 1965, 30, 400–405. [Google Scholar] [CrossRef]
- Kırca, A.; Cemeroğlu, B. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chem. 2003, 81, 583–587. [Google Scholar] [CrossRef]
- Debicki-Pospišil, J.L.T.; Trinajstić, N.; Sabljić, A. Anthocyanin Degradation in the Presence of Furfural and 5-Hydroxymethylfurfural. J. Food Sci. 1983, 48, 411–416. [Google Scholar] [CrossRef]
- Markaris, P.; Livingston, G.E.; Fellers, C.R. Quantitative aspects of strawberry pigment degradation a,b. J. Food Sci. 1957, 22, 117–130. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation Kinetics of Anthocyanin in Blueberry Juice during Thermal Treatment. J. Food Sci. 2010, 75, C173–C176. [Google Scholar] [CrossRef]
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of pasteurization process and storage on color and shelf-life of pomegranate juices. LWT-Food Sci. Technol. 2013, 54, 592–596. [Google Scholar] [CrossRef]
- Ziabakhsh Deylami, M.; Abdul Rahman, R.; Tan, C.P.; Bakar, J.; Olusegun, L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: A kinetic study. J. Food Eng. 2016, 178, 12–19. [Google Scholar] [CrossRef]
- Verbeyst, L.; Crombruggen, K.V.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Anthocyanin degradation kinetics during thermal and high pressure treatments of raspberries. J. Food Eng. 2011, 105, 513–521. [Google Scholar] [CrossRef]
- Dubrović, I.; Herceg, Z.; Režek Jambrak, A.; Badanjak, M.; Dragović-Uzelac, V. Effect of high intensity ultrasound and pasteurization on anthocyanin content in strawberry juice. Food Technol. Biotechnol. 2011, 49, 196–204. [Google Scholar]
- Gu, K.-D.; Wang, C.-K.; Hu, D.-G.; Hao, Y.-J. How do anthocyanins paint our horticultural products? Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Janna, O.A.; Khairul, A.K.; Maziah, M. Anthocyanin stability studies in Tibouchina semidecandra L. Food Chem. 2007, 101, 1640–1646. [Google Scholar] [CrossRef]
- Bakhshayeshi, M.A.; Khayami, M.; Heidari, R.; Jamei, R. The effects of light, storage temperature, pH and variety on stability of anthocyanin pigments in four Malus varieties. Pak. J. Biol. Sci. 2006, 9, 428–433. [Google Scholar] [CrossRef]
- Roobha, J.; Marappan, S.; Aravindhan, K.M.; Devi, P.S. The effect of light, temperature, pH on stability of anthocyanin pigments in Musa acuminata bract. Res. Plant Biol. 2011, 1, 5–12. [Google Scholar]
- Ochoa, M.R.; Kesseler, A.G.; Vullioud, M.B.; Lozano, J.E. Physical and Chemical Characteristics of Raspberry Pulp: Storage Effect on Composition and Color. LWT-Food Sci. Technol. 1999, 32, 149–153. [Google Scholar] [CrossRef]
- Morais, H.; Ramos, C.; Forgács, E.; Cserháti, T.; Oliviera, J. Influence of storage conditions on the stability of monomeric anthocyanins studied by reversed-phase high-performance liquid chromatography. J. Chromatogr. B 2002, 770, 297–301. [Google Scholar] [CrossRef]
- Sui, X.; Bary, S.; Zhou, W. Changes in the color, chemical stability and antioxidant capacity of thermally treated anthocyanin aqueous solution over storage. Food Chem. 2016, 192, 516–524. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Woodward, G.; Kroon, P.; Cassidy, A. Anthocyanin Stability and Recovery: Implications for the Analysis of Clinical and Experimental Samples. J. Agric. Food Chem. 2009, 57, 5271–5278. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Kruszewski, B.; Skąpska, S. The Effect of High Pressure Techniques on the Stability of Anthocyanins in Fruit and Vegetables. Int. J. Mol. Sci. 2017, 18, 277. [Google Scholar] [CrossRef]
- Sarni, P.; Fulcrand, H.; Souillol, V.; Souquet, J.-M.; Cheynier, V. Mechanisms of anthocyanin degradation in grape must-like model solutions. J. Sci. Food Agric. 1995, 69, 385–391. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in Anthocyanins and Polyphenolics During Juice Processing of Highbush Blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [Google Scholar] [CrossRef]
- Jaiswal, V.; DerMarderosian, A.; Porter, J.R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L.). Food Chem. 2010, 118, 11–16. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.-Y.; Kim, B.G.; Cha, S.W.; Jeong, E.J.; Kerr, W.L.; Choi, S.-G. Thermal processing under oxygen–free condition of blueberry puree: Effect on anthocyanin, ascorbic acid, antioxidant activity, and enzyme activities. Food Chem. 2021, 342, 128345. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Ratanapoompinyo, J.; Nguyen, L.T.; Devkota, L.; Shrestha, P. The effects of selected metal ions on the stability of red cabbage anthocyanins and total phenolic compounds subjected to encapsulation process. J. Food Process. Preserv. 2017, 41, e13234. [Google Scholar] [CrossRef]
- Robert, P.; Fredes, C. The Encapsulation of Anthocyanins from Berry-Type Fruits. Trends Foods Mol. 2015, 20, 5875–5888. [Google Scholar]
- Cai, X.; Du, X.; Cui, D.; Wang, X.; Yang, Z.; Zhu, G. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. 2019, 91, 238–245. [Google Scholar] [CrossRef]
- Tan, C.; Celli, G.B.; Abbaspourrad, A. Copigment-polyelectrolyte complexes (PECs) composite systems for anthocyanin stabilization. Food Hydrocoll. 2018, 81, 371–379. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Passamonti, S. Role of the Stomach in Anthocyanin Absorption. Anthocyanins Nat. Sources Exploit. Target. Deliv. Improv. Health 2019, 12, 216–246. [Google Scholar]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2020, 9, 2. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- Lang, Y.; Tian, J.; Meng, X.; Si, X.; Tan, H.; Wang, Y.; Shu, C.; Chen, Y.; Zang, Z.; Zhang, Y.; et al. Effects of α-Casein on the Absorption of Blueberry Anthocyanins and Metabolites in Rat Plasma Based on Pharmacokinetic Analysis. J. Agric. Food Chem. 2021, 69, 6200–6213. [Google Scholar] [CrossRef]
- Koh, J.; Xu, Z.; Wicker, L. Blueberry pectin and increased anthocyanins stability under in vitro digestion. Food Chem. 2020, 302, 125343. [Google Scholar] [CrossRef] [PubMed]
- Ribnicky, D.M.; Roopchand, D.E.; Oren, A.; Grace, M.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Oliveira, H.; Brás, N.F.; Fernandes, I.; Cruz, L.; De Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins—Impact of structural complexity and phase II metabolization. Food Chem. 2020, 317, 126398. [Google Scholar] [CrossRef]
- Gamel, T.H.; Wright, A.J.; Tucker, A.J.; Pickard, M.; Rabalski, I.; Podgorski, M.; Di Ilio, N.; O’Brien, C.; Abdel-Aal, E.-S.M. Absorption and metabolites of anthocyanins and phenolic acids after consumption of purple wheat crackers and bars by healthy adults. J. Cereal Sci. 2019, 86, 60–68. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free. Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef] [PubMed]
- Fornasaro, S.; Ziberna, L.; Gasperotti, M.; Tramer, F.; Vrhovšek, U.; Mattivi, F.; Passamonti, S. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci. Rep. 2016, 6, 22815. [Google Scholar] [CrossRef]
- Oliveira, H.; Wu, N.; Zhang, Q.; Wang, J.; Oliveira, J.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Bioavailability studies and anticancer properties of malvidin based anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food Funct. 2016, 7, 2462–2468. [Google Scholar] [CrossRef]
- Oliveira, H.; Fernandes, I.; Brás, N.F.; Faria, A.; De Freitas, V.; Calhau, C.; Mateus, N. Experimental and Theoretical Data on the Mechanism by Which Red Wine Anthocyanins Are Transported through a Human MKN-28 Gastric Cell Model. J. Agric. Food Chem. 2015, 63, 7685–7692. [Google Scholar] [CrossRef]
- Kuntz, S.; Asseburg, H.; Dold, S.; Römpp, A.; Fröhling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, T.; Kirsch, V.; Schipp, D.; Galan, J.; Richling, E. Absorption of Anthocyanin Rutinosides after Consumption of a Blackcurrant (Ribes nigrum L.) Extract. J. Agric. Food Chem. 2019, 67, 6792–6797. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, C.; Évora, A.; Cruz, L.; de Freitas, V.; Calhau, C.; Faria, A.; Mateus, N. Pharmacokinetics of table and Port red wine anthocyanins: A crossover trial in healthy men. Food Funct. 2017, 8, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lee, S.G.; Vance, T.M.; Wang, Y.; Kim, B.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of aronia berry extract. Food Chem. 2016, 211, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Motilva, M.-J.; Macià, A.; Romero, M.-P.; Rubió, L.; Mercader, M.; González-Ferrero, C. Human bioavailability and metabolism of phenolic compounds from red wine enriched with free or nano-encapsulated phenolic extract. J. Funct. Foods 2016, 25, 80–93. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Huang, Y.; Xiao, D.; Park, E.; Edirisinghe, I.; Burton-Freeman, B. Pharmacokinetic Characterization and Bioavailability of Strawberry Anthocyanins Relative to Meal Intake. J. Agric. Food Chem. 2016, 64, 4891–4899. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical uptake following human consumption of Montmorency tart cherry (L Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar]
- Sigurdson, G.T.; Giusti, M.M. Chapter 7. The Stability and Absorption of Anthocyanins in the Mouth. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef]
- Atnip, A.A.; Sigurdson, G.T.; Bomser, J.; Giusti, M.M. Time, Concentration, and pH-Dependent Transport and Uptake of Anthocyanins in a Human Gastric Epithelial (NCI-N87) Cell Line. Int. J. Mol. Sci. 2017, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, B.N.; Oprea, E.; Mititelu, M.; Ruta, L.L.; Farcasanu, I.C. Dietary Anthocyanins and Stroke: A Review of Pharmacokinetic and Pharmacodynamic Studies. Nutrients 2019, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono- and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health, in Oxidative Stress: Diagnostics, Prevention, and Therapy; American Chemical Society: New York, NY, USA, 2011; pp. 1–37. [Google Scholar]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of dietary anthocyanins on biomarkers of oxidative stress and antioxidative capacity: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2020, 68, 103912. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Tang, S.; Kan, J.; Sun, R.; Cai, H.; Hong, J.; Jin, C.; Zong, S. Anthocyanins from purple sweet potato alleviate doxorubicin-induced cardiotoxicity in vitro and in vivo. J. Food Biochem. 2021, 45, e13869. [Google Scholar] [CrossRef]
- Tian, J.; Si, X.; Wang, Y.; Gong, E.; Xie, X.; Zhang, Y.; Shu, C.; Li, B. Cyanidin-3-O-glucoside protects human gastric epithelial cells against Helicobacter pylori lipopolysaccharide-induced disorders by modulating TLR-mediated NF-κB pathway. J. Funct. Foods 2020, 68, 103899. [Google Scholar] [CrossRef]
- Kim, M.J.; Paramanantham, A.; Lee, W.S.; Yun, J.W.; Chang, S.H.; Kim, D.C.; Park, H.S.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins Derived from Vitis coignetiae Pulliat Contributes Anti-Cancer Effects by Suppressing NF-κB Pathways in Hep3B Human Hepatocellular Carcinoma Cells and In Vivo. Molecules 2020, 25, 5445. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, Q.; Yu, Z.; Gao, Z.; Hu, H.; Chen, W.; Zheng, X.; Yu, T. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int. J. Food Sci. Nutr. 2014, 65, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, J.; Zhang, Q.; Tian, J.; Wang, Y.; Meng, X. Cyanidin-3-glucoside attenuates silica-induced pulmonary inflammatory responses by modulating T cell immune responses and STAT1/STAT3 signaling. J. Funct. Foods 2020, 68, 103911. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Sun, H.; Qi, W.; Li, A. Bilberry anthocyanin-rich extract protects against retinal photooxidative damage via activation of HO-1 and inhibition of NF-κB. Food Agric. Immunol. 2019, 30, 829–840. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Wu, K.; Sun, Z.; Tang, Z.; Li, X.; Zhang, B. Anthocyanins’ effects on diabetes mellitus and islet transplantation. Crit. Rev. Food Sci. Nutr. 2022, 1–24. [Google Scholar] [CrossRef]
- Additives, E.P.o.F.; Food, N.S.a.t. Scientific Opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013, 11, 3145. [Google Scholar]
- Suttisansanee, U.; Charoenkiatkul, S.; Jongruaysup, B.; Tabtimsri, S.; Siriwan, D.; Temviriyanukul, P. Mulberry Fruit Cultivar ‘Chiang Mai’ Prevents Beta-Amyloid Toxicity in PC12 Neuronal Cells and in a Drosophila Model of Alzheimer’s Disease. Molecules 2020, 25, 1837. [Google Scholar] [CrossRef]
- Sasaki, K.; Geribaldi-Doldan, N.; Szele, F.G.; Isoda, H. Grape skin extract modulates neuronal stem cell proliferation and improves spatial learning in senescence-accelerated prone 8 mice. Aging 2021, 13, 18131–18149. [Google Scholar] [CrossRef]
- Qian, F.; Wang, M.; Wang, J.; Lu, C. Anthocyanin-Rich Blueberry Extract Ameliorates the Behavioral Deficits of MPTP-Induced Mouse Model of Parkinson¡¯s Disease via Anti-Oxidative Mechanisms. Yangtze Med. 2019, 3, 7. [Google Scholar] [CrossRef][Green Version]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2021, 77, 109–131. [Google Scholar] [CrossRef]
- Su, H.; Xie, L.; Xu, Y.; Ke, H.; Bao, T.; Li, Y.; Chen, W. Pelargonidin-3-O-glucoside Derived from Wild Raspberry Exerts Antihyperglycemic Effect by Inducing Autophagy and Modulating Gut Microbiota. J. Agric. Food Chem. 2020, 68, 13025–13037. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-Biofortified Colored Wheat Prevents High Fat Diet–Induced Alterations in Mice: Nutrigenomics Studies. Mol. Nutr. Food Res. 2020, 64, 1900999. [Google Scholar] [CrossRef]
- Wei, T.; Ji, X.; Xue, J.; Gao, Y.; Zhu, X.; Xiao, G. Cyanidin-3-O-glucoside represses tumor growth and invasion in vivo by suppressing autophagy via inhibition of the JNK signaling pathways. Food Funct. 2021, 12, 387–396. [Google Scholar] [CrossRef]
- Jing, N.; Song, J.; Liu, Z.; Wang, L.; Jiang, G. Glycosylation of anthocyanins enhances the apoptosis of colon cancer cells by handicapping energy metabolism. BMC Complement. Med. Ther. 2020, 20, 312. [Google Scholar] [CrossRef]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, I.D.; Venskutonis, P.R.; Georgiev, M.I.; Clément, C.; et al. Anthocyanins, multi-functional natural products of industrial relevance: Recent biotechnological advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Chatham, L.A.; Howard, J.E.; Juvik, J.A. A natural colorant system from corn: Flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2020, 310, 125734. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.; Ferreira, I.C. Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: Optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef] [PubMed]
- Agcam, E.; Akyıldız, A.; Balasubramaniam, V.M. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. 2017, 237, 461–470. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.S.P.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules 2019, 24, 2181. [Google Scholar] [CrossRef]
- Yang, W.; Kaimainen, M.; Järvenpää, E.; Sandell, M.; Huopalahti, R.; Yang, B.; Laaksonen, O. Red beet (Beta vulgaris) betalains and grape (Vitis vinifera) anthocyanins as colorants in white currant juice—Effect of storage on degradation kinetics, color stability and sensory properties. Food Chem. 2021, 348, 128995. [Google Scholar] [CrossRef]
- Yang, S.; Mi, L.; Wu, J.; Liao, X.; Xu, Z. Strategy for anthocyanins production: From efficient green extraction to novel microbial biosynthesis. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rakić, V.; Ulrih, N.P. Influence of pH on color variation and stability of cyanidin and cyanidin 3-O-β-glucopyranoside in aqueous solution. CyTA-J. Food 2021, 19, 174–182. [Google Scholar] [CrossRef]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Gonçalves, M.S.T. Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods 2020, 9, 771. [Google Scholar] [CrossRef]
- Nemetz, N.J.; Schieber, A.; Weber, F. Application of Crude Pomace Powder of Chokeberry, Bilberry, and Elderberry as a Coloring Foodstuff. Molecules 2021, 26, 2689. [Google Scholar] [CrossRef]
- Backes, E.; Leichtweis, M.G.; Pereira, C.; Carocho, M.; Barreira, J.C.M.; Kamal Genena, A.; José Baraldi, I.; Filomena Barreiro, M.; Barros, L.; Ferreira, I.C. Ficus carica L. and Prunus spinosa L. extracts as new anthocyanin-based food colorants: A thorough study in confectionery products. Food Chem. 2020, 333, 127457. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef] [PubMed]
- Direito, R.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M. Phenolic Compounds Impact on Rheumatoid Arthritis, Inflammatory Bowel Disease and Microbiota Modulation. Pharmaceutics 2021, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Lichteinstein, G.R.G.E.; Vanderpool, J.; Hark, L.M.G. Gastrointestinal disease. In Medical Nutrition and Disease: A Case-Based Approach; Wiley-Blackwell: New York, NY, USA, 2009; pp. 278–311. [Google Scholar]
- Pramitasari, R.; Sujonoputri, F.R.; Waturangi, D.E. Magna, Prebiotic activity of Indonesian black soybean (Glycine max (L.) Merr) anthocyanin. Sci. Adv. Biol. Pharm. 2021, 2, 37–041. [Google Scholar] [CrossRef]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an Anthocyanin and Prebiotic Blend on Intestinal Environment in Obese Male and Female Subjects. J. Nutr. Metab. 2018, 2018, 7497260. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Fracassetti, D.; Taverniti, V.; Del Bo’, C.; Vendrame, S.; Klimis-Zacas, D.; Arioli, S.; Riso, P.; Porrini, M. Differential Modulation of Human Intestinal Bifidobacterium Populations after Consumption of a Wild Blueberry (Vaccinium angustifolium) Drink. J. Agric. Food Chem. 2013, 61, 8134–8140. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, Y.; Tang, J.; Mi, J.; Lu, L.; Li, X.; Ran, L.; Zeng, X.; Cao, Y. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J. Funct. Foods 2018, 48, 533–541. [Google Scholar] [CrossRef]
- Choung, M.-G.; Baek, I.-Y.; Kang, S.-T.; Han, W.-Y.; Shin, D.-C.; Moon, H.-P.; Kang, K.-H. Isolation and Determination of Anthocyanins in Seed Coats of Black Soybean (Glycine max (L.) Merr.). J. Agric. Food Chem. 2001, 49, 5848–5851. [Google Scholar] [CrossRef]
- Ribeiro, L.R.; Salvadori, D.M.F. Dietary components may prevent mutation-related diseases in humans. Mutat. Res./Rev. Mutat. Res. 2003, 544, 195–201. [Google Scholar] [CrossRef]
- Takahashi, R.; Ohmori, R.; Kiyose, C.; Momiyama, Y.; Ohsuzu, F.; Kondo, K. Antioxidant Activities of Black and Yellow Soybeans against Low Density Lipoprotein Oxidation. J. Agric. Food Chem. 2005, 53, 4578–4582. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 1934578X1100600136. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Zduńczyk, Z. Anthocyanins in Strawberry Polyphenolic Extract Enhance the Beneficial Effects of Diets with Fructooligosaccharides in the Rat Cecal Environment. PLoS ONE 2016, 11, e0149081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, M.; He, S.; Cao, X.; Sun, H.; Chen, X.; Xie, Y.; Lou, Q.; Wang, X.; Ye, Y. Extraction and probiotic properties of newanthocyanins from purple sweet potato (Solanum tuberosum). Curr. Top. Nutraceutical Res. 2016, 14, 153. [Google Scholar]
- Mathur, R.; Barlow, G.M. Obesity and the microbiome. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1087–1099. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef]
- Bajzer, M.; Seeley, R.J. Obesity and gut flora. Nature 2006, 444, 1009–1010. [Google Scholar] [CrossRef]
- Tsuda, T. Recent Progress in Anti-Obesity and Anti-Diabetes Effect of Berries. Antioxidants 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.-E.; Kang, D.-W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors 2017, 43, 507–516. [Google Scholar] [CrossRef]
- Cenac, N.; Andrews, C.N.; Holzhausen, M.; Chapman, K.; Cottrell, G.; Andrade-Gordon, P.; Steinhoff, M.; Barbara, G.; Beck, P.; Bunnett, N.W.; et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Investig. 2007, 117, 636–647. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response. Nutrients 2017, 9, 1341. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef]
- Vasile, M.; Adelina, M.; Enachi, E.; Barbu, V.; Circiumaru, A.; Bahrim, G.E.; Rapeanu, G.; Nicoleta, S. Functional Enhancement of Bioactives from Black Beans and Lactic Acid Bacteria into an Innovative Food Ingredient by Comicroencapsulation. Food Bioprocess Technol. 2020, 13, 978–987. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Cornelian Cherry Fruits and Lactic Acid Bacteria in Biopolymeric Matrices by Freeze-Drying: Evidences on Functional Properties and Applications in Food. Polymers 2020, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-encapsulation of probiotics with prebiotics and their application in functional/synbiotic dairy products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- Westfall, A.; Sigurdson, G.T.; Giusti, M.M. Antioxidant, UV Protection, and Antiphotoaging Properties of Anthocyanin-Pigmented Lipstick Formulations. J. Cosmet. Sci. 2019, 70, 63–76. [Google Scholar] [PubMed]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant (Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef]
- Phan, K.; Van Den Broeck, E.; Van Speybroeck, V.; De Clerck, K.; Raes, K.; De Meester, S. The potential of anthocyanins from blueberries as a natural dye for cotton: A combined experimental and theoretical study. Dye. Pigment. 2020, 176, 108180. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.; Zhou, W. A method for dyeing cotton fabric with anthocyanin dyes extracted from mulberry (Morus rubra) fruits. Color. Technol. 2016, 132, 222–231. [Google Scholar] [CrossRef]
- Haddar, W.; Ben Ticha, M.; Meksi, N.; Guesmi, A. Application of anthocyanins as natural dye extracted from Brassica oleracea L. var. capitata f. rubra: Dyeing studies of wool and silk fibres. Nat. Prod. Res. 2018, 32, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Trevisol, T.C.; Pelicioli, J.; Terrazas, M.B.; Monteiro, A.R.; Valencia, G.A. Preparation and Characterization of Colorimetric Indicator Films Based on Chitosan/Polyvinyl Alcohol and Anthocyanins from Agri-Food Wastes. J. Polym. Environ. 2020, 29, 1616–1629. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, T.-S.; Yan, H.; Hu, X.; Ren, T. Novel pH-sensitive films based on starch/polyvinyl alcohol and food anthocyanins as a visual indicator of shrimp deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776. [Google Scholar] [CrossRef]
- Lee, J.M.; Jin, M. The Role of Glucosides on the Thermal Stability of Color Produced by Anthocyanins from Red Cabbage. Food Eng. Prog. 2015, 19, 172–176. [Google Scholar] [CrossRef]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Ağçam, E.; Akyıldız, A. Effects of different solvents and acid concentrations on extraction of anthocyanins from black carrot pomace. Food 2015, 40, 149–156. [Google Scholar]
- Okur, İ.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the Effect of Different Extraction Techniques on Sour Cherry Pomace Phenolic Content and Antioxidant Activity and Determination of Phenolic Compounds by FTIR and HPLC. Waste Biomass Valorization 2019, 10, 3545–3555. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Allaw, M.; Hellström, J.; Granato, D.; Manconi, M. Jabuticaba (Myrciaria jaboticaba) Peel as a Sustainable Source of Anthocyanins and Ellagitannins Delivered by Phospholipid Vesicles for Alleviating Oxidative Stress in Human Keratinocytes. Molecules 2021, 26, 6697. [Google Scholar] [CrossRef] [PubMed]
- Wathon, M.H.; Beaumont, N.; Benohoud, M.; Blackburn, R.S.; Rayner, C.M. Extraction of anthocyanins from Aronia melanocarpa skin waste as a sustainable source of natural colorants. Color. Technol. 2019, 135, 5–16. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Waltman, L.; van Eck, N.J.; Noyons, E.C.M. A unified approach to mapping and clustering of bibliometric networks. J. Informetr. 2010, 4, 629–635. [Google Scholar] [CrossRef]
- Philip, T. An anthocyanin recovery system from grape wastes. J. Food Sci. 1974, 39, 859. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.S.; Dufossé, L.; Morales-Oyervides, L. Editorial: Sustainable Production of Bioactive Pigments. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Gamlath, S.; Wakeling, L. Non-Thermal food Processing: Impact on Chemical, Nutritional and Bioactive Components; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 1–91. [Google Scholar]
- Pires, C.S.P.T.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, C.F.R.I. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications - A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.P.; Pereira, E.; Melgar, B.; Stojković, D.; Sokovic, M.; Calhelha, R.C.; Pereira, C.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Barros, L. Eggplant Fruit (Solanum melongena L.) and Bio-Residues as a Source of Nutrients, Bioactive Compounds, and Food Colorants, Using Innovative Food Technologies. Appl. Sci. 2021, 11, 151. [Google Scholar] [CrossRef]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as high-performance green co-solvents in the aqueous extraction of polyphenols and anthocyanin pigments from solid onion waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Pasquel Reátegui, J.L.; Machado, A.P.d.F.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- da Silva Frasao, B.; Lima dos Santos Rosario, A.I.; Leal Rodrigues, B.; Abreu Bitti, H.; Diogo Baltar, J.; Nogueira, R.I.; Pereira da Costa, M.; Conte-Junior, C.A. Impact of juçara (Euterpe edulis) fruit waste extracts on the quality of conventional and antibiotic-free broiler meat. Poult. Sci. 2021, 100, 101232. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Christiano, F.D.P.; Marczak, L.D.F.; Tessaro, I.C.; Thys, R.C.S. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT-Food Sci. Technol. 2014, 58, 497–501. [Google Scholar] [CrossRef]
- Sofroniou, A. Relational Databases and Distributed Systems, 1st ed.; Lulu.com: Raleigh, NC, USA, 2018; p. 184. [Google Scholar]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. U.S., in Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory Home Page. 2008. Available online: http://www.ars.usda.gov/nutrientdata/isoflav (accessed on 2 December 2022).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0, a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Kiely, M.; Black, L.J.; Plumb, J.; Kroon, P.A.; Hollman, P.C.; Larsen, J.C.; Speijers, G.J.; Kapsokefalou, M.; Sheehan, D.; Gry, J.; et al. EuroFIR eBASIS: Application for health claims submissions and evaluations. Eur. J. Clin. Nutr. 2010, 64, S101–S107. [Google Scholar] [CrossRef]
- Plumb, J.; Pigat, S.; Bompola, F.; Cushen, M.; Pinchen, H.; Nørby, E.; Astley, S.; Lyons, J.; Kiely, M.; Finglas, P. eBASIS (Bioactive Substances in Food Information Systems) and Bioactive Intakes: Major Updates of the Bioactive Compound Composition and Beneficial Bioeffects Database and the Development of a Probabilistic Model to Assess Intakes in Europe. Nutrients 2017, 9, 320. [Google Scholar] [CrossRef]
- Igwe, E.; Neale, E.; Charlton, K.E.; Morton, K.; Probst, Y.C. First stage development of an Australian anthocyanin food composition database for dietary studies—A systematic process and its challenges. J. Food Compos. Anal. 2017, 64, 33–38. [Google Scholar] [CrossRef]


| Reference | Colored Medium | Source | Final Color | Reported Results |
|---|---|---|---|---|
| Primo da Silva et al. (2019) [116] | Donut | Rubus ulmifolius extract
|  (L* = 57.5, a* = 10.8, b* = 10.9) |
|
| Dias et al. (2020) [120] | Soy-based yogurt | Red radish extract
|  (L* = 69.43, a* = 7.45, b* = 0.61) |
|
| Nemetz et al. (2021) [121] | Yogurt | Chokeberry pomace powder
| ΔE = 1.87, ho = 1.79, C* = 19.74 |
|
Bilberry pomace powder
| ΔE = 2.17, ho = 10.42, C* = 16.78 | |||
Elderberry pomace powder
| ΔE = 4.89, ho = 3.59, C* = 15.16 | |||
| Yang et al. (2021) [117] | White currant juice | Solution of grape anthocyanins
|  (L* = 31.1, a* = 18.7, b* = 2.56, C* = 18.9, ho = 7.64) |
|
| Albuquerque et al. (2020) [114] | Macaron | Jabuticaba epicarp
|  (L* = 80.9, a* = 6.3, b* = 7.6) |
|
| Backes et al. (2020) [122] | Bakery products (Icing and Beijinhos) | Fig peels and blackthorn fruit extracts
|  (Icing; L* = 62, a* = 13, b* = 15)  (Beijinhos; L* = 66, a* = 10, b* = 15) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2023, 12, 48. https://doi.org/10.3390/antiox12010048
Ayvaz H, Cabaroglu T, Akyildiz A, Pala CU, Temizkan R, Ağçam E, Ayvaz Z, Durazzo A, Lucarini M, Direito R, et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants. 2023; 12(1):48. https://doi.org/10.3390/antiox12010048
Chicago/Turabian StyleAyvaz, Huseyin, Turgut Cabaroglu, Asiye Akyildiz, Cigdem Uysal Pala, Riza Temizkan, Erdal Ağçam, Zayde Ayvaz, Alessandra Durazzo, Massimo Lucarini, Rosa Direito, and et al. 2023. "Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential" Antioxidants 12, no. 1: 48. https://doi.org/10.3390/antiox12010048
APA StyleAyvaz, H., Cabaroglu, T., Akyildiz, A., Pala, C. U., Temizkan, R., Ağçam, E., Ayvaz, Z., Durazzo, A., Lucarini, M., Direito, R., & Diaconeasa, Z. (2023). Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants, 12(1), 48. https://doi.org/10.3390/antiox12010048











