Identification and Functional Analysis of CAD Gene Family in Pomegranate (Punica granatum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. RNA Extraction, Library Construction, and Sequencing
2.3. Identification of CAD Gene Family Members and Physicochemical Properties Analysis in Pomegranate
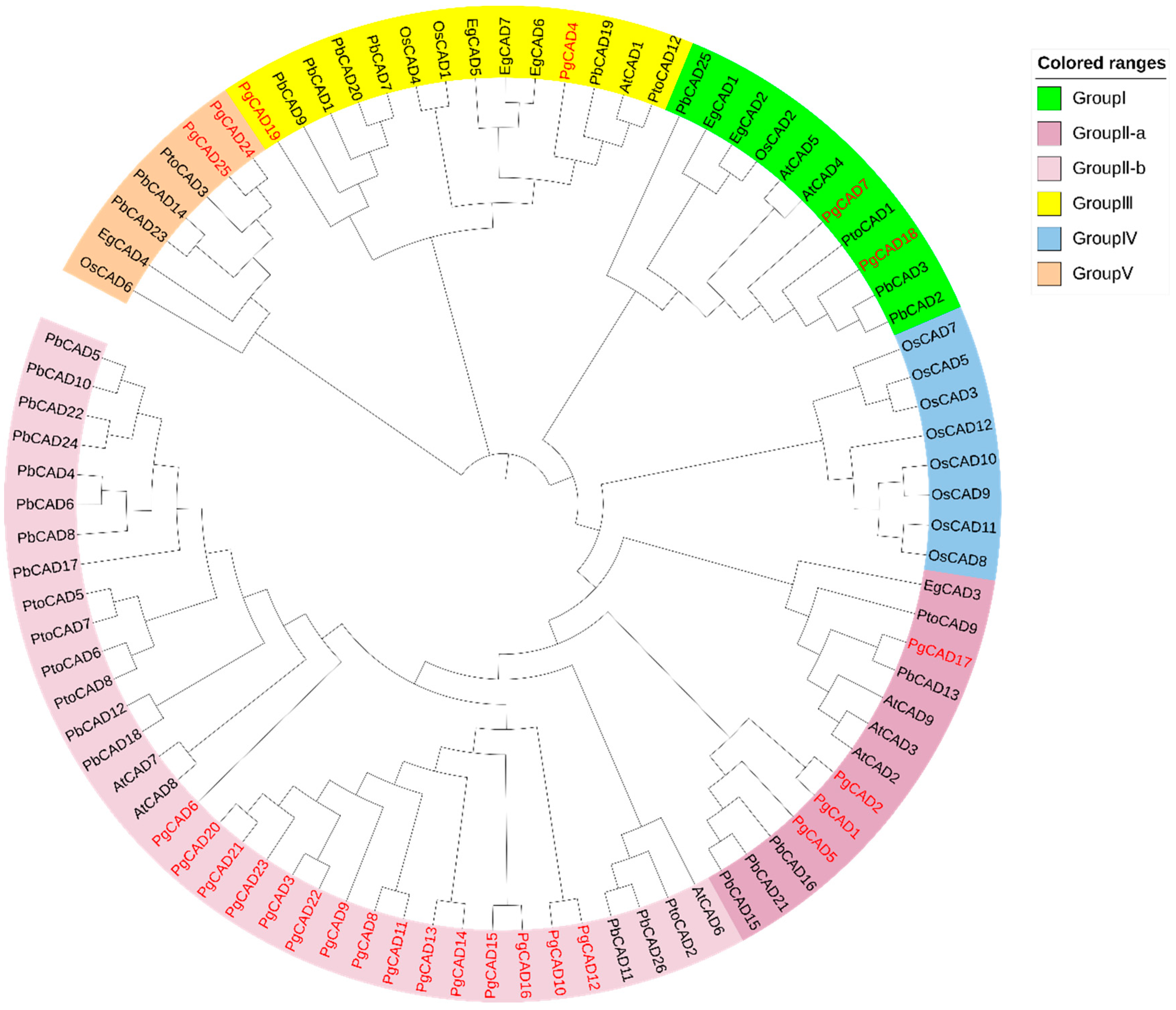
2.4. Phylogenetic Analysis
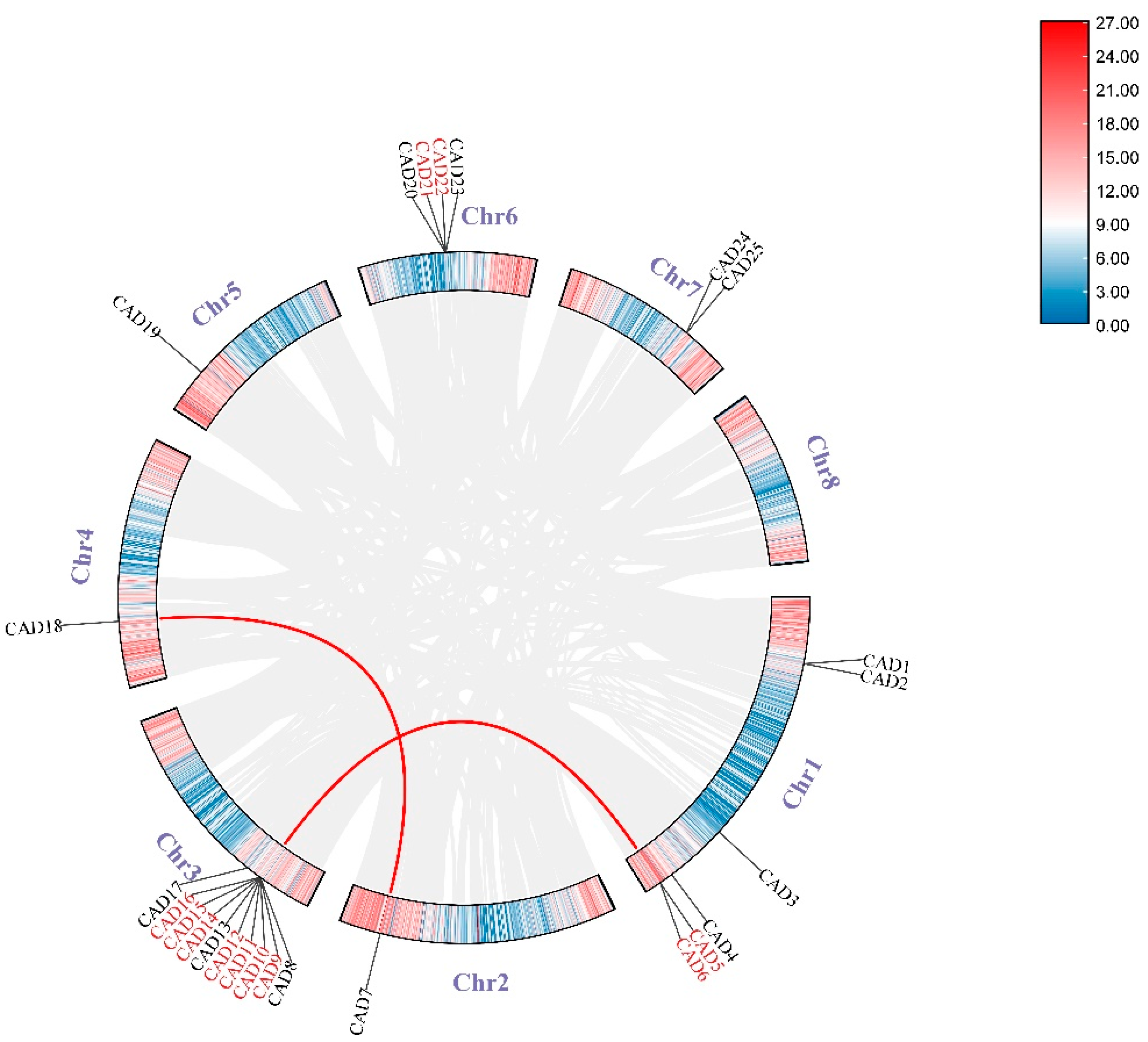
2.5. Chromosomal Position, Duplication Mode, and Collinearity Analysis
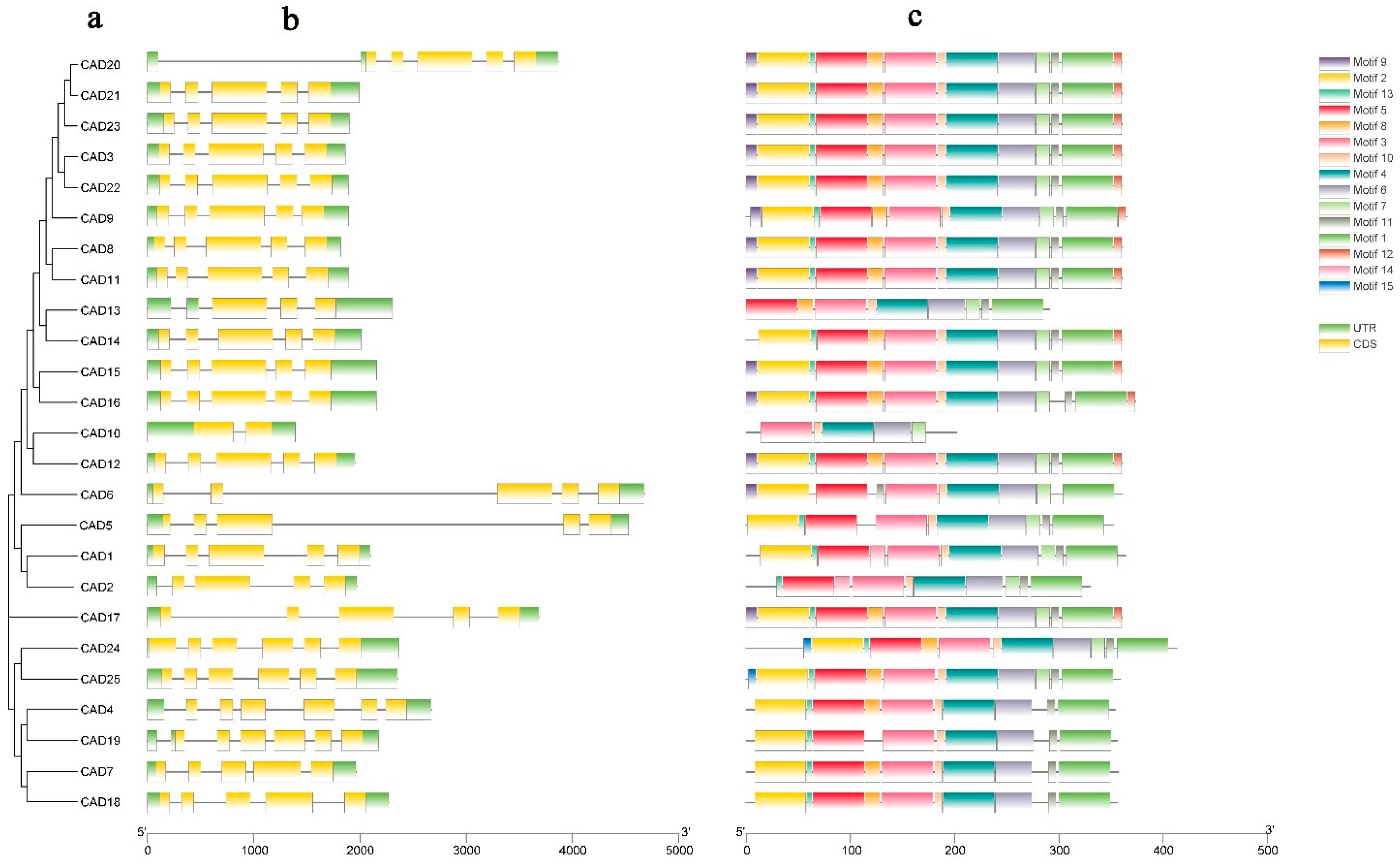
2.6. Gene Structure and Protein Motif Analysis
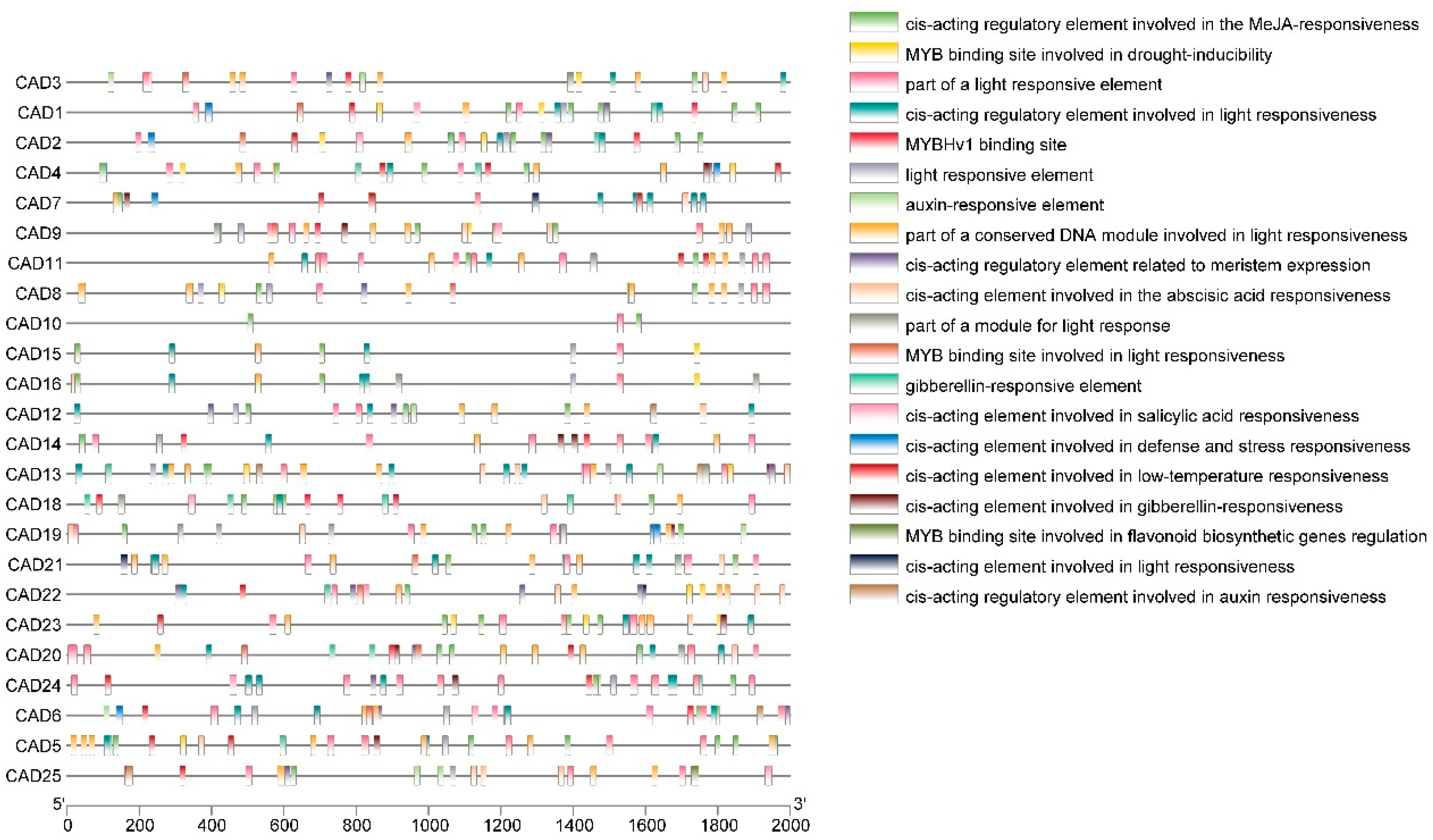
2.7. Analysis of Promoter Cis-Acting Elements
2.8. Expression of CAD Gene in Pomegranate
3. Results
3.1. Physicochemical Properties of Pomegranate CAD Proteins
3.2. Phylogenetic Analysis of the CAD Gene Family in Pomegranate
3.3. Chromosomal Position, Duplication Mode, and Collinearity Analysis
3.4. The Gene Structures and Protein Domains Analysis of the CAD Gene Family in Pomegranate
3.5. Promoter Analysis of Pomegranate CAD Genes
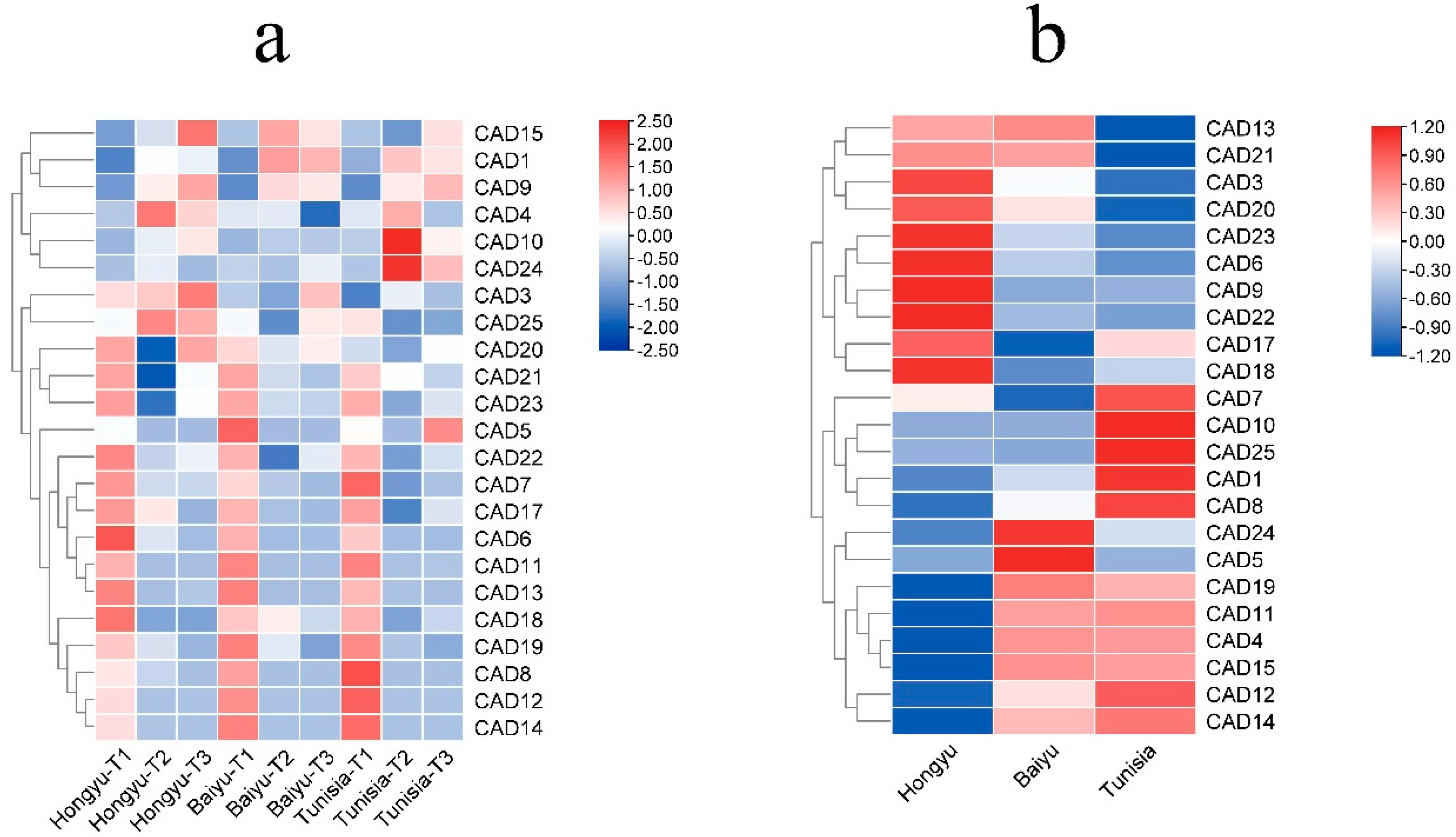
3.6. Expression of PgCAD genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, N.V.; Abburi, V.L.; Ramajayam, D.; Kumar, R.; Chandra, R.; Sharma, K.K.; Sharma, J.; Babu, K.D.; Pal, R.K.; Mundewadikar, D.M.; et al. Genetic diversity and association mapping of bacterial blight and other horticulturally important traits with microsatellite markers in pomegranate from India. Mol. Genet. Genom. 2015, 290, 1393–1402. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Ren, Y.; Wang, Y.; Yuan, Z. Fruit Breeding in Regard to Color and Seed Hardness: A Genomic View from Pomegranate. Agronomy 2020, 10, 991. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Harakeh, S.; Ramadan, W.S.; Al Muhayawi, M.S.; Al Jaouni, S.; Mousa, S. Pomegranate peel extract lessens histopathologic changes and restores antioxidant homeostasis in the hippocampus of rats with aluminium chloride-induced Alzheimer’s disease. Asian Pac. J. Trop. Med. 2020, 13, 456. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Wang, Y.; Zhang, X.; Zhao, X.; Yuan, Z. Genome-Wide Identification and Expression Analysis of MIKC-Type MADS-Box Gene Family in Punica granatum L. Agronomy 2020, 10, 1197. [Google Scholar] [CrossRef]
- Morittu, V.M.; Mastellone, V.; Tundis, R.; Loizzo, M.R.; Tudisco, R.; Figoli, A.; Cassano, A.; Musco, N.; Britti, D.; Infascelli, F.; et al. Antioxidant, Biochemical, and In-Life Effects of Punica granatum L. Natural Juice vs. Clarified Juice by Polyvinylidene Fluoride Membrane. Foods 2020, 9, 242. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Huang, R.; Isha, A.; Dong, L. Generation and analysis of expressed sequence tag sequences from a soft-seeded pomegranate cDNA library. Plant Breed. 2017, 136, 994–999. [Google Scholar] [CrossRef]
- Gomez-Casati, D.F.; Busi, M.V.; Barchiesi, J.; Peralta, D.A.; Hedin, N.; Bhadauria, V. Applications of Bioinformatics to Plant Biotechnology. Curr. Issues Mol. Biol. 2018, 27, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Poudel, K.; Luo, X.; Chen, L.; Jing, D.; Xia, X.; Tang, L.; Li, H.; Cao, S. Identification of the SUT Gene Family in Pomegranate (Punica granatum L.) and Functional Analysis of PgL0145810.1. Int. J. Mol. Sci. 2020, 21, 6608. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-Y.; Niu, J.; Cao, D.; Li, H.-X.; Xue, H.; Chen, L.-N.; Zhang, F.-H.; Zhao, D.-G. Comparative proteomics analysis of pomegranate seeds on fruit maturation period (Punica granatum L.). J. Integr. Agric. 2015, 14, 2558–2564. [Google Scholar] [CrossRef]
- Qin, G.; Xu, C.; Ming, R.; Tang, H.; Guyot, R.; Kramer, E.M.; Hu, Y.; Yi, X.; Qi, Y.; Xu, X.; et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef]
- Niu, J.; Cao, D.; Li, H.; Xue, H.; Chen, L.; Liu, B.; Cao, S. Quantitative proteomics of pomegranate varieties with contrasting seed hardness during seed development stages. Tree Genet. Genomes 2018, 14, 14. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.; Wu, Z.; Yao, W.; Zhao, P.; Cao, D.; Yu, H.; Li, K.; Poudel, K.; Zhao, D.; et al. The pomegranate (Punica granatum L.) draft genome dissects genetic divergence between soft- and hard-seeded cultivars. Plant Biotechnol. J. 2019, 18, 955–968. [Google Scholar] [CrossRef]
- Dai, H.; Han, G.; Yan, Y.; Zhang, F.; Liu, Z.; Li, X.; Li, W.; Ma, Y.; Li, H.; Liu, Y.; et al. Transcript Assembly and Quantification by RNA-Seq Reveals Differentially Expressed Genes between Soft-Endocarp and Hard-Endocarp Hawthorns. PLoS ONE 2013, 8, e72910. [Google Scholar] [CrossRef]
- Xue, H.; Cao, S.; Li, H.; Zhang, J.; Niu, J.; Chen, L.; Zhang, F.; Zhao, D. De novo transcriptome assembly and quantification reveal differentially expressed genes between soft-seed and hard-seed pomegranate (Punica granatum L.). PLoS ONE 2017, 12, e0178809. [Google Scholar] [CrossRef]
- Luo, X.; Cao, D.; Zhang, J.; Chen, L.; Xia, X.; Li, H.; Zhao, D.; Zhang, F.; Xue, H.; Chen, L.; et al. Integrated microRNA and mRNA expression profiling reveals a complex network regulating pomegranate (Punica granatum L.) seed hardness. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Zarei, A.; Zamani, Z.; Fatahi, R.; Mousavi, A.; Salami, S.A.; Avila, C.; Cánovas, F.M. Differential expression of cell wall related genes in the seeds of soft- and hard-seeded pomegranate genotypes. Sci. Hortic. 2016, 205, 7–16. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.-M.; Hawkins, S.; Rochange, S. The polymorphism of the genes/enzymes involved in the last two reductive steps of monolignol synthesis: What is the functional significance? C. R. Biol. 2004, 327, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.; Camacho, R.; Moinuddin, S.G.A.; Lee, C.; Davin, L.B.; Lewis, N.G.; Kang, C. Crystal structures and catalytic mechanism of the Arabidopsis cinnamyl alcohol dehydrogenases AtCAD5 and AtCAD4. Org. Biomol. Chem. 2006, 4, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Luderitz, T.; Grisebach, H. Enzymic Synthesis of Lignin Precursors Comparison of Cinnamoyl-CoA Reductase and Cinnamyl Alcohol: NADP+ Dehydrogenase from Spruce (Picea abies L.) and Soybean (Glycine max L.). JBIC J. Biol. Inorg. Chem. 1981, 119, 115–124. [Google Scholar] [CrossRef]
- Knight, M.E.; Halpin, C.; Schuch, W. Identification and characterisation of cDNA clones encoding cinnamyl alcohol dehydrogenase from tobacco. Plant Mol. Biol. 1992, 19, 793–801. [Google Scholar] [CrossRef]
- Raes, J.; Rohde, A.; Christensen, J.H.; Van de Peer, Y.; Boerjan, W. Genome-Wide Characterization of the Lignification Toolbox in Arabidopsis. Plant Physiol. 2003, 133, 1051–1071. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, M.-R.; Bedgar, D.L.; Moinuddin, S.G.A.; Cardenas, C.L.; Davin, L.B.; Kang, C.; Lewis, N.G. Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Tronchet, M.; Balaguã, C.; Kroj, T.; Jouanin, L.; Roby, D. Cinnamyl alcohol dehydrogenases-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol. Plant Pathol. 2010, 11, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sibout, R.; Eudes, A.; Pollet, B.; Goujon, T.; Mila, I.; Granier, F.; Séguin, A.; Lapierre, C.; Jouanin, L. Expression Pattern of Two Paralogs Encoding Cinnamyl Alcohol Dehydrogenases in Arabidopsis. Isolation and Characterization of the Corresponding Mutants. Plant Physiol. 2003, 132, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Bagniewska-Zadworna, A.; Choi, A.; Plakkat, U.; DiLoreto, D.S.; Yellanki, P.; E Carlson, J. The cinnamyl alcohol dehydrogenase gene family in Populus: Phylogeny, organization, and expression. BMC Plant Biol. 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, M.; Li, D.; Zhang, J.; Jin, Q.; Sheng, L.; Cai, Y.; Lin, Y. Correction: Characterization and analysis of CCR and CAD gene families at the whole-genome level for lignin synthesis of stone cells in pear (Pyrus bretschneideri) fruit. Biol. Open 2020, 9, 1602–1613. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, X.F.; Yang, S.L.; Wang, C.H.; Lu, G.L.; Wang, R.; Yang, Y.J.; Li, D.L. Heterogenous expression of Pyrus pyrifolia PpCAD2 and PpEXP2 in tobacco impacts lignin accumulation in transgenic plants. Gene 2017, 637, 181–189. [Google Scholar] [CrossRef]
- Li, M.T.; Cheng, C.X.; Zhang, X.F.; Zhou, S.P.; Li, L.X.; Yang, S.L. Overexpression of Pear (Pyrus pyrifolia) CAD2 in Tomato Affects Lignin Content. Molecules 2019, 24, 2595. [Google Scholar] [CrossRef]
- Wang, P.; Yang, J.; Li, Z.-Y.; Zhu, J.-J.; Gao, Q.-H.; Ni, D.-A.; Duan, K. Genome-wide identification and expression analysis revealed cinnamyl alcohol dehydrogenase genes correlated with fruit-firmness in strawberry1. J. Berry Res. 2021, 11, 447–464. [Google Scholar] [CrossRef]
- Li, Y.K.; Wang, R.; Pei, Y.K.; Yu, W.W.; Wu, W.J.; Li, D.; Hu, Z.N. Phylogeny and functional characterization of the cinnamyl alcohol dehydrogenase gene family in Phryma leptostachya. Int. J. Biol. Macromol. 2022, 217, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.; Yu, T.; Hou, C.; Liu, L.; Zhang, L. Genome-wide analysis of the lignin toolbox for morus and the roles of lignin related genes in response to zinc stress. Peerj 2021, 9, e11964. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.; Huang, S.; Kang, X.R.; Yidilisi, K.; Dai, M.; Liu, L. Systematic functional characterization of cinnamyl alcohol dehydrogenase family members revealed their functional divergence in lignin biosynthesis and stress responses in mulberry. Plant Physiol. Biochem. 2022, 186, 145–156. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Qi, K.; Song, X.; Yuan, Y.; Bao, J.; Gong, X.; Huang, X.; Khanizadeh, S.; Zhang, S.; Tao, S. CAD Genes: Genome-Wide Identification, Evolution, and Their Contribution to Lignin Biosynthesis in Pear (Pyrus bretschneideri). Plants 2021, 10, 1444. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Qin, G.; Liu, C.; Cao, Z.; Jia, B.; Xu, Y.; Li, G.; Yang, Y.; Su, Y.; et al. Characterization of the ABC Transporter G Subfamily in Pomegranate and Function Analysis of PgrABCG14. Int. J. Mol. Sci. 2022, 23, 11661. [Google Scholar] [CrossRef]
- Dong, L.; Xiong, F.; Liu, N.; Wang, Q.; Zhang, S. Molecular Cloning and Expression Analysis of PgLAC in Pomegranate. Am. J. Mol. Biol. 2018, 8, 145–155. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Wu, S.; Chang, X.; Wang, X.; Zhao, Y.; Xia, Y.; Trigiano, R.N.; Jiao, Y.; Chen, F. The ancient wave of polyploidization events in flowering plants and their facilitated adaptation to environmental stress. Plant Cell Environ. 2020, 43, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Bae, J.M.; Huh, G.-H. Transcriptional regulation of the cinnamyl alcohol dehydrogenase gene from sweetpotato in response to plant developmental stage and environmental stress. Plant Cell Rep. 2010, 29, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.T.; Mahmood, M.; Seman, I.A.; Wong, M.-Y. The Phenylpropanoid Pathway and Lignin in Defense against Ganoderma boninense Colonized Root Tissues in Oil Palm (Elaeis guineensis Jacq.). Front. Plant Sci. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Yusuf, C.Y.L.; Nabilah, N.S.; Taufik, N.A.A.M.; Abu Seman, I.; Abdullah, M.P. Genome-wide analysis of the CAD gene family reveals two bona fide CAD genes in oil palm. 3 Biotech 2022, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, O.; Botha, C.E.; Bradley, G. In silico analysis of cis-acting regulatory elements in 5′ regulatory regions of sucrose transporter gene families in rice (Oryza sativa Japonica) and Arabidopsis thaliana. Comput. Biol. Chem. 2010, 34, 268–283. [Google Scholar] [CrossRef]
- Khaksar, G.; Tabatabaei, B.E.S.; Arzani, A.; Ghobadi, C.; Ebrahimie, E. Functional Analysis of a Pomegranate (Punica granatum L.) MYB Transcription Factor Involved in the Regulation of Anthocyanin Biosynthesis. Iran. J. Biotechnol. 2015, 13, 17–25. [Google Scholar] [CrossRef][Green Version]

| Name | Gene ID | No. of Amino Acide | Mol. Wt (kDa) | Isoelectric Point (pI) | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| PgCAD1 | XM_031521130.1 | 364 | 39,091.80 | 5.94 | 31.86 | 91.04 | 0.008 | Cytoplasm |
| PgCAD2 | XM_031521131.1 | 330 | 35,401.70 | 5.70 | 29.57 | 94.21 | 0.050 | Cytoplasm |
| PgCAD3 | XM_031518621.1 | 361 | 39,241.30 | 5.73 | 32.26 | 87.76 | 0.023 | Cytoplasm |
| PgCAD4 | XM_031521510.1 | 354 | 39,203.86 | 7.54 | 38.20 | 77.60 | −0.244 | Cytoplasm |
| PgCAD5 | XM_031549529.1 | 352 | 37,611.48 | 6.21 | 27.43 | 98.64 | 0.168 | Cytoplasm |
| PgCAD6 | XM_031549518.1 | 361 | 38,767.91 | 6.67 | 32.29 | 96.62 | 0.096 | Cytoplasm |
| PgCAD7 | XM_031526044.1 | 357 | 38,864.78 | 5.46 | 22.55 | 90.28 | −0.003 | Cytoplasm |
| PgCAD8 | XM_031530115.1 | 361 | 38,658.47 | 5.95 | 28.74 | 92.02 | 0.066 | Cytoplasm |
| PgCAD9 | XM_031530113.1 | 365 | 39,148.38 | 6.70 | 29.78 | 89.70 | 0.043 | Cytoplasm |
| PgCAD10 | XM_031530121.1 | 202 | 21,601.50 | 7.78 | 28.40 | 114.41 | 0.279 | Cytoplasm |
| PgCAD11 | XM_031530114.1 | 361 | 38,783.69 | 6.06 | 27.40 | 92.02 | 0.044 | Cytoplasm |
| PgCAD12 | XM_031532959.1 | 361 | 38,792.61 | 5.70 | 38.79 | 92.88 | 0.015 | Cytoplasm |
| PgCAD13 | XM_031532961.1 | 291 | 31,297.27 | 6.14 | 24.01 | 95.05 | 0.084 | Cytoplasm |
| PgCAD14 | XM_031532960.1 | 361 | 38,892.13 | 6.31 | 23.22 | 93.91 | 0.099 | Cytoplasm |
| PgCAD15 | XM_031532956.1 | 374 | 40,503.65 | 6.31 | 28.21 | 92.46 | 0.021 | Cytoplasm |
| PgCAD16 | XM_031532957.1 | 361 | 39,055.97 | 6.43 | 29.04 | 92.27 | −0.025 | Cytoplasm |
| PgCAD17 | XM_031532280.1 | 361 | 39,130.21 | 6.79 | 29.49 | 91.47 | 0.005 | Cytoplasm |
| PgCAD18 | XM_031536024.1 | 356 | 38,861.85 | 5.72 | 29.12 | 91.91 | −0.055 | Cytoplasm |
| PgCAD19 | XM_031540038.1 | 356 | 38,754.64 | 5.65 | 26.95 | 84.86 | −0.027 | Cytoplasm |
| PgCAD20 | XM_031545715.1 | 361 | 39,273.56 | 6.46 | 25.15 | 97.45 | 0.023 | Cytoplasm |
| PgCAD21 | XM_031544474.1 | 361 | 39,245.50 | 6.46 | 25.39 | 96.93 | 0.017 | Cytoplasm |
| PgCAD22 | XM_031544478.1 | 361 | 39,238.27 | 5.73 | 29.67 | 90.69 | 0.021 | Cytoplasm |
| PgCAD23 | XM_031544479.1 | 361 | 39,143.31 | 6.13 | 28.53 | 93.66 | 0.017 | Cytoplasm |
| PgCAD24 | XM_031548045.1 | 413 | 45,082.65 | 8.65 | 31.19 | 83.80 | −0.138 | Cytoplasm |
| PgCAD25 | XM_031551278.1 | 359 | 38,947.64 | 6.59 | 28.04 | 91.78 | −0.035 | Cytoplasm |
| Gene Pair | Ka | Ks | Ka/Ks |
|---|---|---|---|
| PgCAD5-PgCAD8 | 0.280640639 | 3.285317089 | 0.085422695 |
| PgCAD6-PgCAD | 0.207931028 | 2.218153296 | 0.093740603 |
| PgCAD7-PgCAD18 | 0.113573012 | 1.141785238 | 0.099469679 |
| Gene Name | Whole Genome Duplication or Segmental | Tandem Duplication | Dispersed Duplication | Proximal Duplication |
|---|---|---|---|---|
| PgCAD1 | √ | |||
| PgCAD2 | ||||
| PgCAD3 | √ | |||
| PgCAD4 | √ | |||
| PgCAD5 | √ | |||
| PgCAD6 | √ | |||
| PgCAD7 | √ | |||
| PgCAD8 | √ | |||
| PgCAD9 | √ | |||
| PgCAD10 | √ | |||
| PgCAD11 | √ | |||
| PgCAD12 | √ | |||
| PgCAD13 | ||||
| PgCAD14 | √ | |||
| PgCAD15 | √ | |||
| PgCAD16 | ||||
| PgCAD17 | √ | |||
| PgCAD18 | √ | |||
| PgCAD19 | √ | |||
| PgCAD20 | ||||
| PgCAD21 | √ | |||
| PgCAD22 | √ | |||
| PgCAD23 | √ | |||
| PgCAD24 | √ | |||
| PgCAD25 | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Zhang, X.; Ni, H.; Yuan, F.; Zhang, S. Identification and Functional Analysis of CAD Gene Family in Pomegranate (Punica granatum). Genes 2023, 14, 26. https://doi.org/10.3390/genes14010026
Hu L, Zhang X, Ni H, Yuan F, Zhang S. Identification and Functional Analysis of CAD Gene Family in Pomegranate (Punica granatum). Genes. 2023; 14(1):26. https://doi.org/10.3390/genes14010026
Chicago/Turabian StyleHu, Lei, Xuan Zhang, Huihui Ni, Fangyu Yuan, and Shuiming Zhang. 2023. "Identification and Functional Analysis of CAD Gene Family in Pomegranate (Punica granatum)" Genes 14, no. 1: 26. https://doi.org/10.3390/genes14010026
APA StyleHu, L., Zhang, X., Ni, H., Yuan, F., & Zhang, S. (2023). Identification and Functional Analysis of CAD Gene Family in Pomegranate (Punica granatum). Genes, 14(1), 26. https://doi.org/10.3390/genes14010026





